Nucleotides BIOMEDICAL IMPORTANCE Building blocks of nucleic acids

Nucleotides
BIOMEDICAL IMPORTANCE • Building blocks of nucleic acids • Part of many coenzymes • Donors of – Phosphoryl groups (eg, ATP or GTP) – Sugars (eg, UDP- or GDP-sugars) – Lipid (eg, CDP-acylglycerol) • Regulatory nucleotides – c. AMP and c. GMP • Control of oxidative phosphorylation – by ADP • Allosteric regulation of enzyme activity – by ATP, AMP, and CTP
BIOMEDICAL IMPORTANCE • For therapy – Chemotherapy of cancer and AIDS – Suppressors of the immune response during organ transplantation
Classification • • PURINES PYRIMIDINES NUCLEOSIDES NUCLEOTIDES
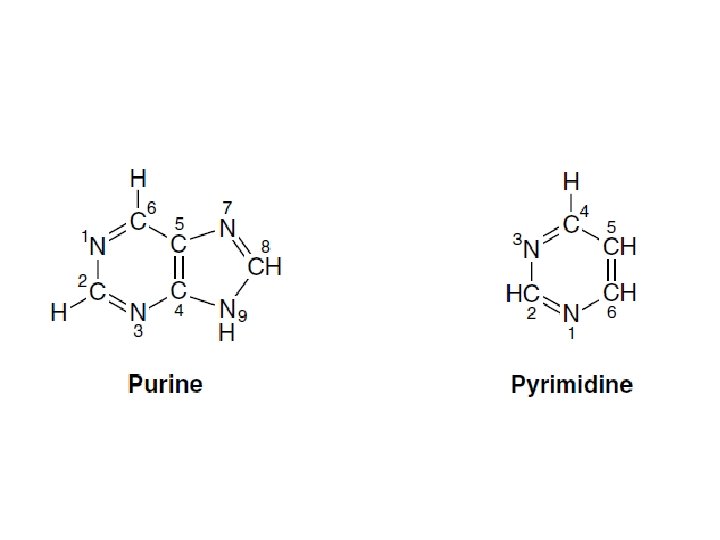
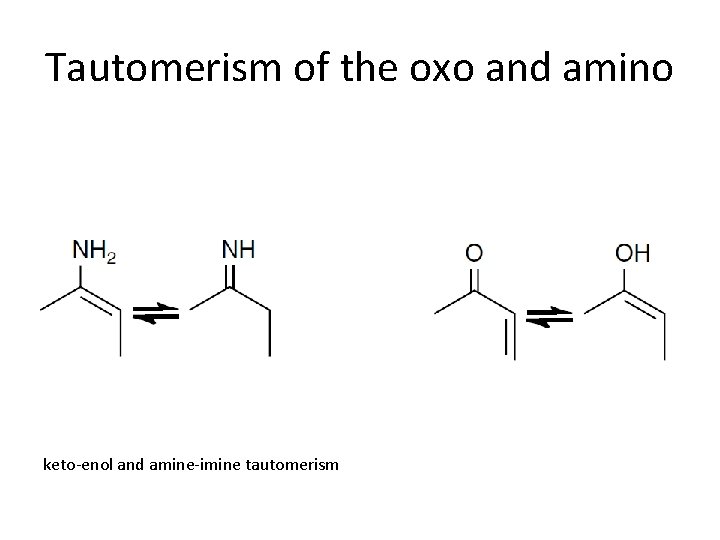
Tautomerism of the oxo and amino keto-enol and amine-imine tautomerism

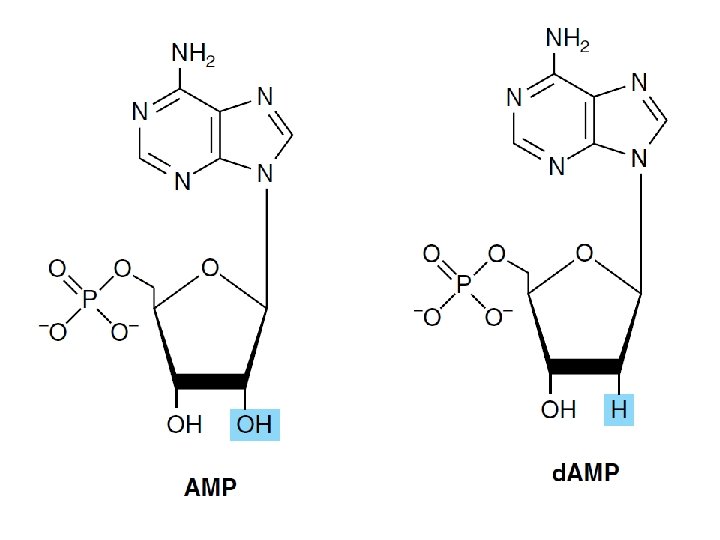
• Nucleoside – Diphosphates – Triphosphates • The sugar moiety – D-ribose or 2 -deoxy-Dribose
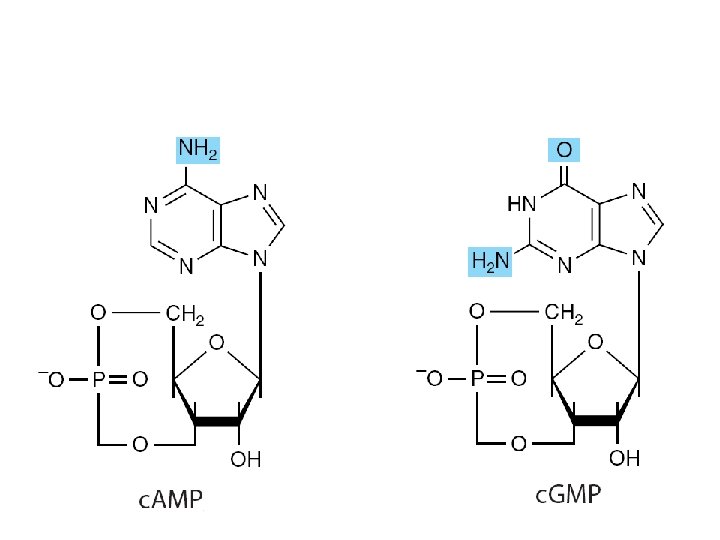
• Nucleoside triphosphates – have high group transfer potential • Participate in covalent bond syntheses. • Cyclic phosphodiesters – c. AMP and c. GMP • Intracellular second messengers
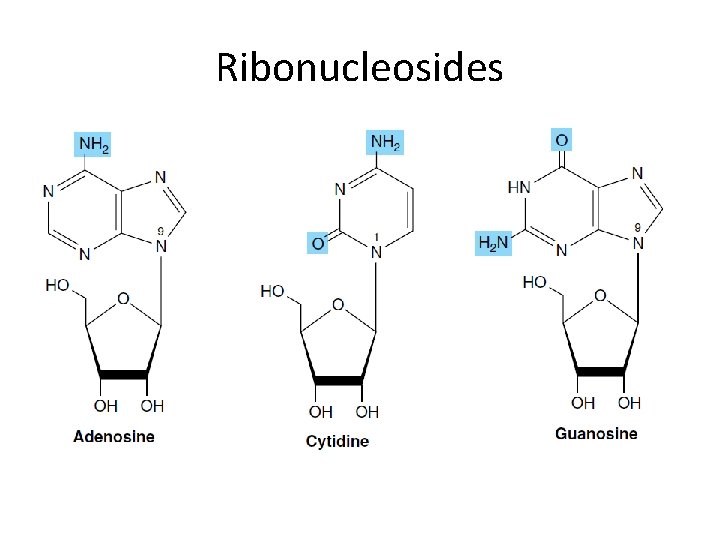
Ribonucleosides
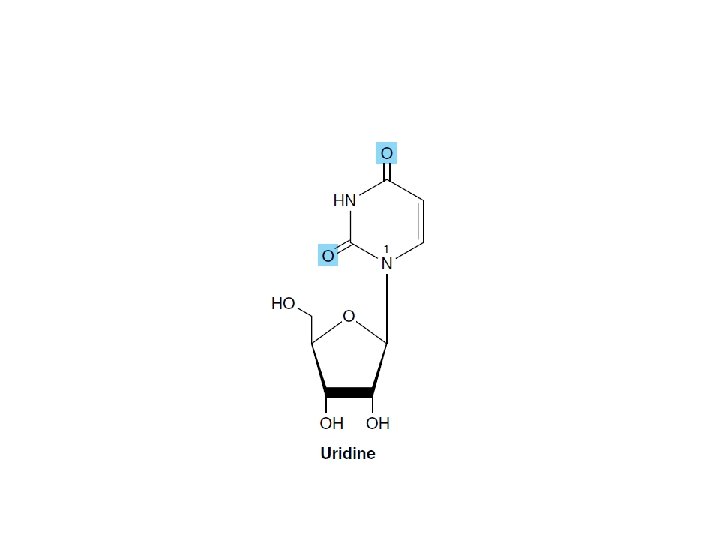
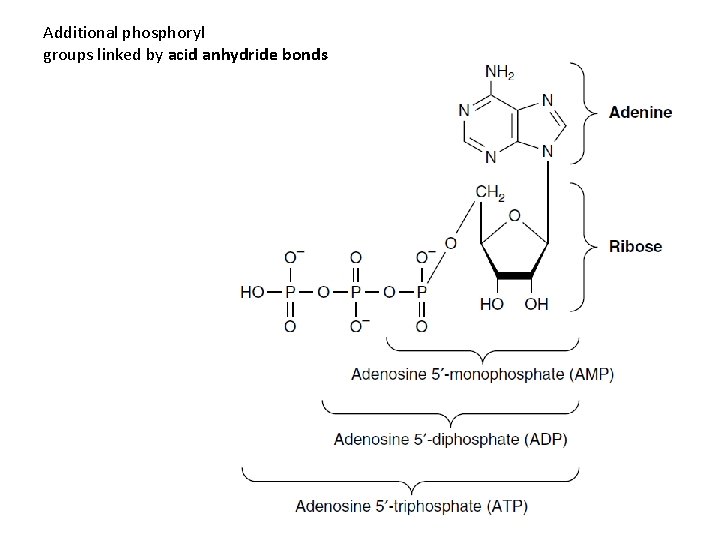
Additional phosphoryl groups linked by acid anhydride bonds
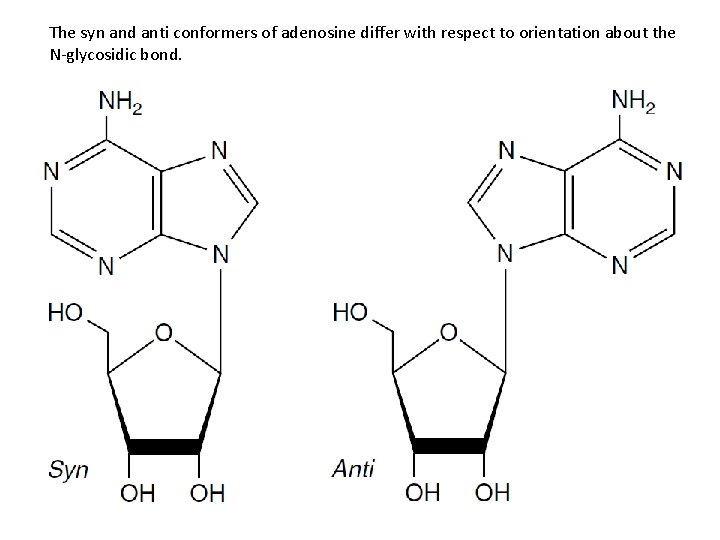
The syn and anti conformers of adenosine differ with respect to orientation about the N-glycosidic bond.
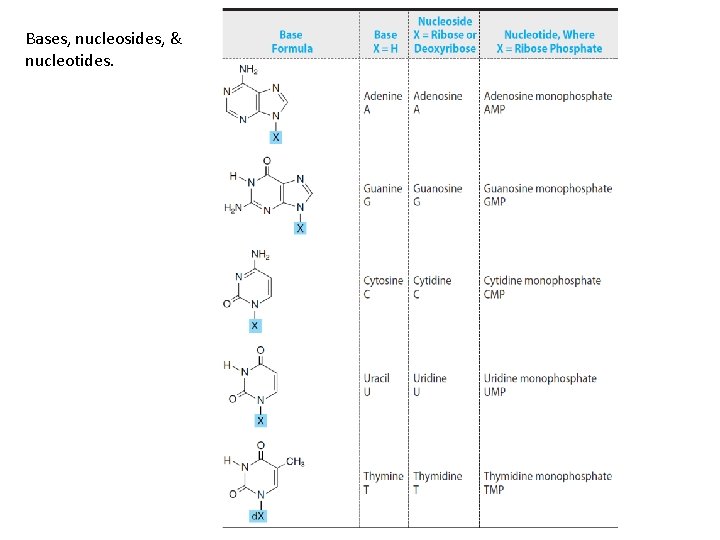
Bases, nucleosides, & nucleotides.
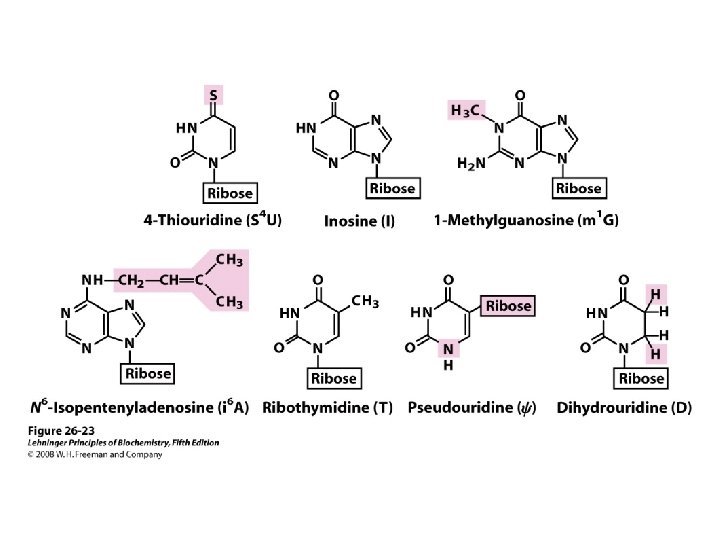
Nucleic Acids Also Contain Additional Bases • 5 -methylcytosine • 5 -hydroxymethylcytosine • Mono- and di-N-methylated adenine & guanine – Mammalian messenger RNAs
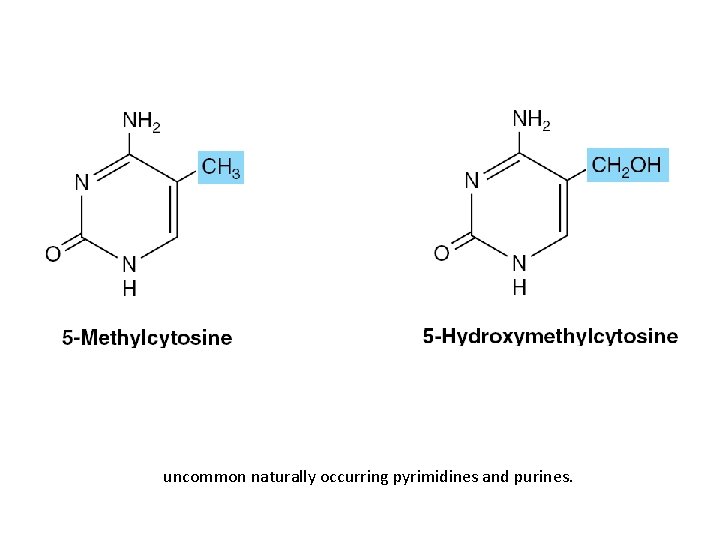
uncommon naturally occurring pyrimidines and purines.
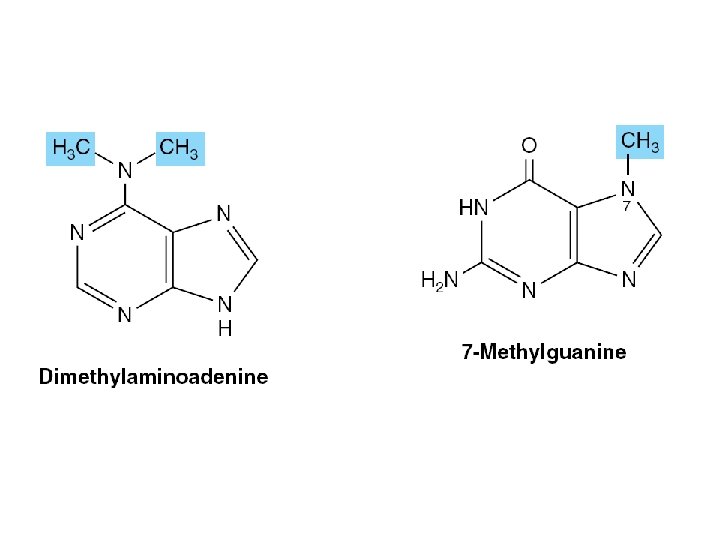
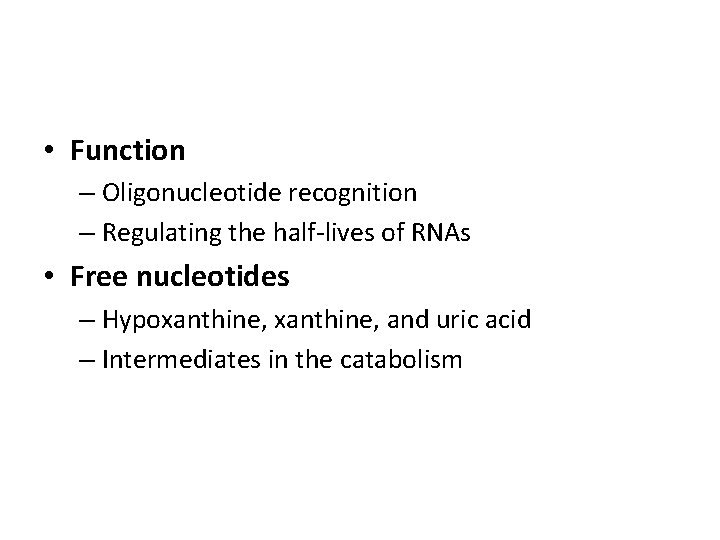
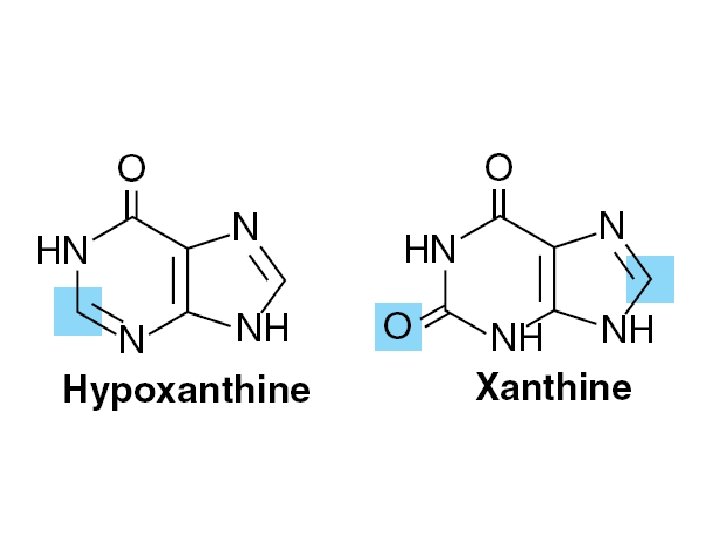
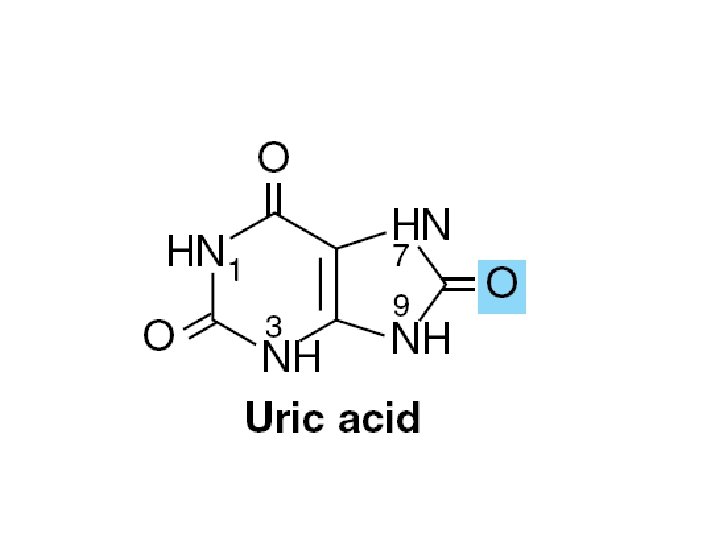
• Function – Oligonucleotide recognition – Regulating the half-lives of RNAs • Free nucleotides – Hypoxanthine, and uric acid – Intermediates in the catabolism
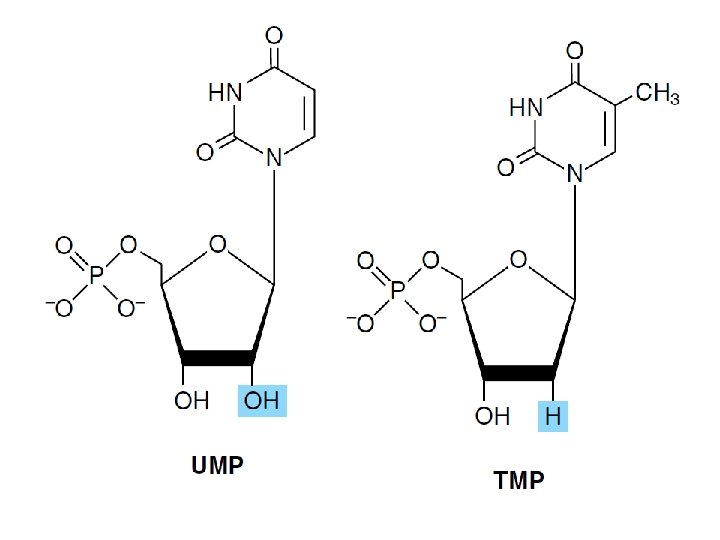
Posttranscriptional modification • Pseudouridine (Ψ) • Methylation by S-adenosylmethionine of a UMP of preformed t. RNA forms TMP
Nucleotides Serve Diverse Physiologic Functions • • Protein synthesis Nucleic acid synthesis Regulatory cascades Signal transduction pathways
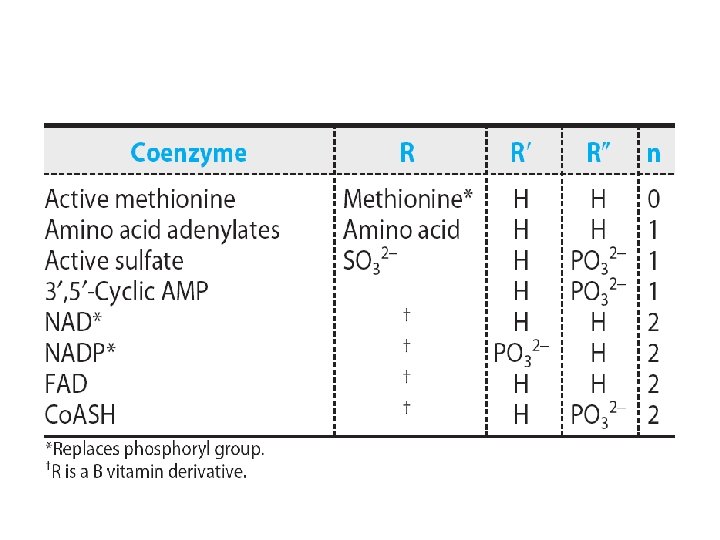
Physiologic functions • As precursors of nucleic acids • Transducer of free energy – ATP • The second messenger – c. AMP • Adenosine 3′-phosphate-5′-phosulfate • Methyl group donor
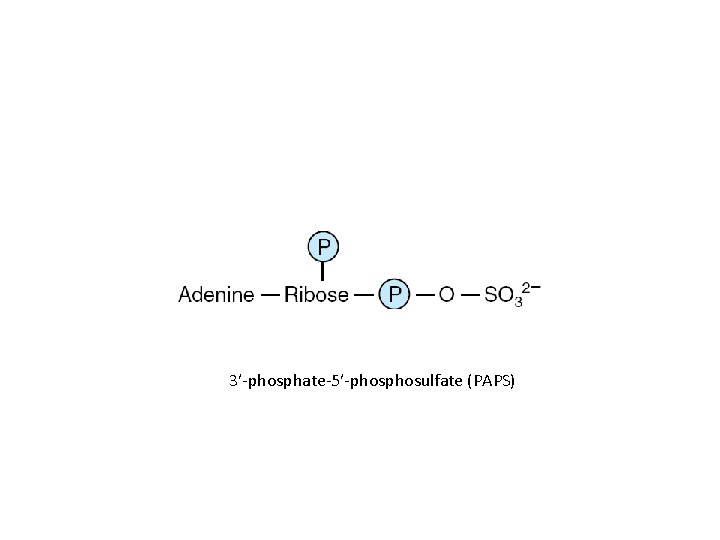
3′-phosphate-5′-phosulfate (PAPS)
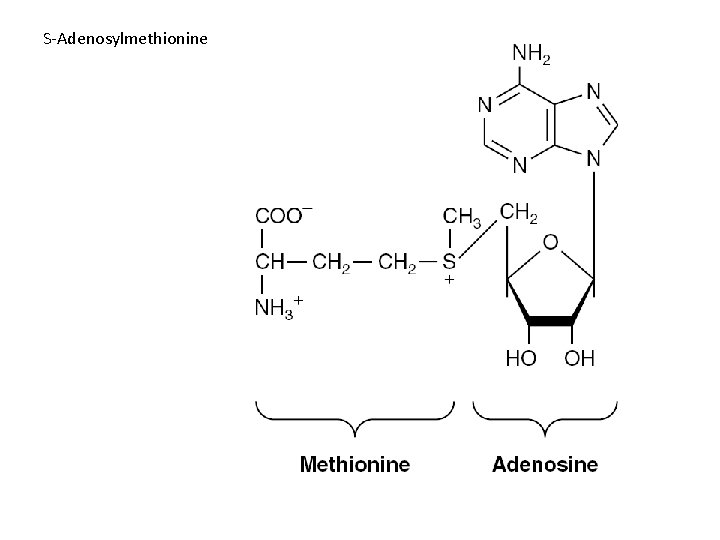
S-Adenosylmethionine
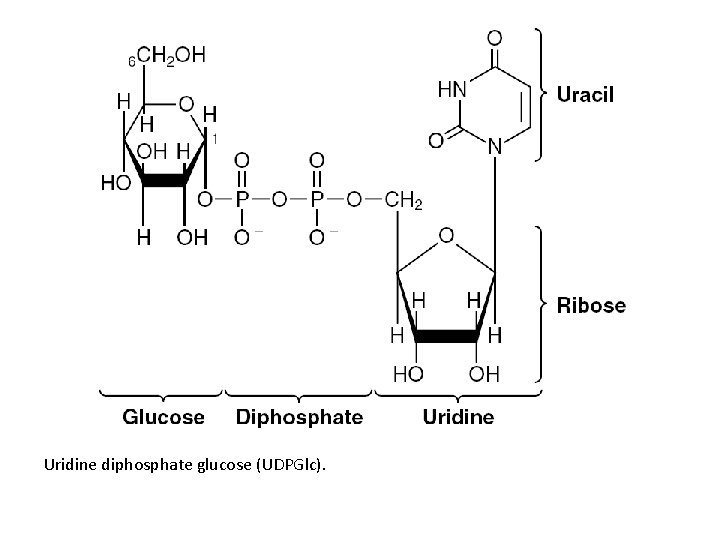
Uridine diphosphate glucose (UDPGlc).
• Energy source for protein synthesis – GTP • UDP-sugar derivatives – Sugar epimerizations – Biosynthesis of glycogen, glucosyl disaccharides, and the oligosaccharides of glycoproteins and proteoglycans
• UDP-glucuronic acid. – Conjugation • Bilirubin • Drugs • CTP – Biosynthesis of phosphoglycerides – Sphingomyelin • Coenzymes
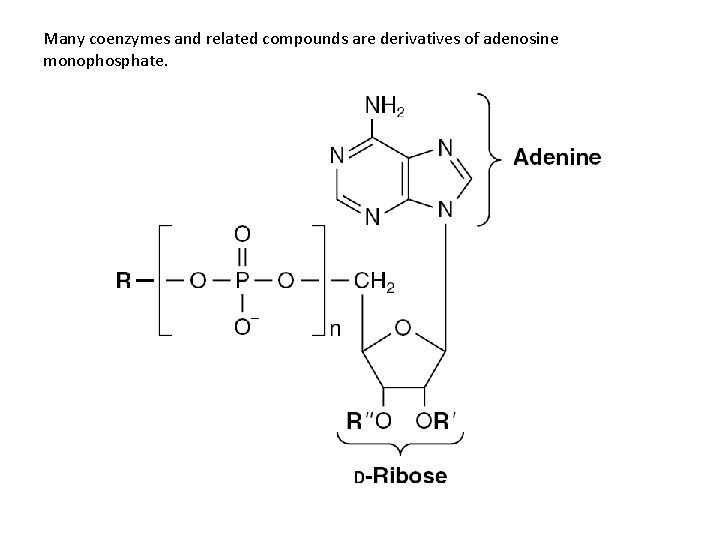
Many coenzymes and related compounds are derivatives of adenosine monophosphate.
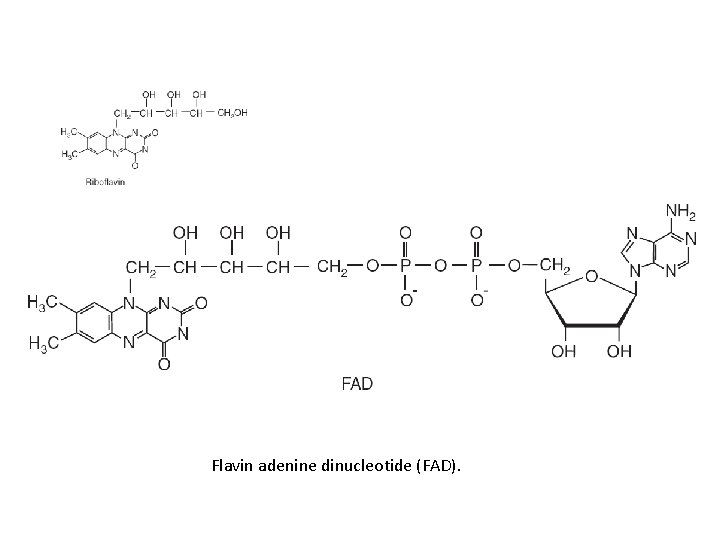
Flavin adenine dinucleotide (FAD).
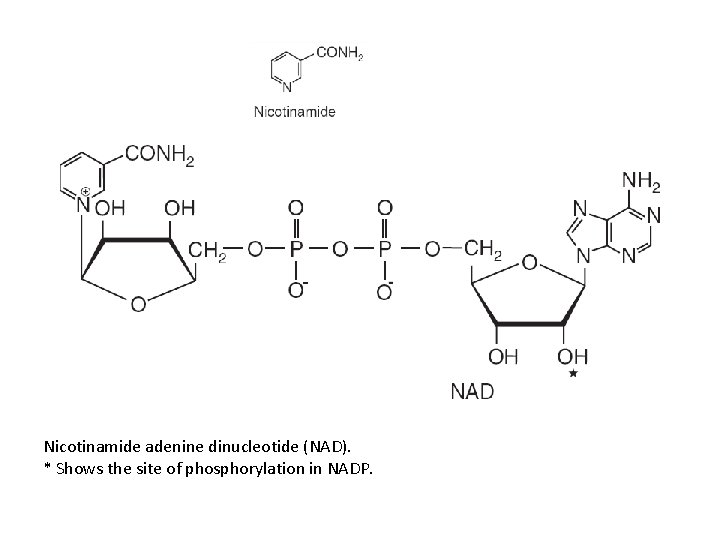
Nicotinamide adenine dinucleotide (NAD). * Shows the site of phosphorylation in NADP.
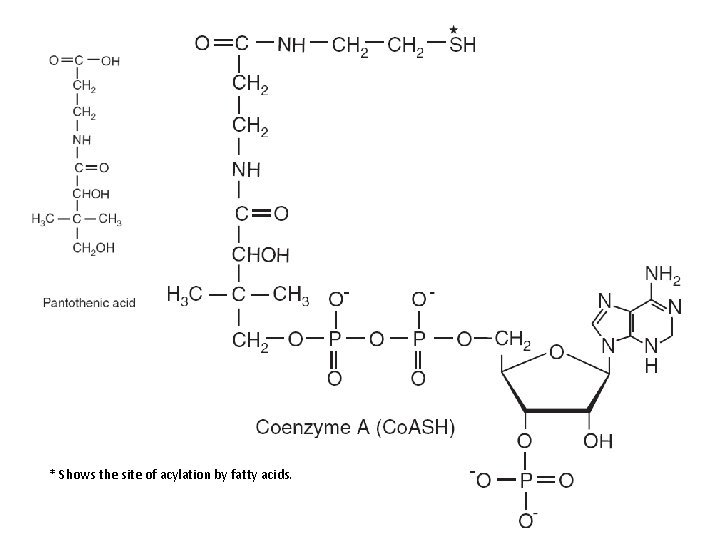
* Shows the site of acylation by fatty acids.

• Nucleotides Are Polyfunctional Acids • Nucleotides Absorb Ultraviolet Light – Close to 260 nm
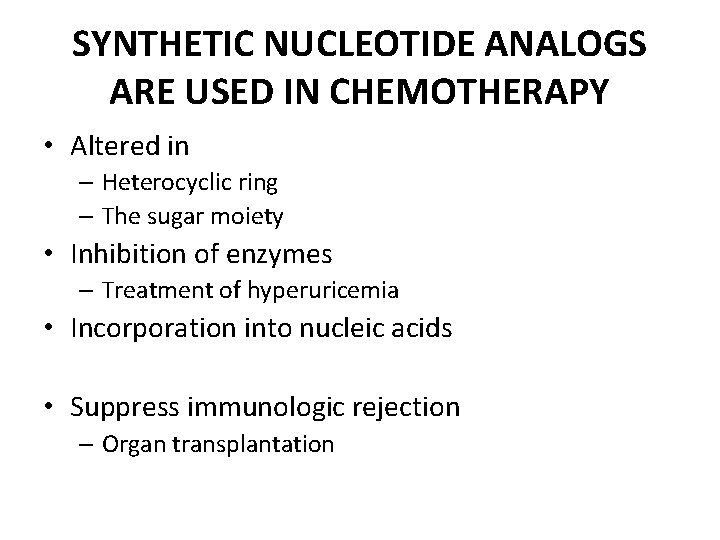
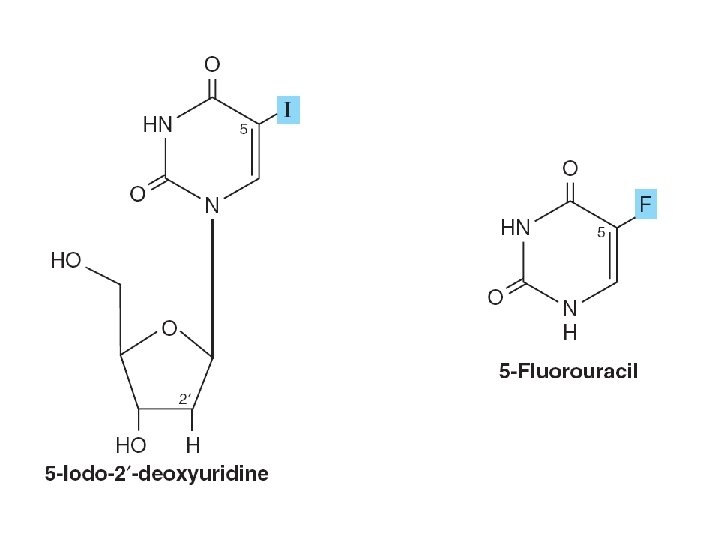
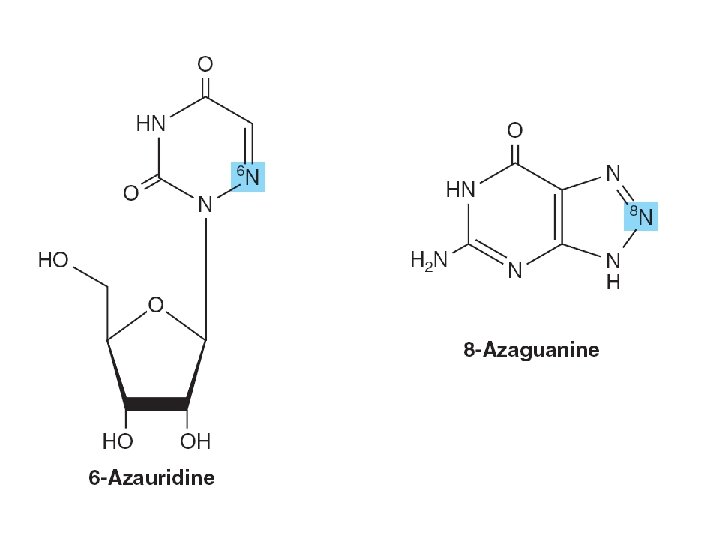
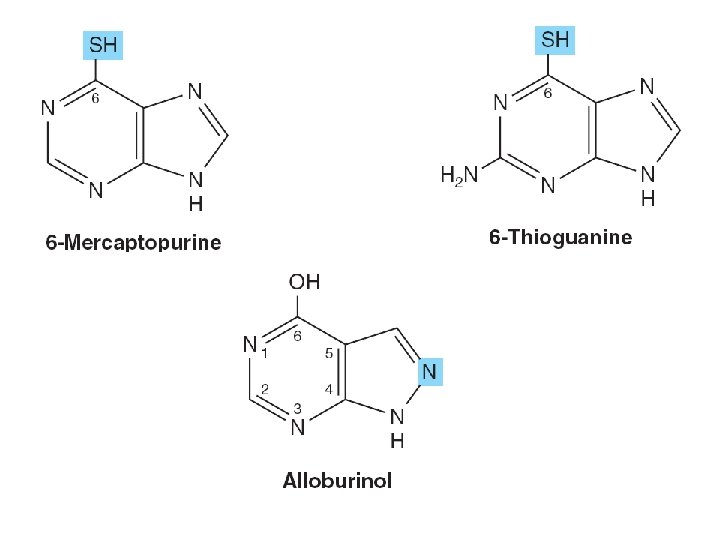
SYNTHETIC NUCLEOTIDE ANALOGS ARE USED IN CHEMOTHERAPY • Altered in – Heterocyclic ring – The sugar moiety • Inhibition of enzymes – Treatment of hyperuricemia • Incorporation into nucleic acids • Suppress immunologic rejection – Organ transplantation
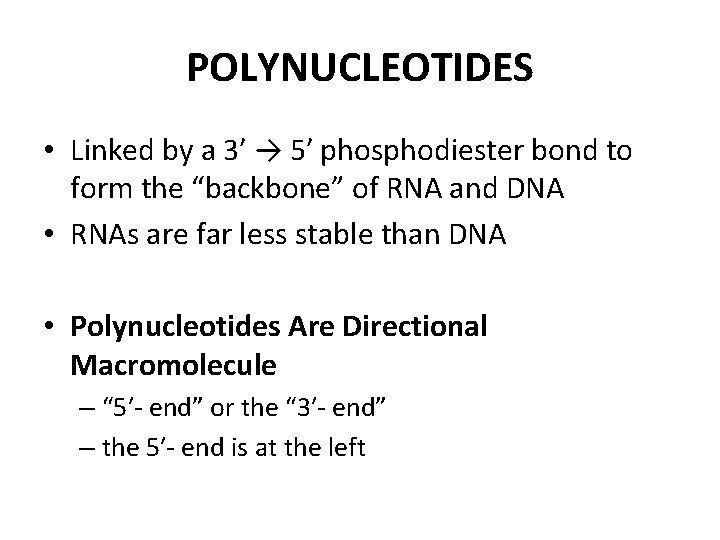
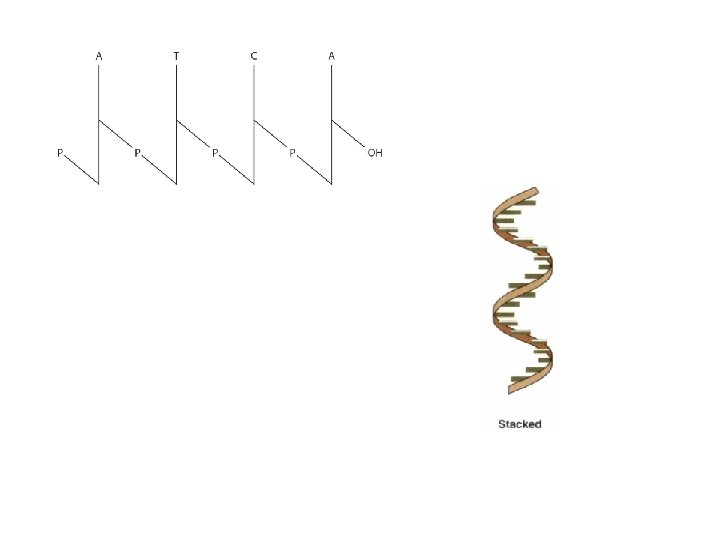
POLYNUCLEOTIDES • Linked by a 3′ → 5′ phosphodiester bond to form the “backbone” of RNA and DNA • RNAs are far less stable than DNA • Polynucleotides Are Directional Macromolecule – “ 5′- end” or the “ 3′- end” – the 5′- end is at the left
Polynucleotides Have Primary Structure • Base sequence – Compact notation • p. Gp. Ap. Tp. Cp. A • GGATCA
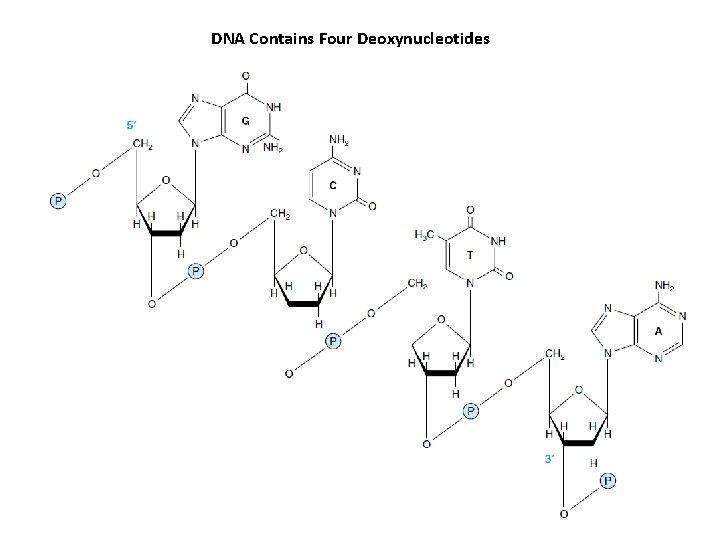
DNA Contains Four Deoxynucleotides
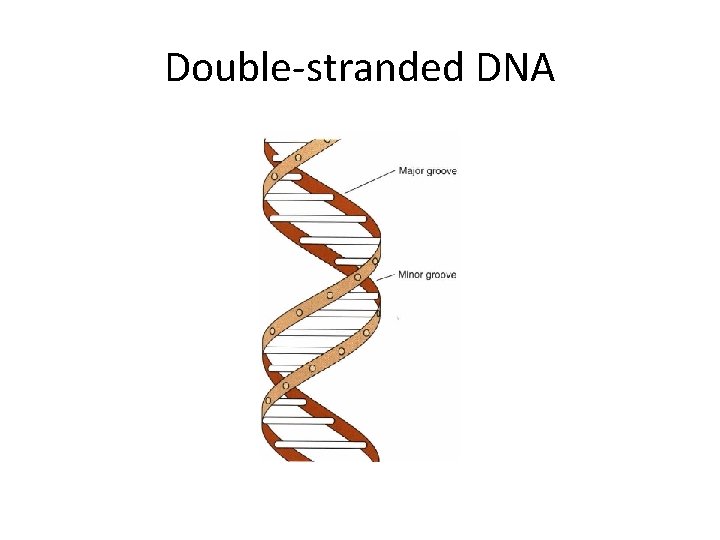
Double-stranded DNA
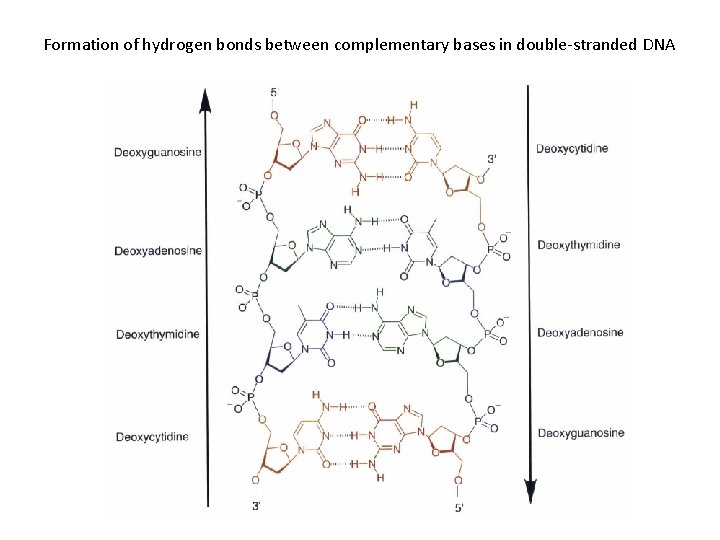
Formation of hydrogen bonds between complementary bases in double-stranded DNA
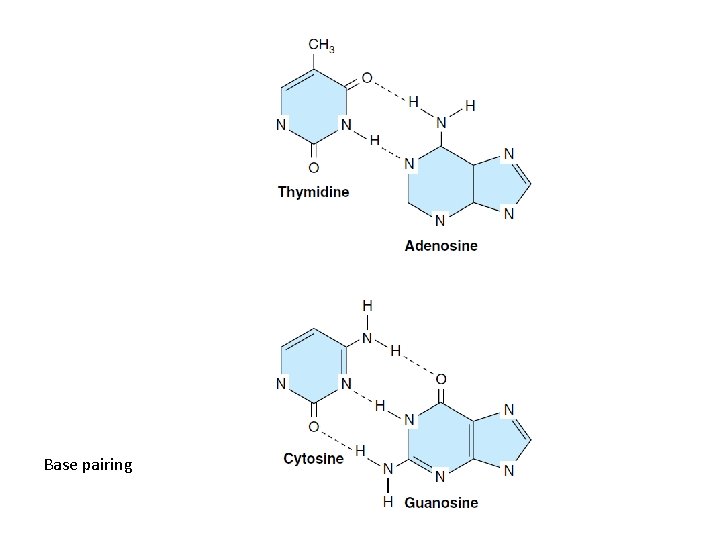
Base pairing
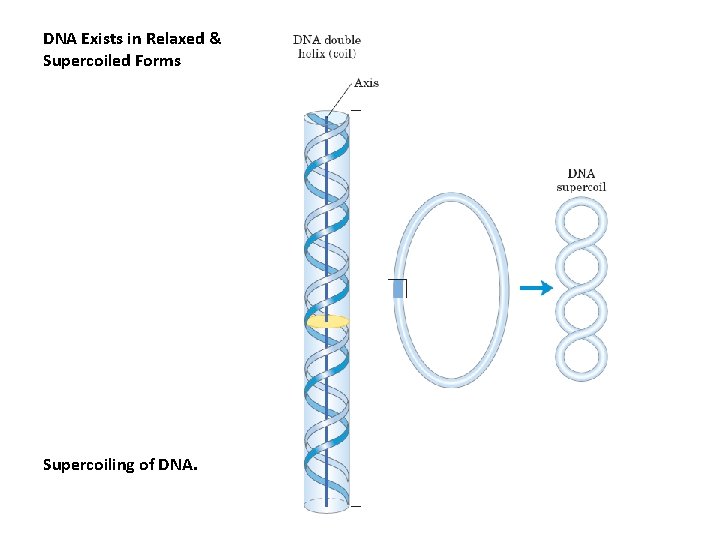
DNA Exists in Relaxed & Supercoiled Forms Supercoiling of DNA.
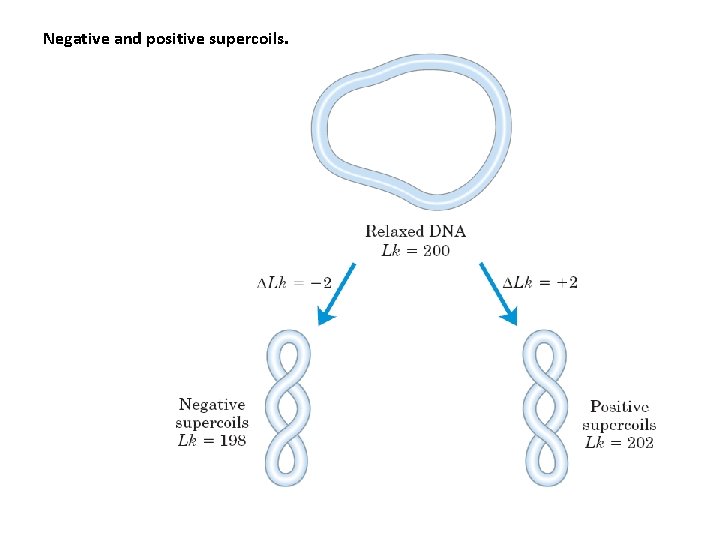
Negative and positive supercoils.
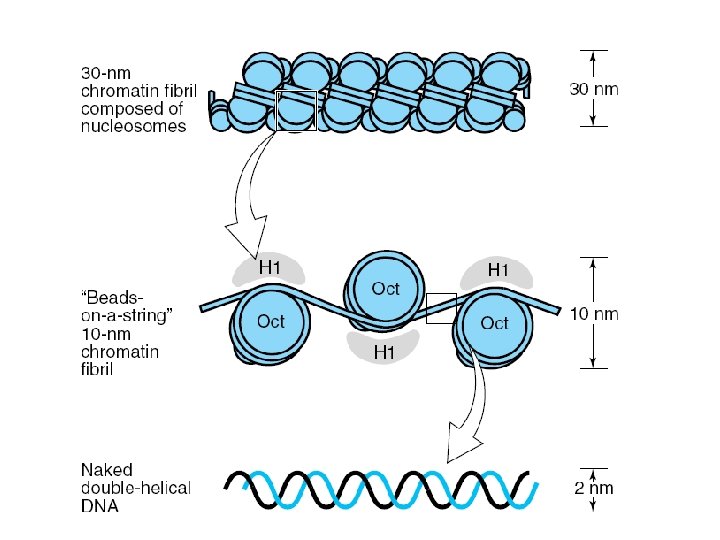
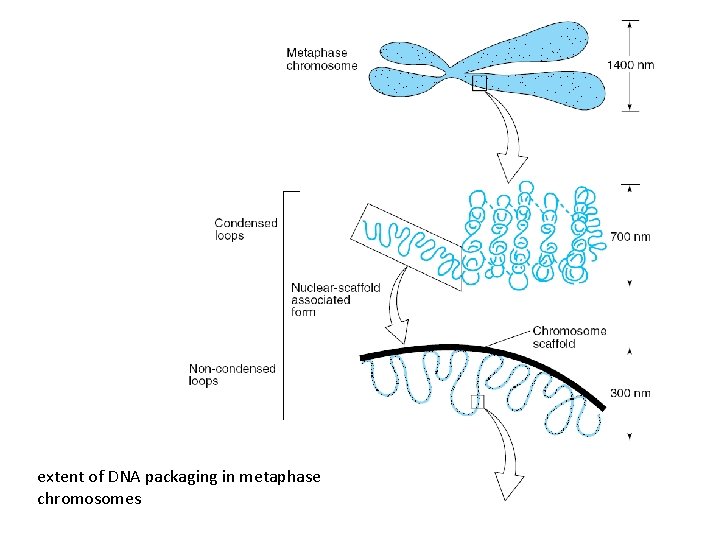
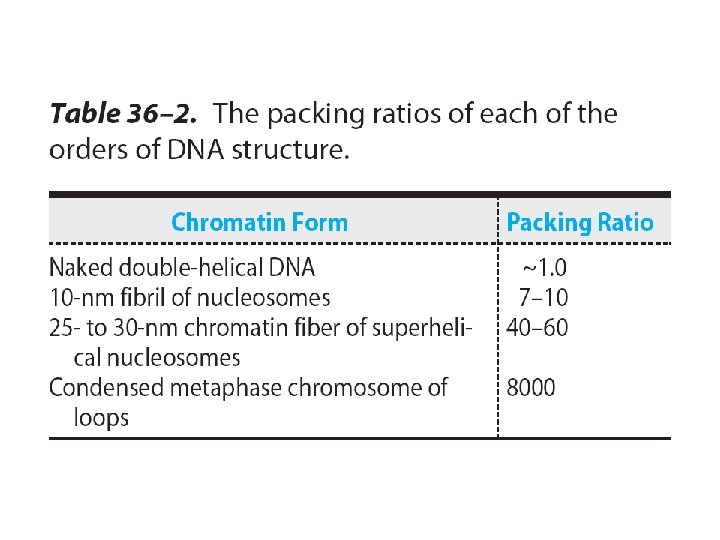
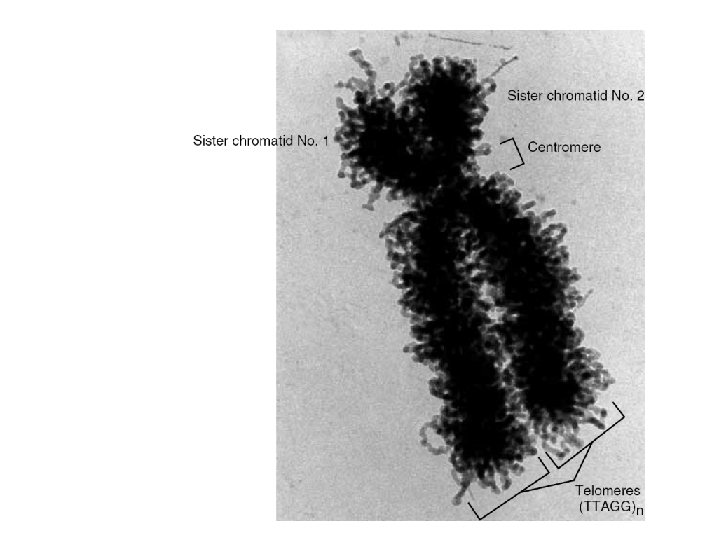
extent of DNA packaging in metaphase chromosomes
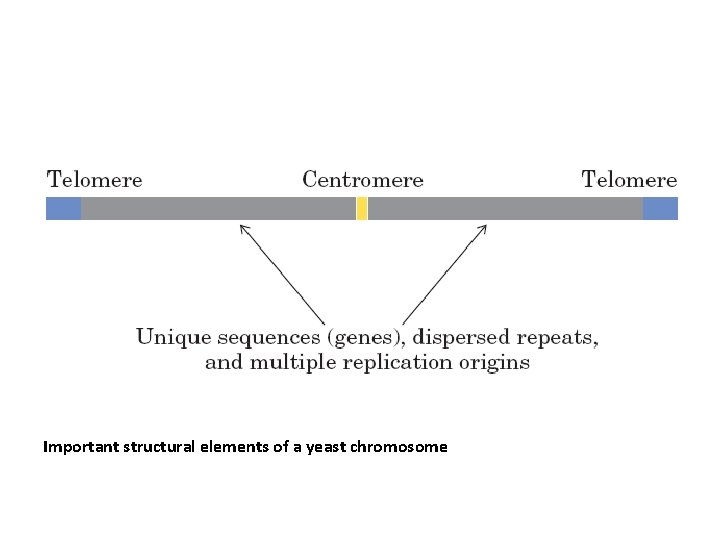
Important structural elements of a yeast chromosome
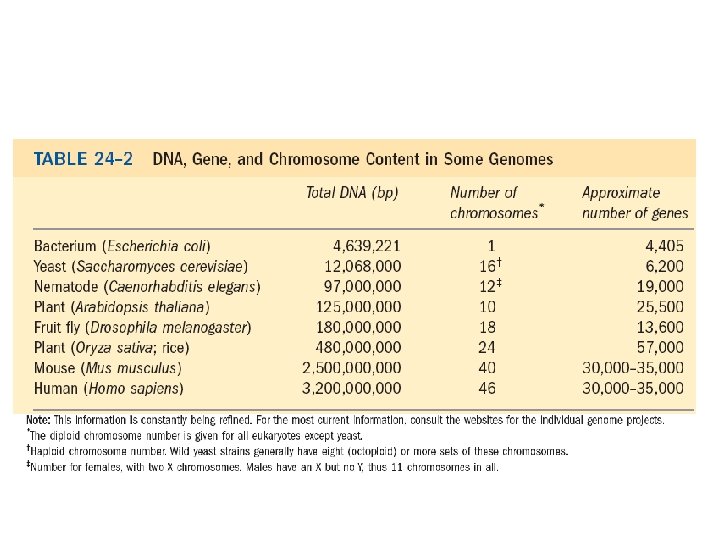

• One of the hallmarks of living organisms is their ability to reproduce. • DNA contains the genetic information
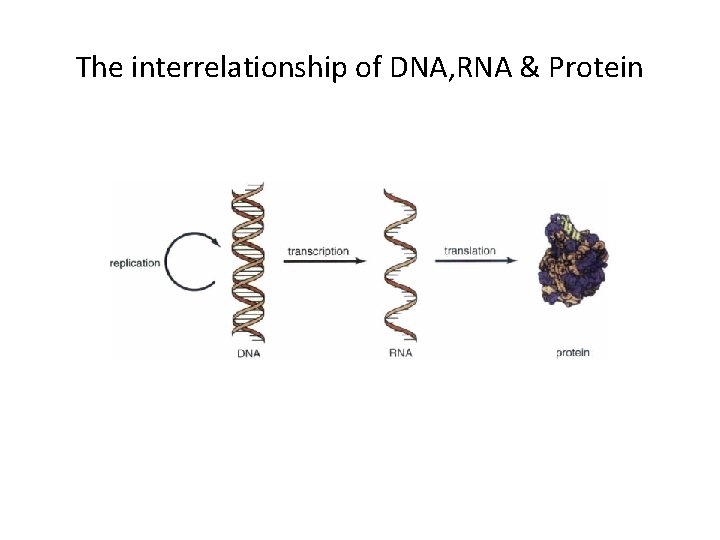
The interrelationship of DNA, RNA & Protein
- Slides: 58