Nucleic Acids Protiens and Enzymes Nucleic Acids Contain



- Slides: 29

Nucleic Acids, Protiens, and Enzymes

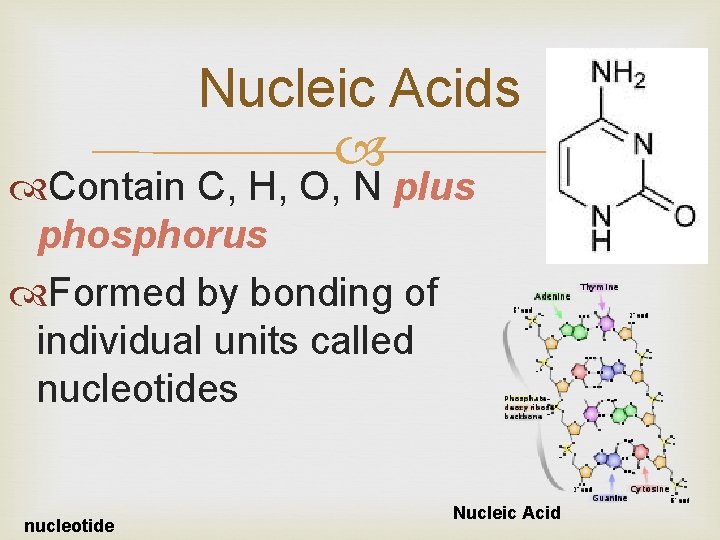
Nucleic Acids Contain C, H, O, N plus phosphorus Formed by bonding of individual units called nucleotides nucleotide Nucleic Acid

Nucleic Acids Store and transmit hereditary information Ex: DNA (deoxyribonucleic acid) RNA (ribonucleic acid)


Proteins Contain C, H, O, plus nitrogen More than 20 amino acids can be joined in any order or number to make countless proteins (think of how many words can be made from 26 letters!) Form unique structures that perform specific functions in living organisms Also known as Enzymes that allow regulation of activities in the body

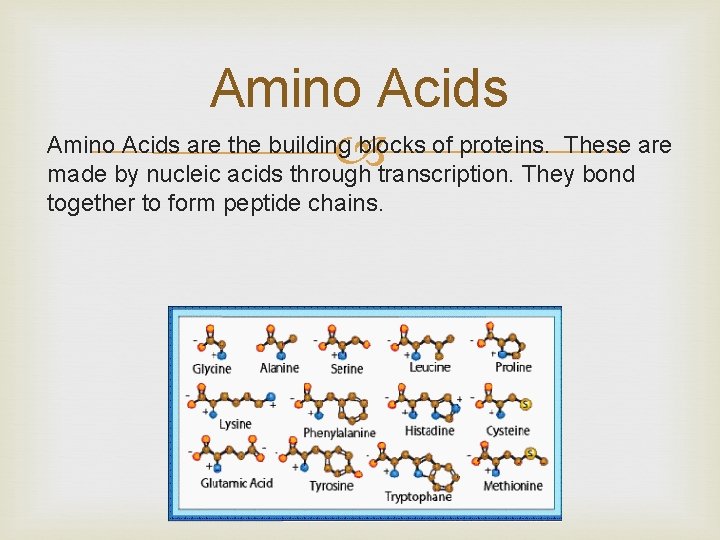
Amino Acids are the building blocks of proteins. These are made by nucleic acids through transcription. They bond together to form peptide chains.

Protein Structure Chains are folded and twisted giving each protein a unique shape Van der Waals forces and hydrogen bonds help maintain protein’s shape The four stages of protein structure (primary, secondary, tertiary, and quaternary) account for a final shape. Shape of protein is important to its function!

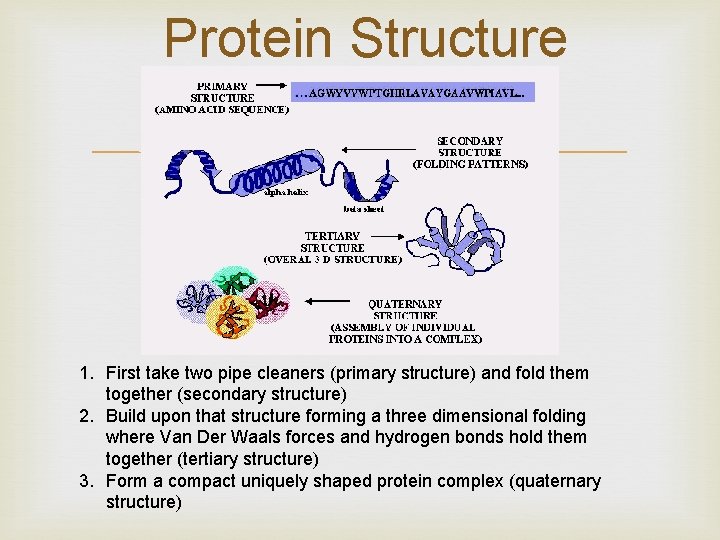
Protein Structure 1. First take two pipe cleaners (primary structure) and fold them together (secondary structure) 2. Build upon that structure forming a three dimensional folding where Van Der Waals forces and hydrogen bonds hold them together (tertiary structure) 3. Form a compact uniquely shaped protein complex (quaternary structure)

Protein Function Provide structure Ex: Collagen- makes up your skin, muscles & bones Aid chemical activities in your body Ex: Enzymes- work to speed up (catalyze) reactions in your body Transport substances into or out of cells Help fight diseases


Rates of Reaction Chemical reactions occur when different atoms or molecules collide: For the reaction to happen the particles must have a certain amount of energy – this is called the ACTIVATION ENERGY. The rate at which the reaction happens depends on four things: 1) The temperature of the reactants, 2) Their concentration 3) Their surface area 4) Whether or not a catalyst is used (Enzymes)

Measuring rate of reaction Two common ways: 1) Measure how fast the products are formed 2) Measure how fast the reactants are used up

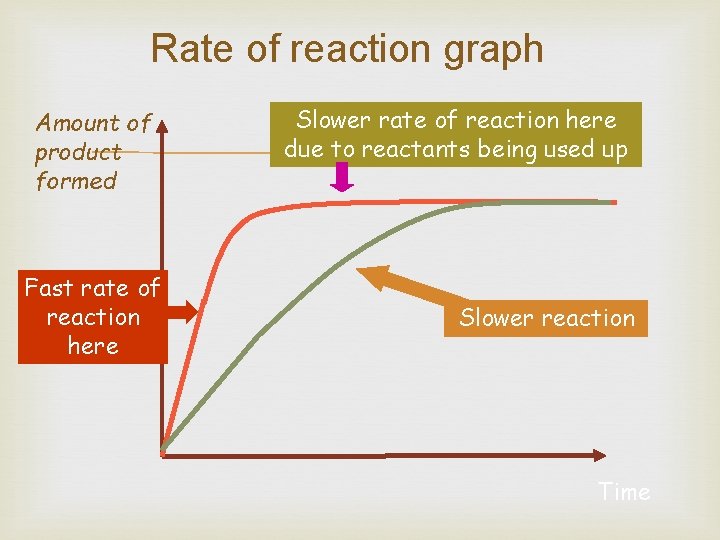
Rate of reaction graph Amount of product formed Fast rate of reaction here Slower rate of reaction here due to reactants being used up Slower reaction Time

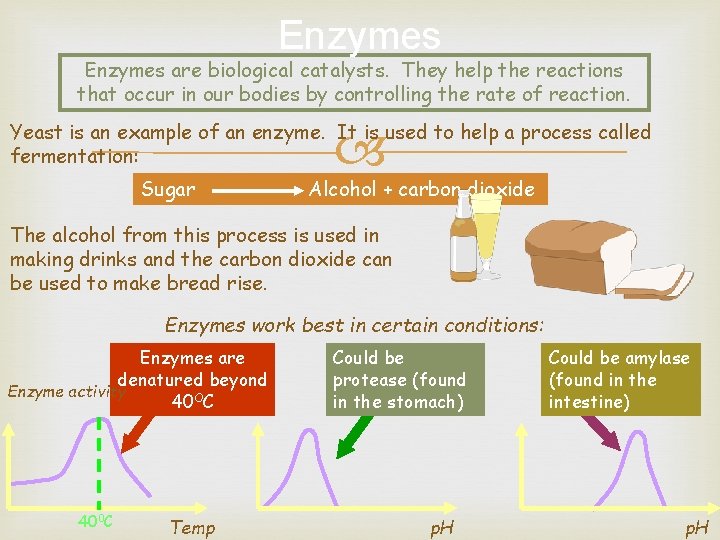
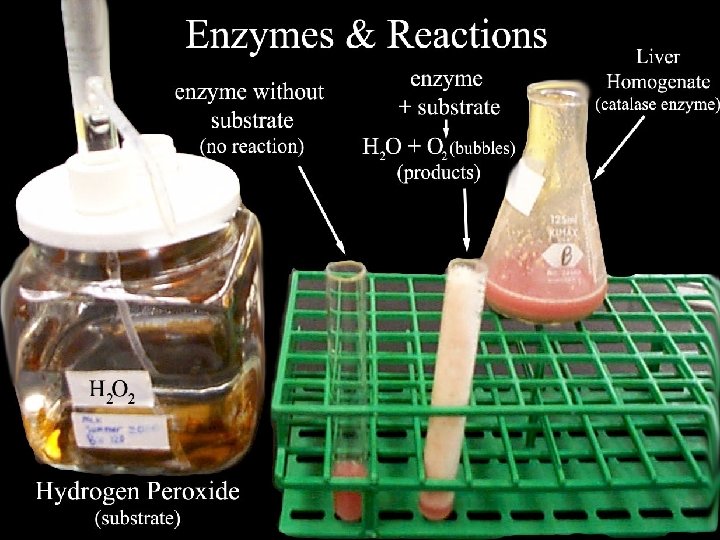
Enzymes are biological catalysts. They help the reactions that occur in our bodies by controlling the rate of reaction. Yeast is an example of an enzyme. It is used to help a process called fermentation: Sugar Alcohol + carbon dioxide The alcohol from this process is used in making drinks and the carbon dioxide can be used to make bread rise. Enzymes work best in certain conditions: Enzymes are denatured beyond Enzyme activity 40 OC 400 C Temp Could be protease (found in the stomach) p. H Could be amylase (found in the intestine) p. H

Uses of enzymes 1) Enzymes are used in washing powders to help digest food stains. Biological washing powders will only work on 400 C or lower. 2) Enzymes are used in baby foods to “pre-digest” the proteins. 3) Enzymes are used to convert starch into sugar which can then be used in food. 4) Conversion of glucose into fructose – glucose and fructose are “isomers” (they have the same chemical formula), but fructose is sweeter.

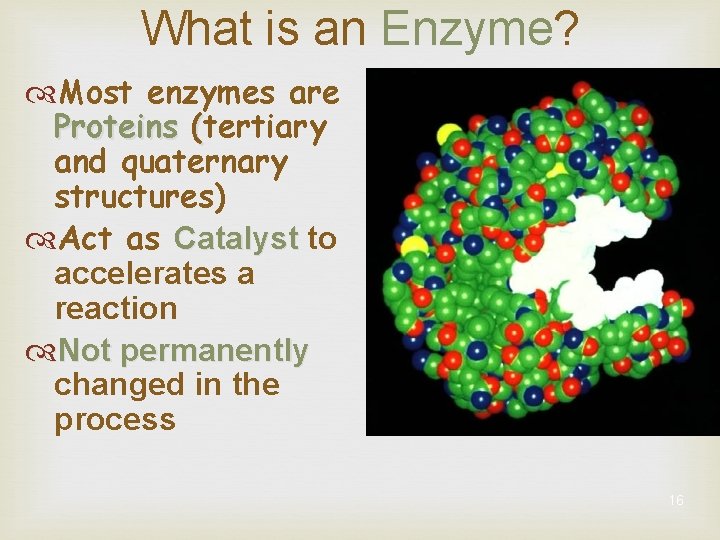
What is an Enzyme? Most enzymes are Proteins (tertiary and quaternary structures) Act as Catalyst to accelerates a reaction Not permanently changed in the process 16

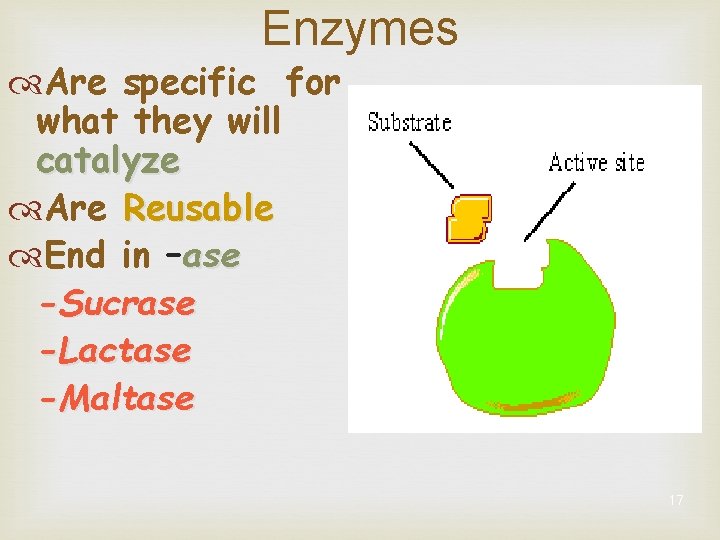
Enzymes Are specific for what they will catalyze Are Reusable End in –ase -Sucrase -Lactase -Maltase 17

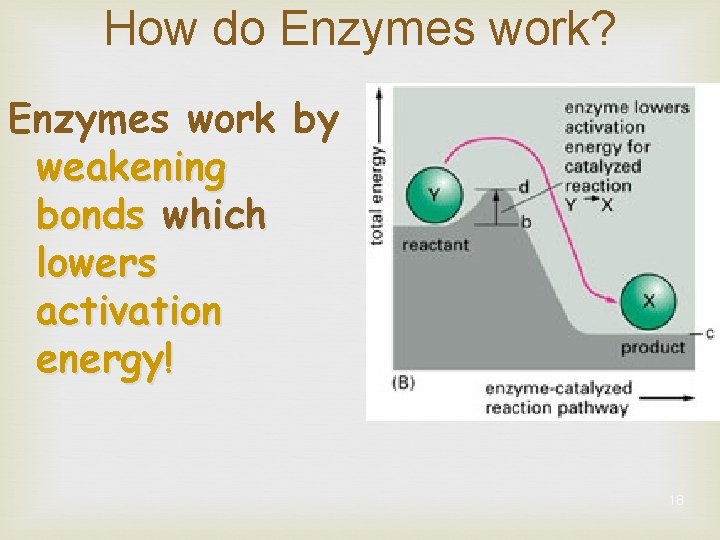
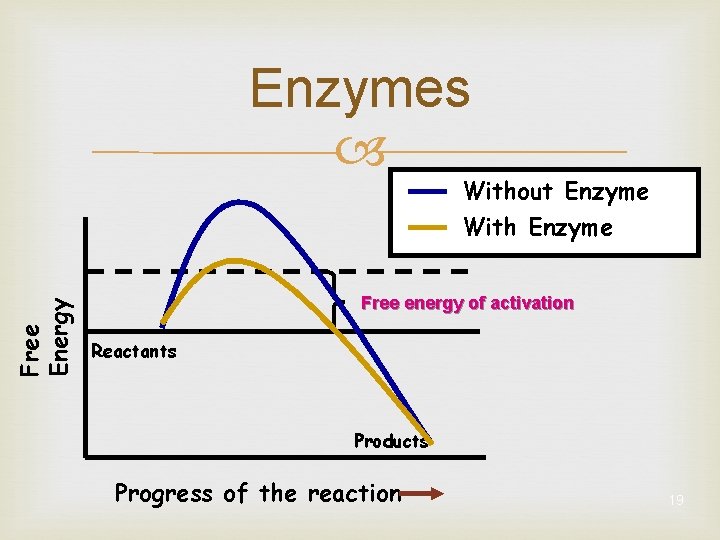
How do Enzymes work? Enzymes work by weakening bonds which lowers activation energy! 18

Enzymes Free Energy Without Enzyme With Enzyme Free energy of activation Reactants Products Progress of the reaction 19

20

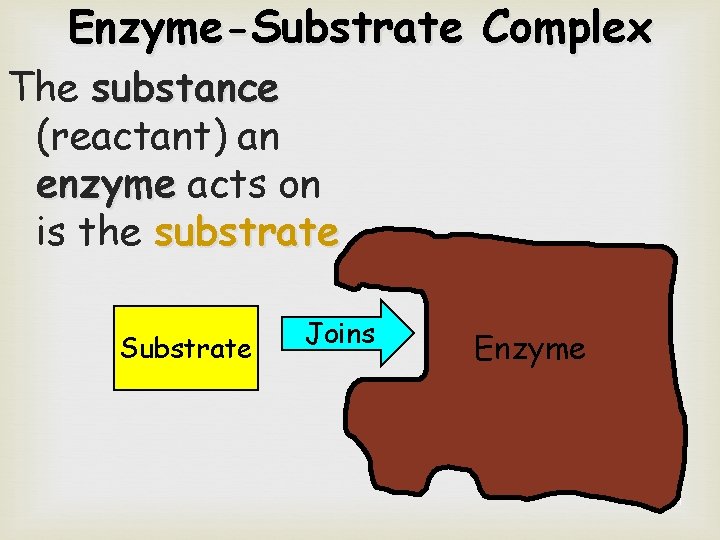
Enzyme-Substrate Complex The substance (reactant) an enzyme acts on is the substrate Substrate Joins Enzyme 21

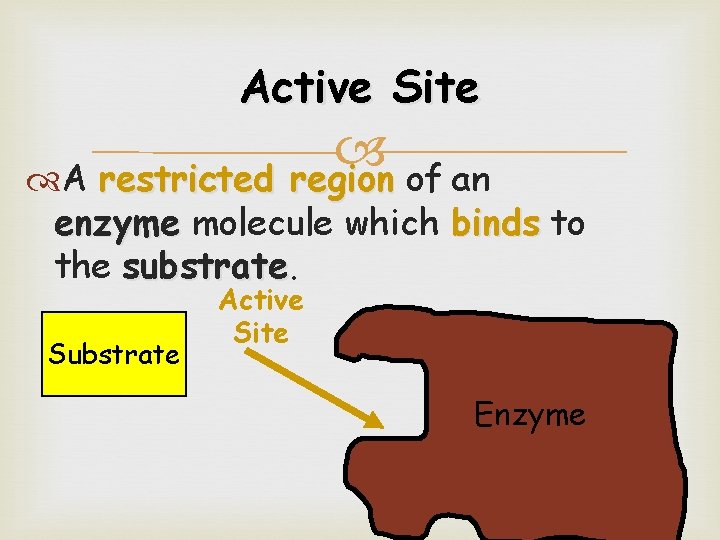
Active Site A restricted region of an restricted region enzyme molecule which binds to the substrate Substrate Active Site Enzyme 22

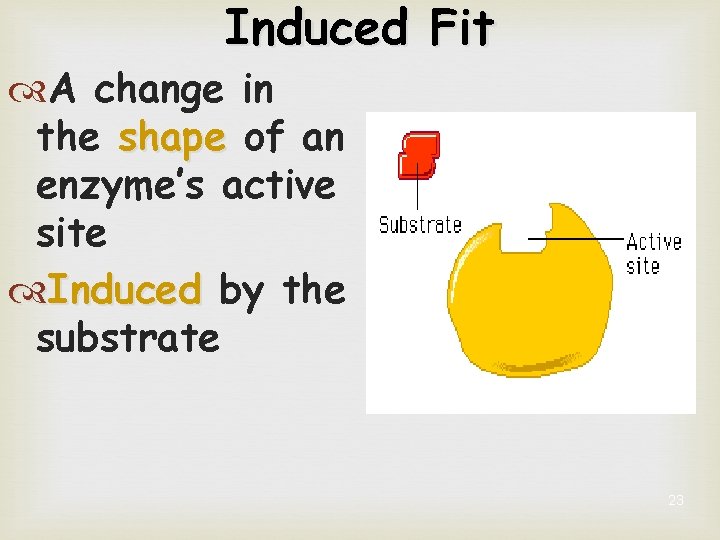
Induced Fit A change in the shape of an enzyme’s active site Induced by the substrate 23

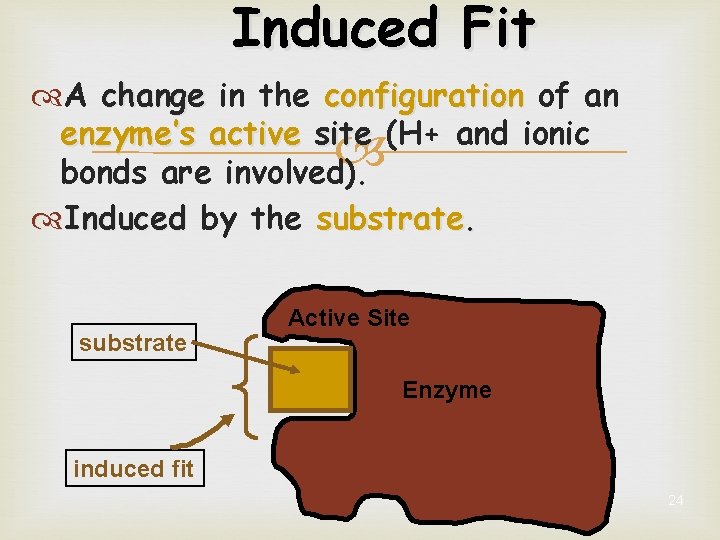
Induced Fit A change in the configuration of an enzyme’s active site (H+ and ionic bonds are involved). Induced by the substrate Active Site Enzyme induced fit 24

What Affects Enzyme Activity? Three factors: 1. Environmental Conditions 2. Cofactors and Coenzymes 3. Enzyme Inhibitors 25

1. Environmental Conditions 1. Extreme Temperature are the most dangerous - high temps may denature (unfold) the enzyme. 2. p. H (most like 6 - 8 p. H near neutral) 3. Ionic concentration (salt ions) 26

2. Cofactors and Coenzymes Inorganic substances (zinc, iron) and vitamins (respectively) are sometimes need for proper enzymatic activity Example: Iron must be present in the quaternary structure - hemoglobin in order for it to pick up oxygen. 27

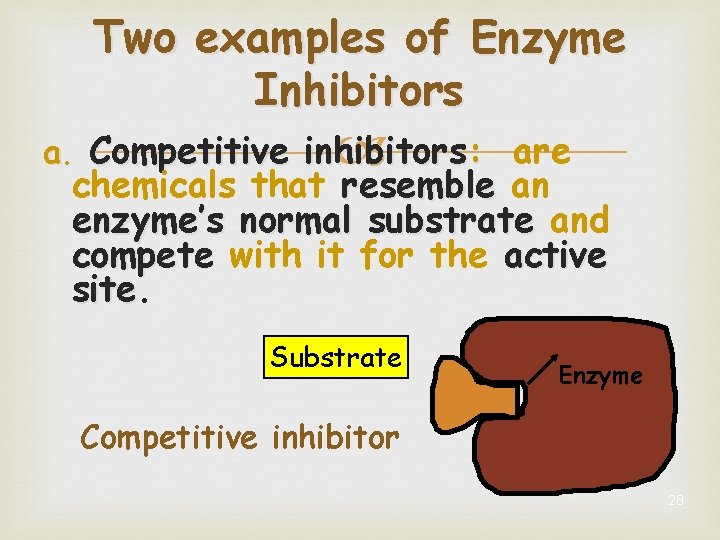
Two examples of Enzyme Inhibitors a. Competitive inhibitors: are chemicals that resemble an enzyme’s normal substrate and compete with it for the active site Substrate Enzyme Competitive inhibitor 28

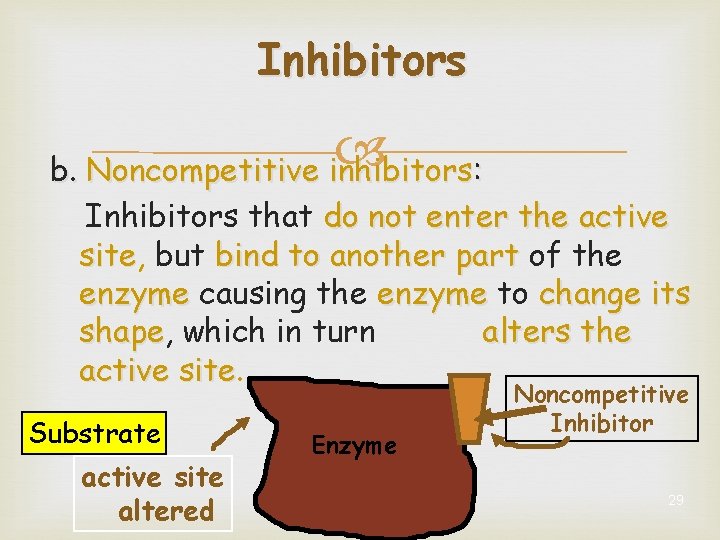
Inhibitors b. Noncompetitive inhibitors: Inhibitors that do not enter the active site, site but bind to another part of the enzyme causing the enzyme to change its shape, alters the shape which in turn active site Substrate active site altered Enzyme Noncompetitive Inhibitor 29