John E Mc Murry Robert C Fay C

John E. Mc. Murry • Robert C. Fay C H E M I S T R Y Fifth Edition Chapter 24 Biochemistry Lecture Notes Alan D. Earhart Southeast Community College • Lincoln, NE Copyright © 2008 Pearson Prentice Hall, Inc.
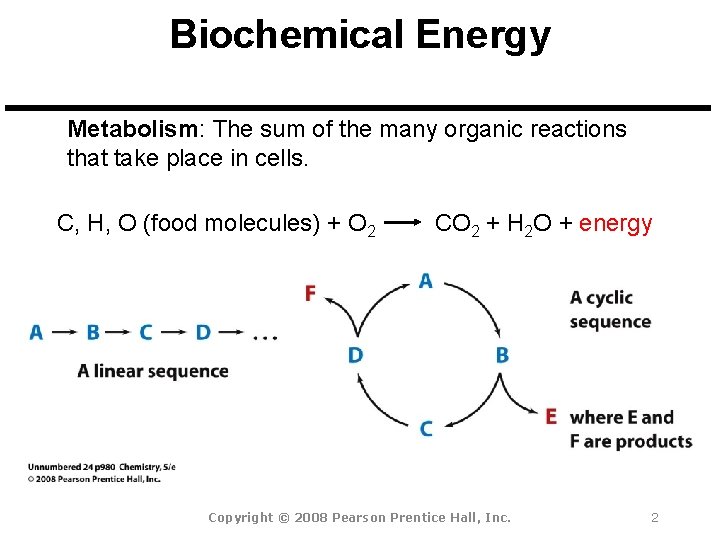
Biochemical Energy Metabolism: The sum of the many organic reactions that take place in cells. C, H, O (food molecules) + O 2 CO 2 + H 2 O + energy Copyright © 2008 Pearson Prentice Hall, Inc. 2
Biochemical Energy Catabolism: The reaction sequences that break molecules apart. These reactions generally release energy. Anabolism: The reaction sequences that put building blocks back together to assemble larger molecules. These reactions generally absorb energy. Copyright © 2008 Pearson Prentice Hall, Inc. 3
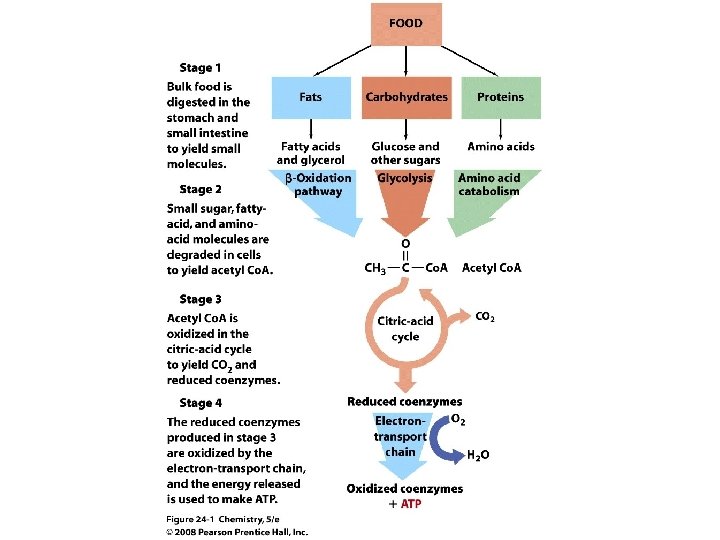
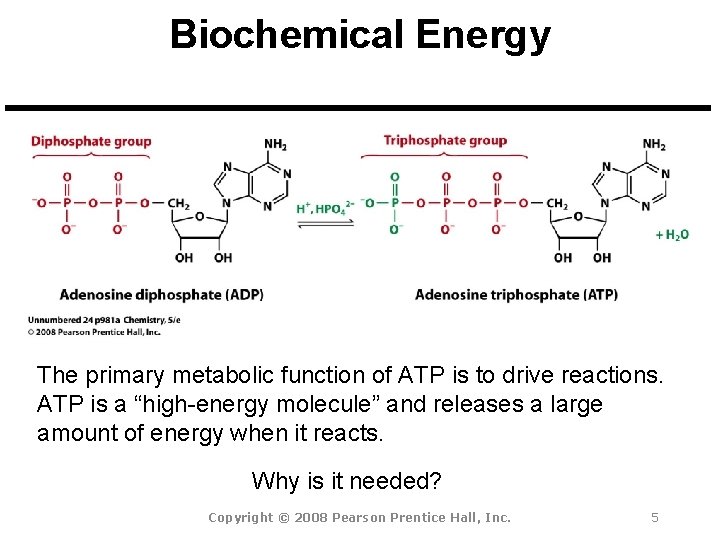
Biochemical Energy The primary metabolic function of ATP is to drive reactions. ATP is a “high-energy molecule” and releases a large amount of energy when it reacts. Why is it needed? Copyright © 2008 Pearson Prentice Hall, Inc. 5
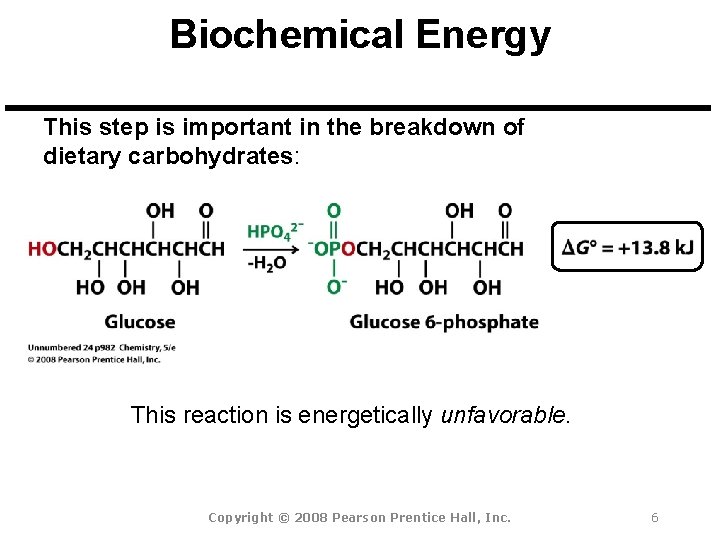
Biochemical Energy This step is important in the breakdown of dietary carbohydrates: This reaction is energetically unfavorable. Copyright © 2008 Pearson Prentice Hall, Inc. 6
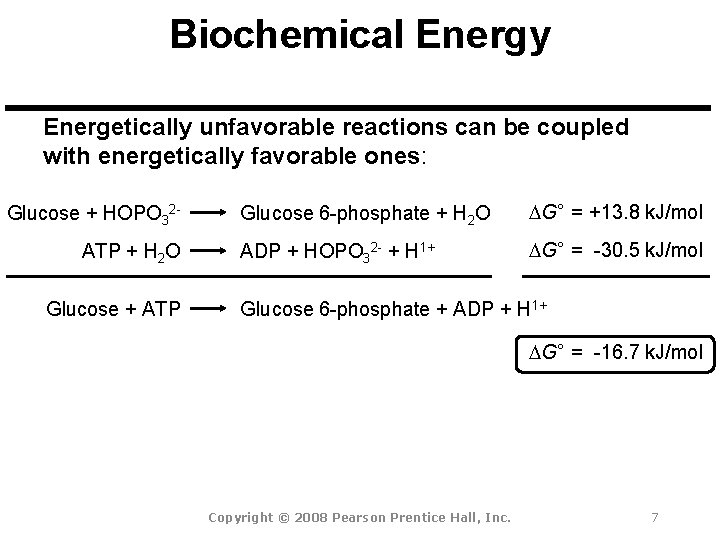
Biochemical Energy Energetically unfavorable reactions can be coupled with energetically favorable ones: Glucose + HOPO 32 ATP + H 2 O Glucose + ATP Glucose 6 -phosphate + H 2 O DG° = +13. 8 k. J/mol ADP + HOPO 32 - + H 1+ DG° = -30. 5 k. J/mol Glucose 6 -phosphate + ADP + H 1+ DG° = -16. 7 k. J/mol Copyright © 2008 Pearson Prentice Hall, Inc. 7
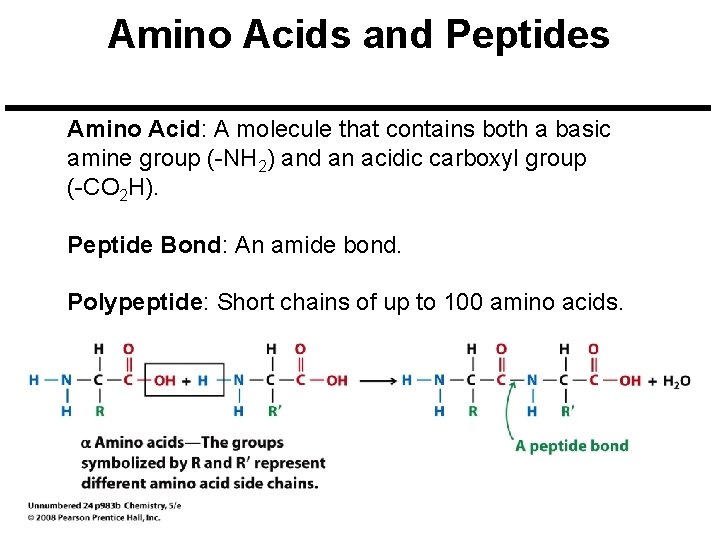
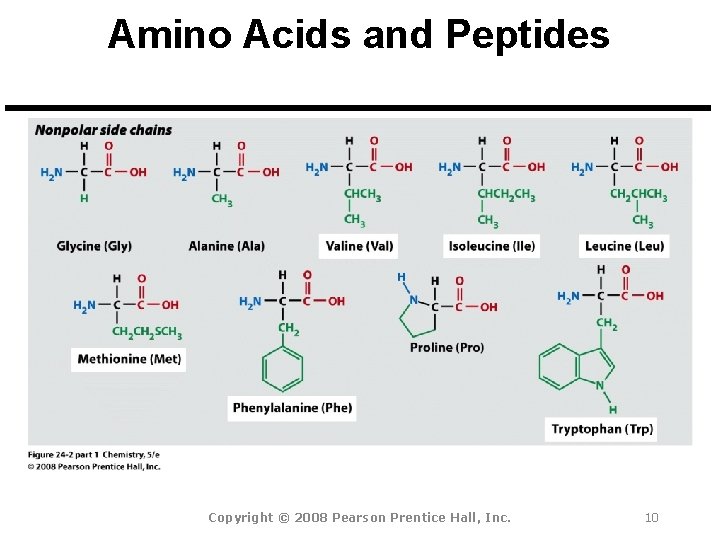
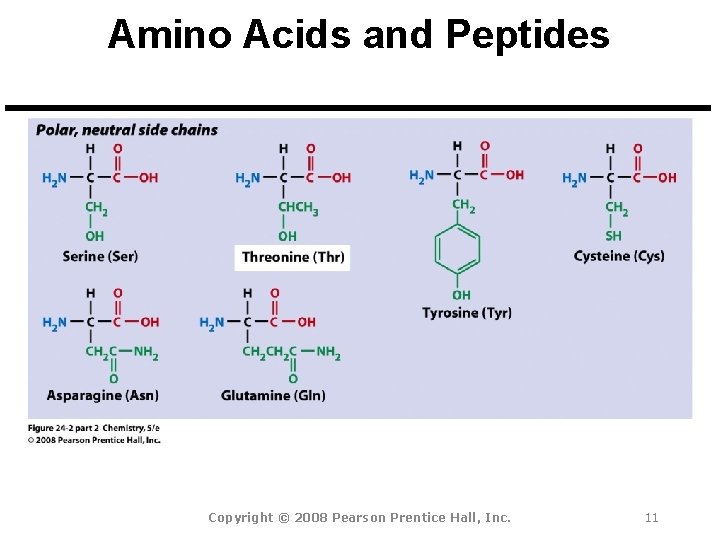
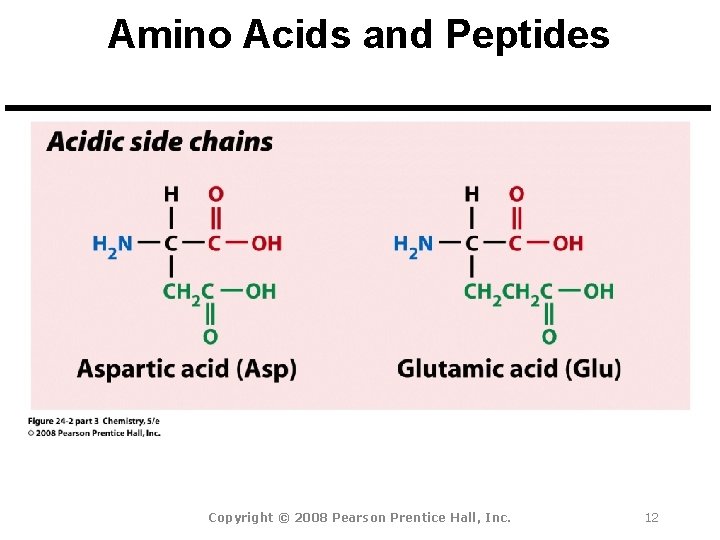
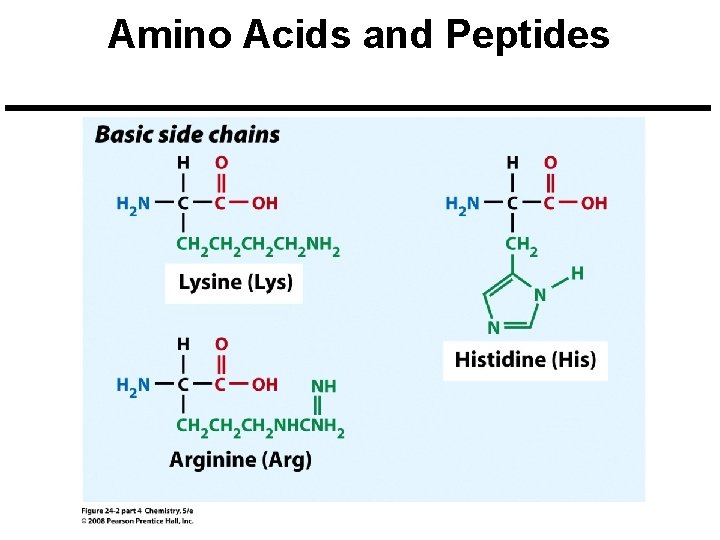
Amino Acids and Peptides Amino Acid: A molecule that contains both a basic amine group (-NH 2) and an acidic carboxyl group (-CO 2 H). Peptide Bond: An amide bond. Polypeptide: Short chains of up to 100 amino acids.
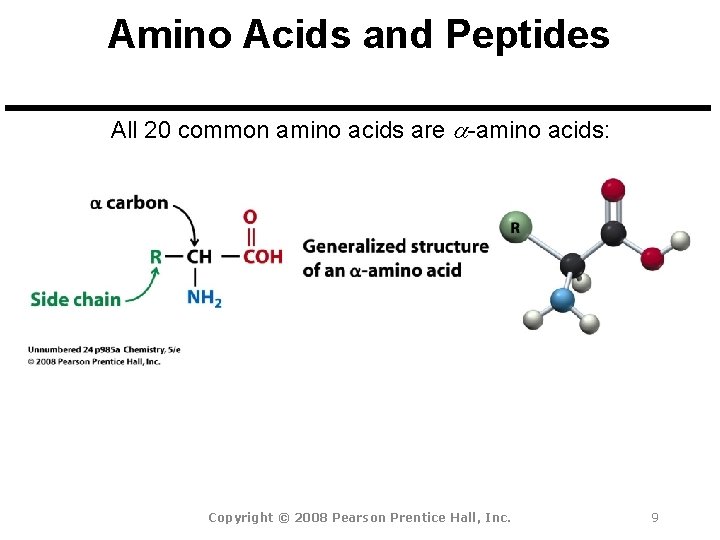
Amino Acids and Peptides All 20 common amino acids are a-amino acids: Copyright © 2008 Pearson Prentice Hall, Inc. 9
Amino Acids and Peptides Copyright © 2008 Pearson Prentice Hall, Inc. 10
Amino Acids and Peptides Copyright © 2008 Pearson Prentice Hall, Inc. 11
Amino Acids and Peptides Copyright © 2008 Pearson Prentice Hall, Inc. 12
Amino Acids and Peptides
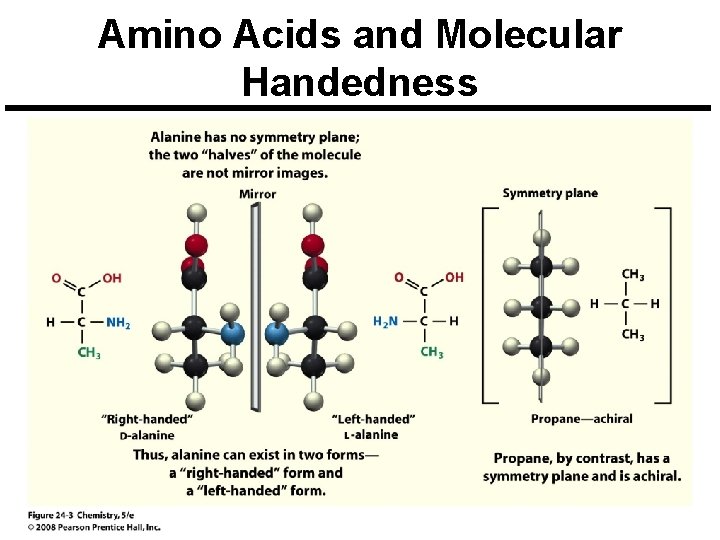
Amino Acids and Molecular Handedness
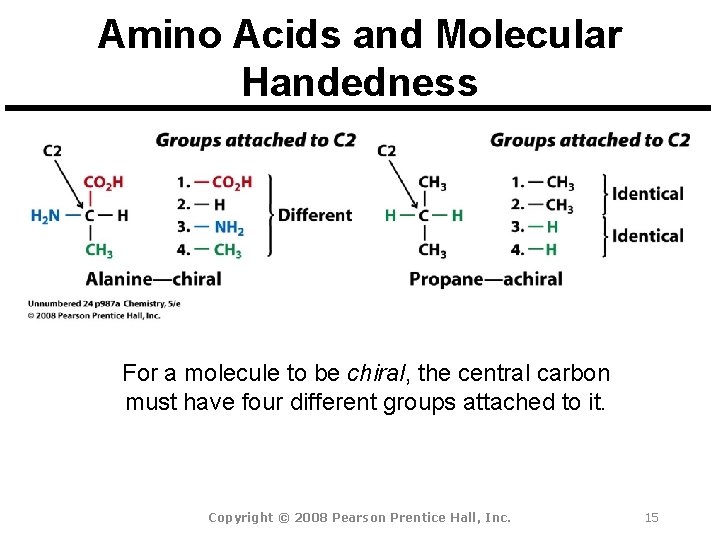
Amino Acids and Molecular Handedness For a molecule to be chiral, the central carbon must have four different groups attached to it. Copyright © 2008 Pearson Prentice Hall, Inc. 15
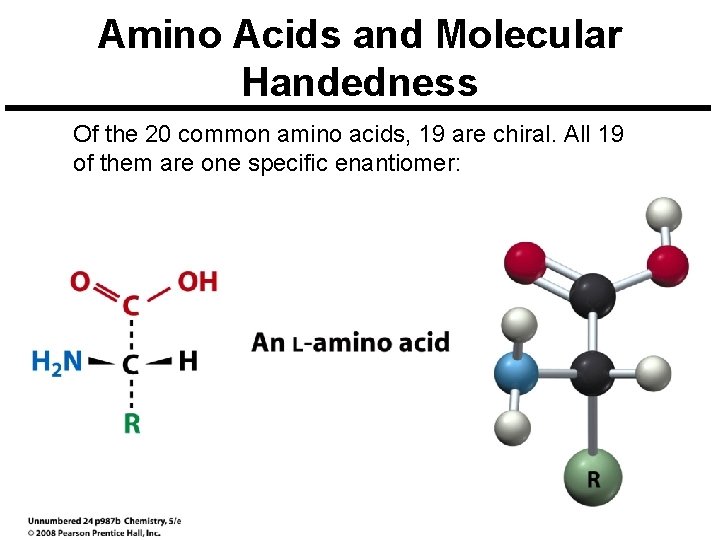
Amino Acids and Molecular Handedness Of the 20 common amino acids, 19 are chiral. All 19 of them are one specific enantiomer:
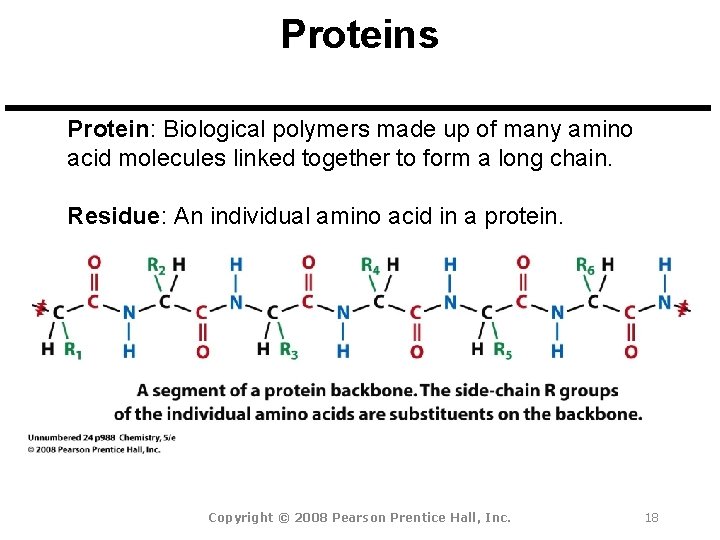
Proteins Protein: Biological polymers made up of many amino acid molecules linked together to form a long chain. Residue: An individual amino acid in a protein. Copyright © 2008 Pearson Prentice Hall, Inc. 18
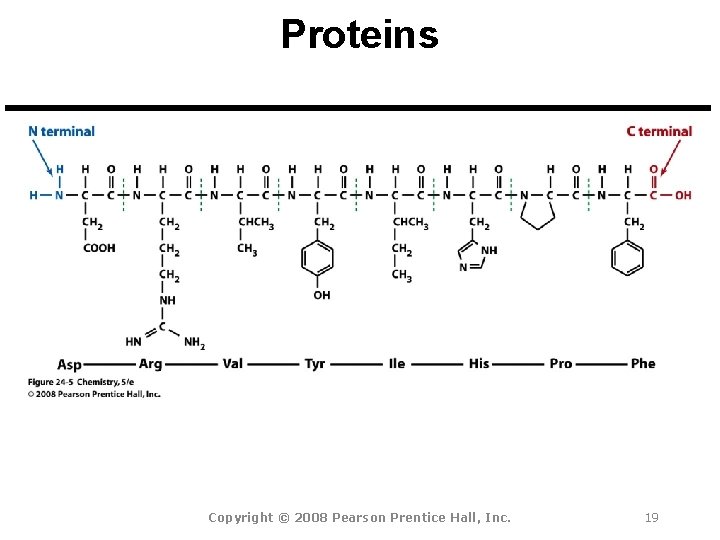
Proteins Copyright © 2008 Pearson Prentice Hall, Inc. 19
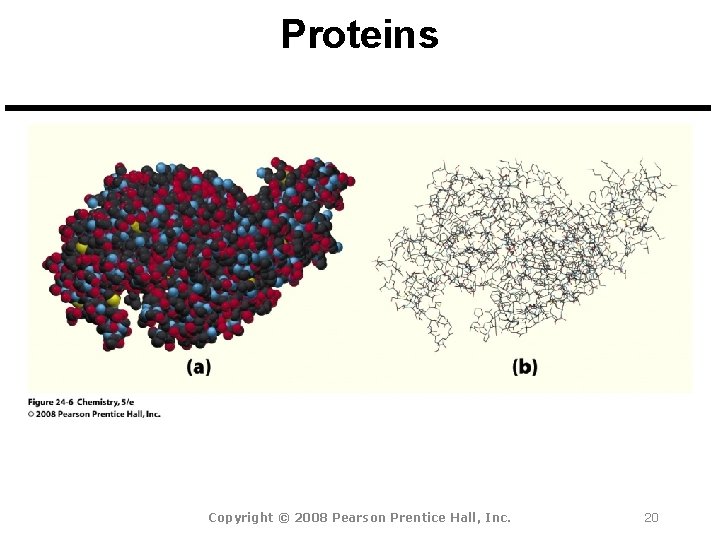
Proteins Copyright © 2008 Pearson Prentice Hall, Inc. 20
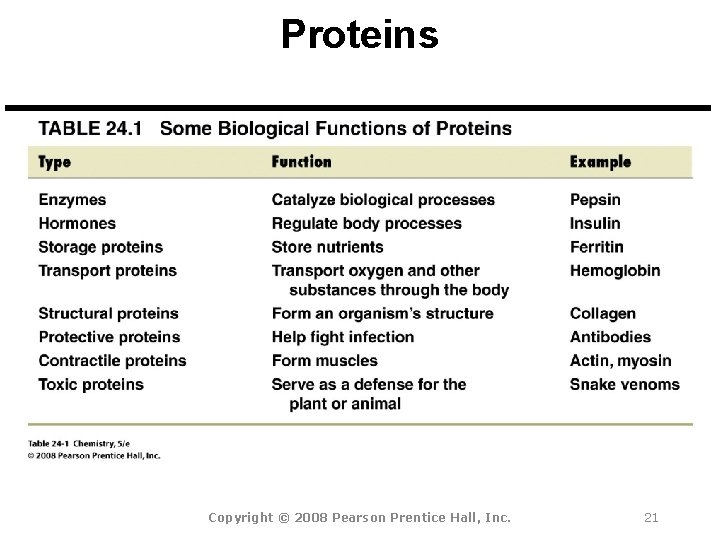
Proteins Copyright © 2008 Pearson Prentice Hall, Inc. 21
Levels of Protein Structure Primary Structure: This specifies the protein’s amino acid sequence which determines its overall shape and function. Secondary Structure: This specifies how segments of the protein chain are oriented into regular patterns. Tertiary Structure: Specifies how the entire protein chain is coiled and folded into a specific threedimensional shape. Quaternary Structure: Specifies how several protein chains can aggregate to form a larger unit. Copyright © 2008 Pearson Prentice Hall, Inc. 22
Levels of Protein Structure Primary Structure How important is the relationship between the sequence of amino acids to the function of the protein? The disease sickle-cell anemia is caused by a genetic defect whereby valine is substituted for glutamic acid at only one position in a chain of 146 amino acids. Copyright © 2008 Pearson Prentice Hall, Inc. 23
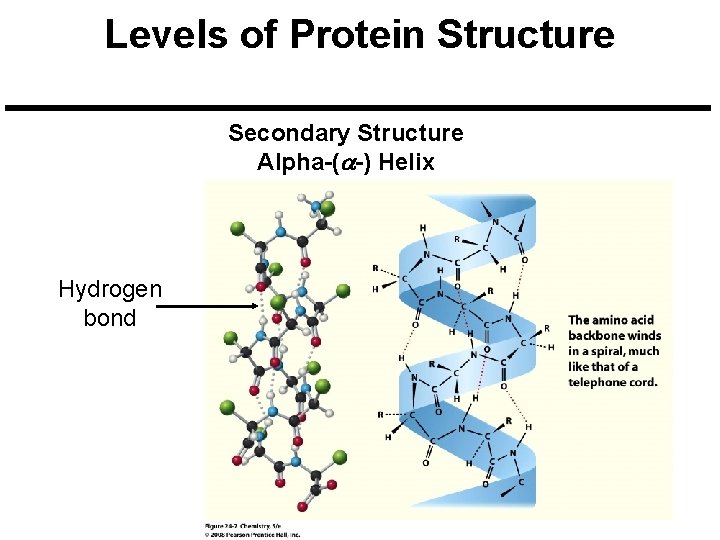
Levels of Protein Structure Secondary Structure Alpha-(a-) Helix Hydrogen bond
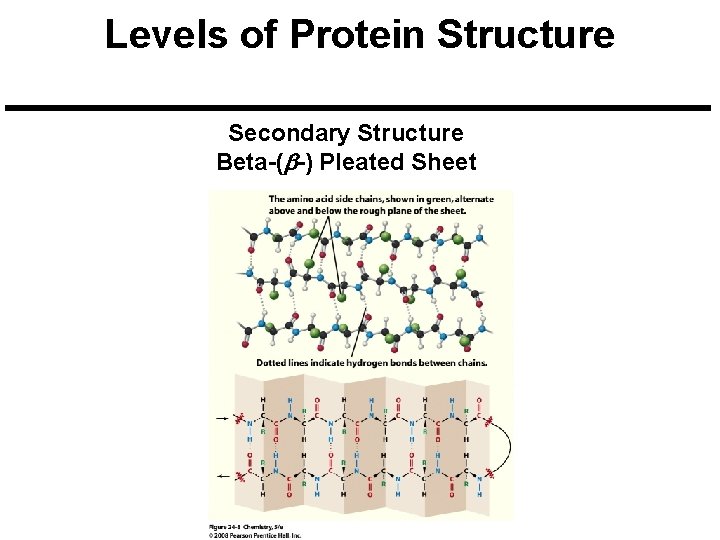
Levels of Protein Structure Secondary Structure Beta-(b-) Pleated Sheet
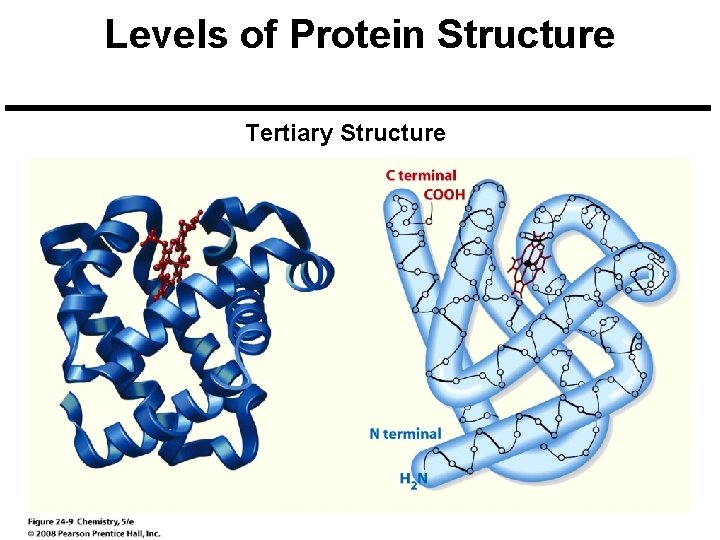
Levels of Protein Structure Tertiary Structure
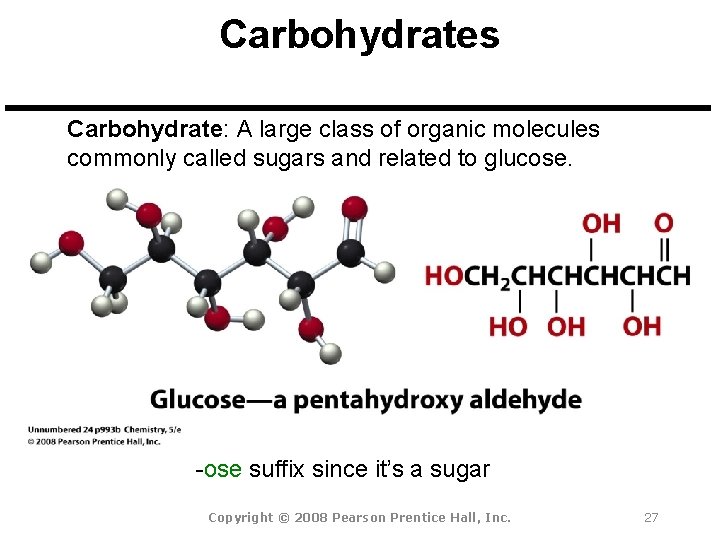
Carbohydrates Carbohydrate: A large class of organic molecules commonly called sugars and related to glucose. -ose suffix since it’s a sugar Copyright © 2008 Pearson Prentice Hall, Inc. 27
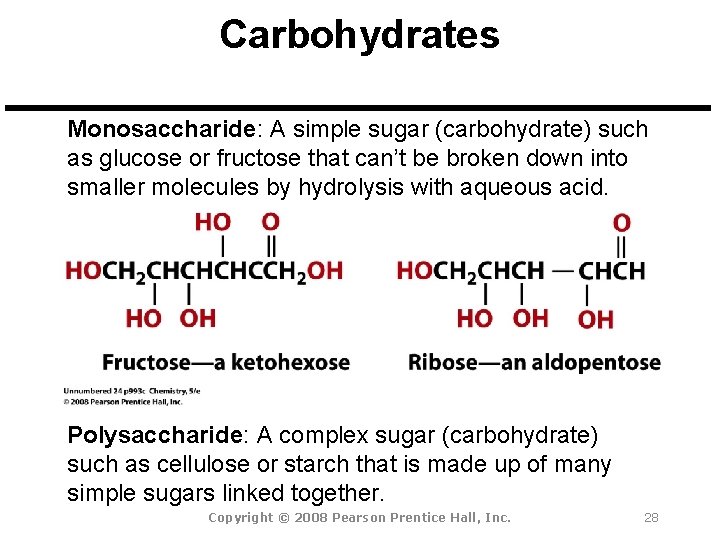
Carbohydrates Monosaccharide: A simple sugar (carbohydrate) such as glucose or fructose that can’t be broken down into smaller molecules by hydrolysis with aqueous acid. Polysaccharide: A complex sugar (carbohydrate) such as cellulose or starch that is made up of many simple sugars linked together. Copyright © 2008 Pearson Prentice Hall, Inc. 28
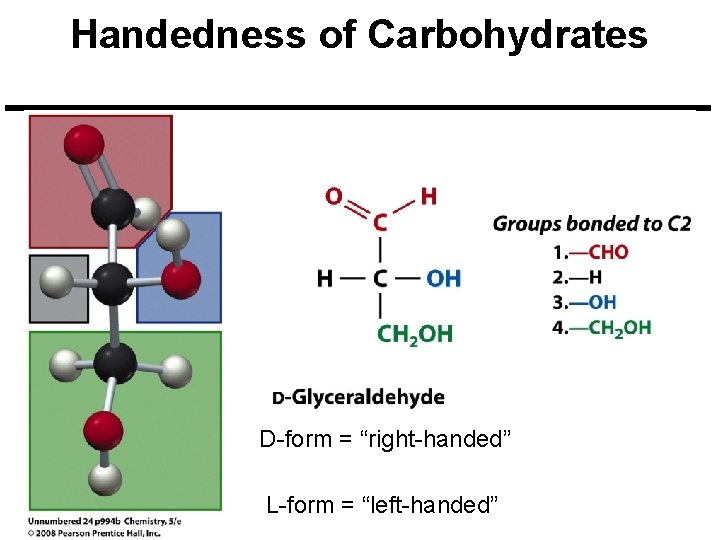
Handedness of Carbohydrates D-form = “right-handed” L-form = “left-handed”
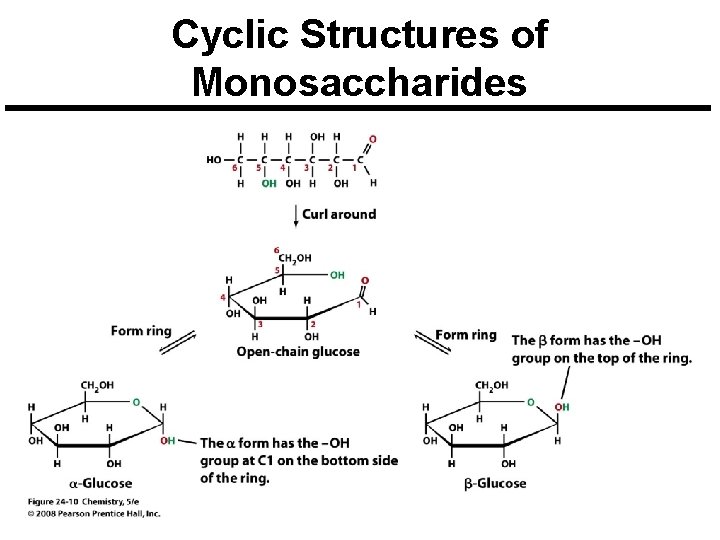
Cyclic Structures of Monosaccharides
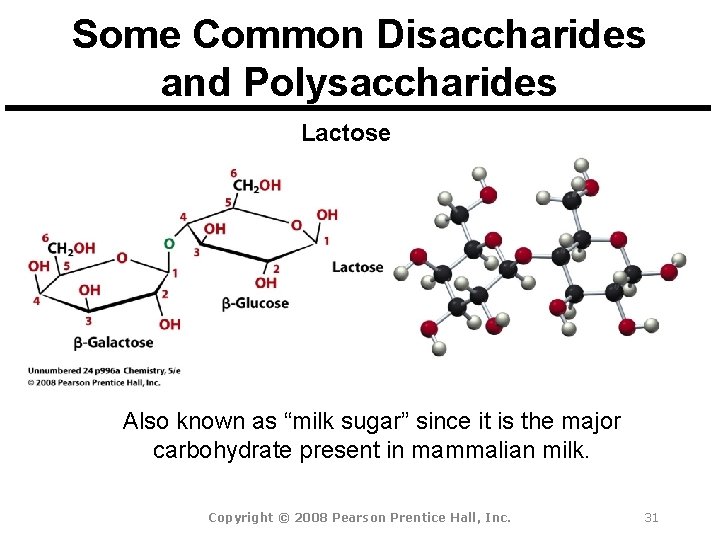
Some Common Disaccharides and Polysaccharides Lactose Also known as “milk sugar” since it is the major carbohydrate present in mammalian milk. Copyright © 2008 Pearson Prentice Hall, Inc. 31
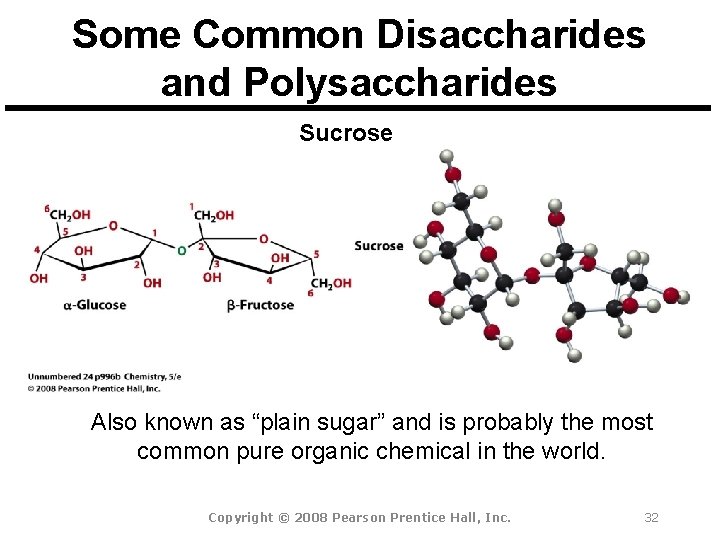
Some Common Disaccharides and Polysaccharides Sucrose Also known as “plain sugar” and is probably the most common pure organic chemical in the world. Copyright © 2008 Pearson Prentice Hall, Inc. 32
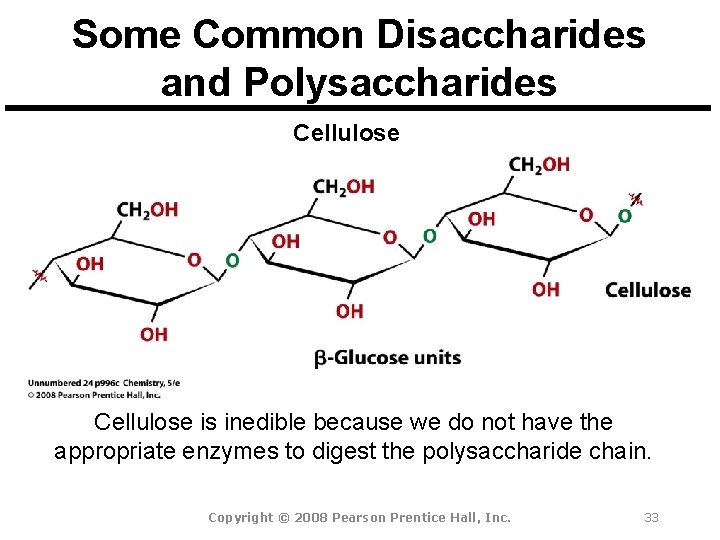
Some Common Disaccharides and Polysaccharides Cellulose is inedible because we do not have the appropriate enzymes to digest the polysaccharide chain. Copyright © 2008 Pearson Prentice Hall, Inc. 33
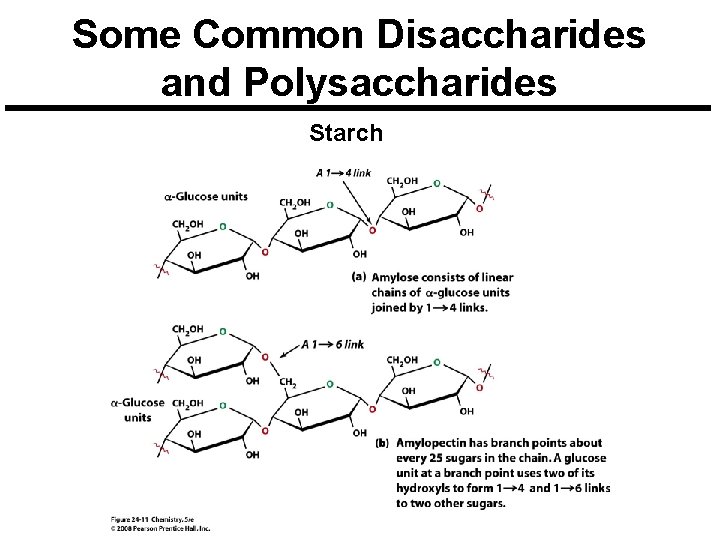
Some Common Disaccharides and Polysaccharides Starch
Some Common Disaccharides and Polysaccharides Glycogen Also known as “animal starch” since it serves the same purpose in animals that starch serves in plants. Copyright © 2008 Pearson Prentice Hall, Inc. 35
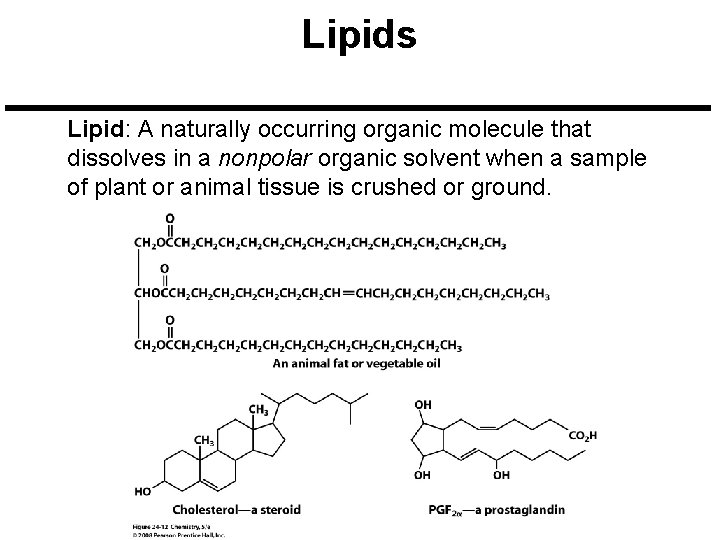
Lipids Lipid: A naturally occurring organic molecule that dissolves in a nonpolar organic solvent when a sample of plant or animal tissue is crushed or ground.
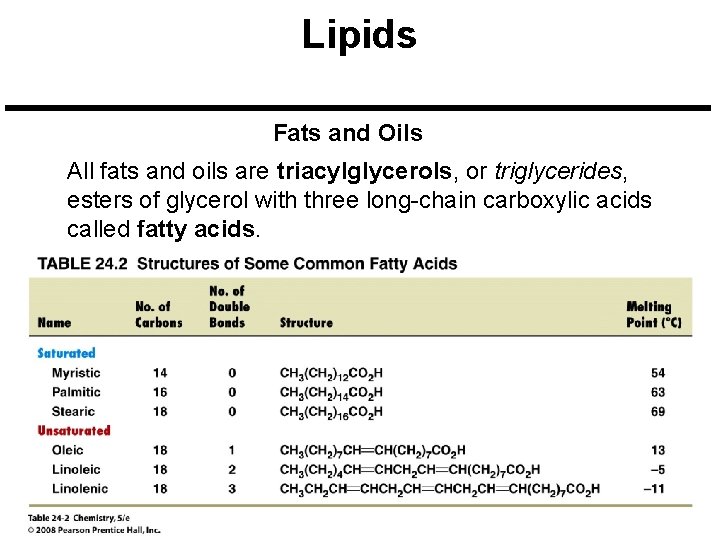
Lipids Fats and Oils All fats and oils are triacylglycerols, or triglycerides, esters of glycerol with three long-chain carboxylic acids called fatty acids.
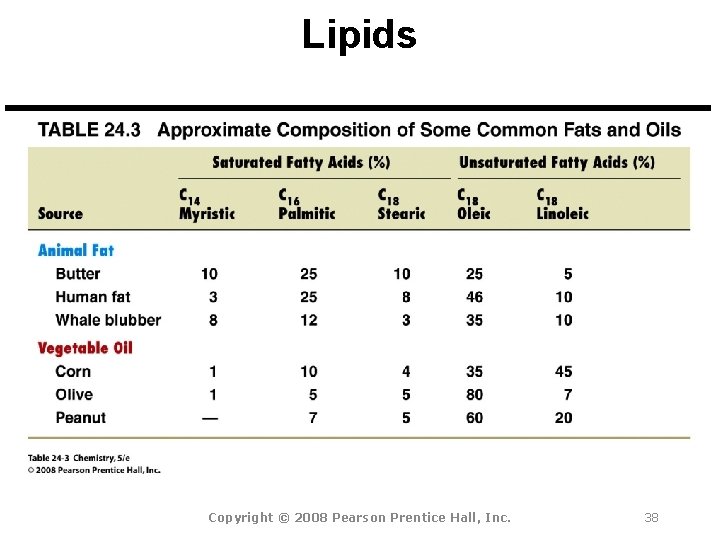
Lipids Copyright © 2008 Pearson Prentice Hall, Inc. 38
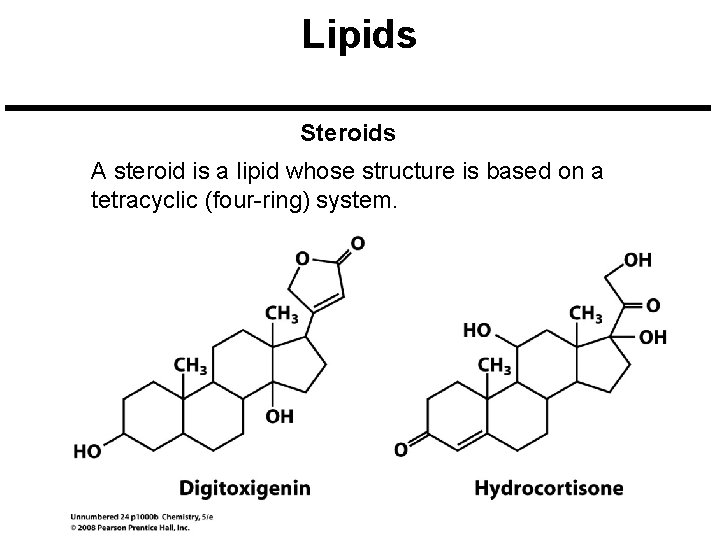
Lipids Steroids A steroid is a lipid whose structure is based on a tetracyclic (four-ring) system.
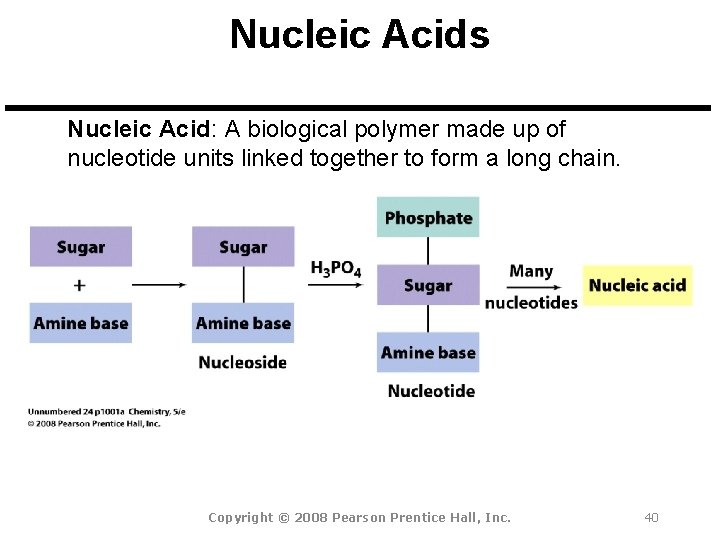
Nucleic Acids Nucleic Acid: A biological polymer made up of nucleotide units linked together to form a long chain. Copyright © 2008 Pearson Prentice Hall, Inc. 40
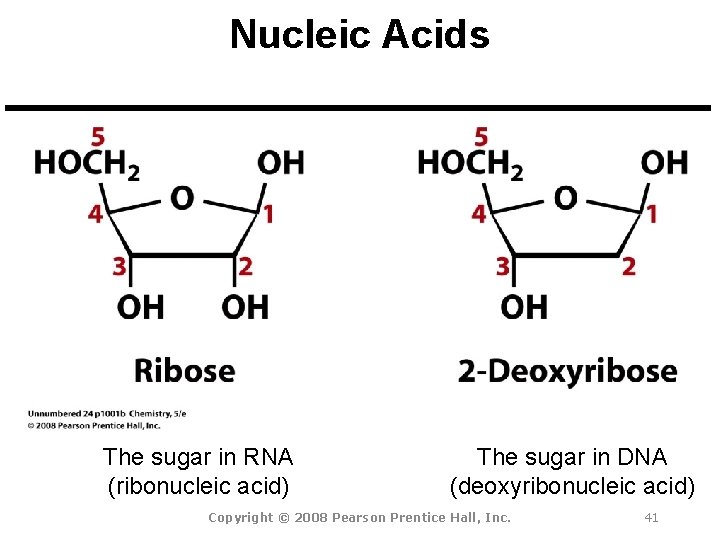
Nucleic Acids The sugar in RNA (ribonucleic acid) The sugar in DNA (deoxyribonucleic acid) Copyright © 2008 Pearson Prentice Hall, Inc. 41
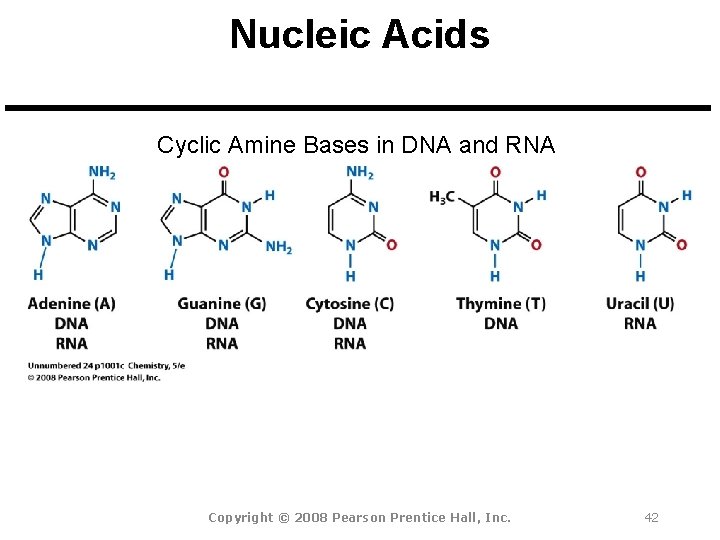
Nucleic Acids Cyclic Amine Bases in DNA and RNA Copyright © 2008 Pearson Prentice Hall, Inc. 42
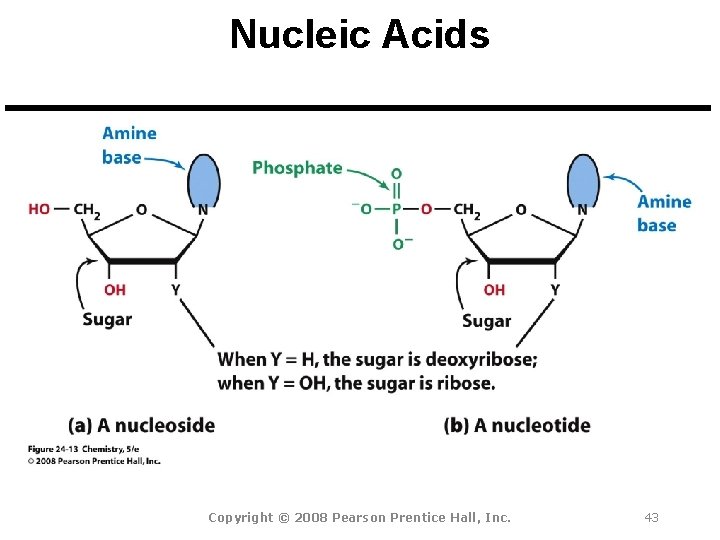
Nucleic Acids Copyright © 2008 Pearson Prentice Hall, Inc. 43
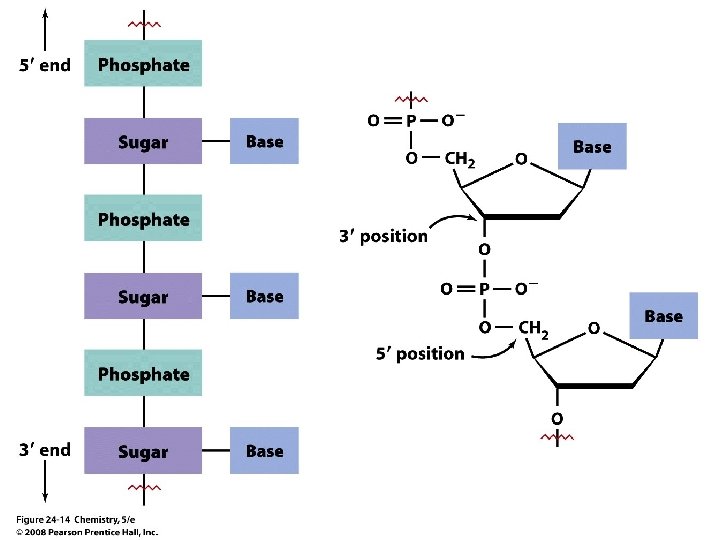
Nucleic Acids
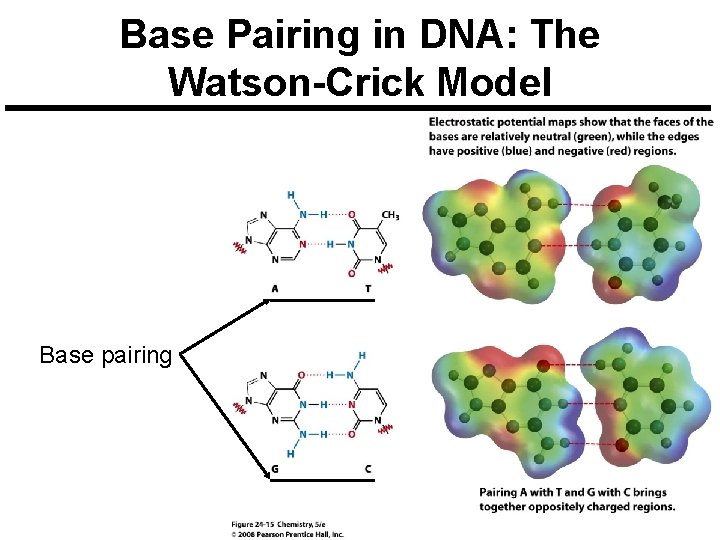
Base Pairing in DNA: The Watson-Crick Model Base pairing

Base Pairing in DNA: The Watson-Crick Model

Nucleic Acids and Heredity Chromosome: A threadlike strand of DNA in the nucleus of a cell. Each chromosome is made up of several thousand genes. Gene: A segment of a DNA chain that contains the instructions necessary to make a specific protein. Transfer of Genetic Information Copyright © 2008 Pearson Prentice Hall, Inc. 47
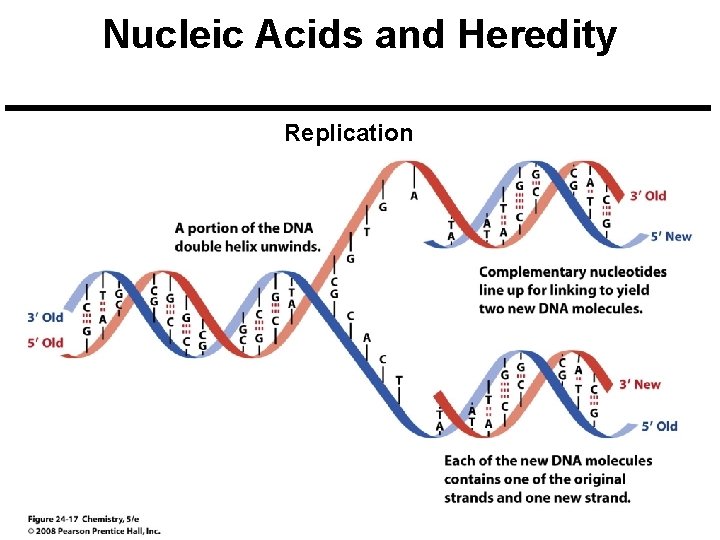
Nucleic Acids and Heredity Replication
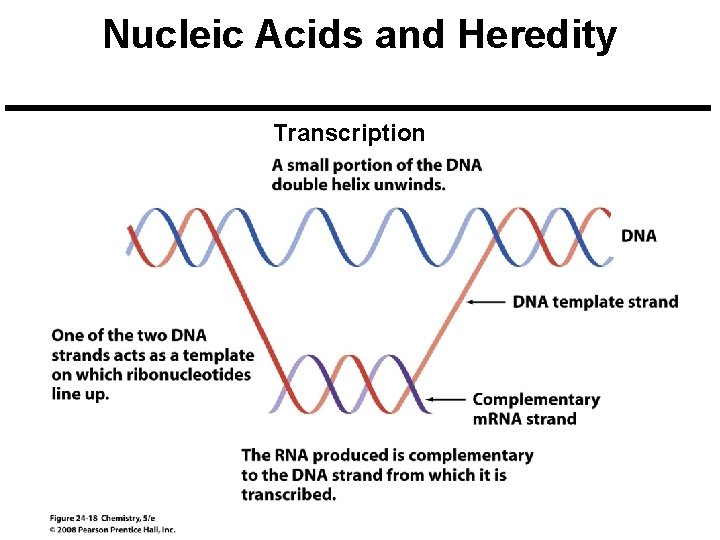
Nucleic Acids and Heredity Transcription
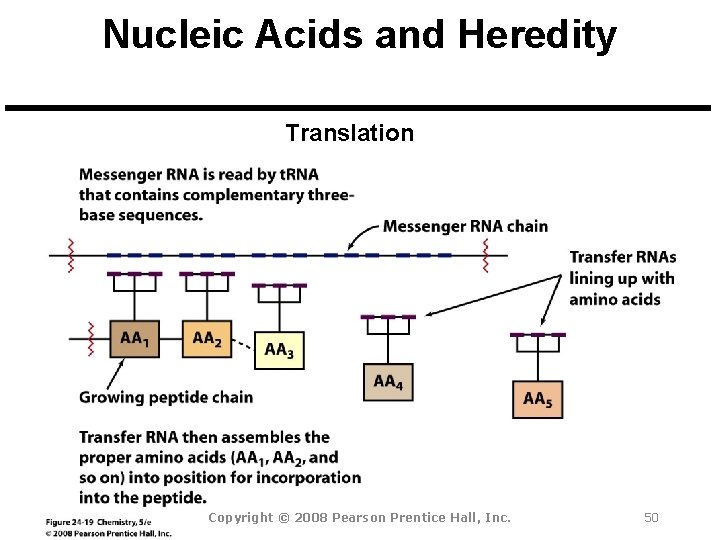
Nucleic Acids and Heredity Translation Copyright © 2008 Pearson Prentice Hall, Inc. 50
- Slides: 50