Chemistry of Nucleic Acids Nucleic acids DNA and






- Slides: 11

Chemistry of Nucleic Acids Ø Nucleic acids (DNA and RNA) are made by joining nucleotides in a repetitive way into long polymers Ø Nucleotides have three components (a) phosphate (b) sugar and (c) base Ø Nucleoside is a sugar-base compound

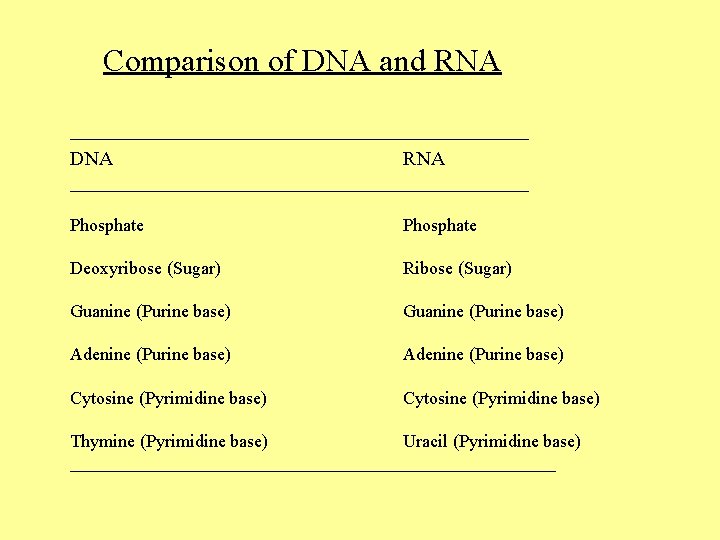
Comparison of DNA and RNA __________________________ DNA RNA __________________________ Phosphate Deoxyribose (Sugar) Ribose (Sugar) Guanine (Purine base) Adenine (Purine base) Cytosine (Pyrimidine base) Thymine (Pyrimidine base) Uracil (Pyrimidine base) ___________________________

Figure 1. Components of nucleic acids (a) phosphate (b) sugar (c) base

Figure 2. The structure of a nucleoside and two nucleotides: nucleoside monophosphate and nucleoside triphosphate

Figure 3. Structure of four deoxyribose nucleotides

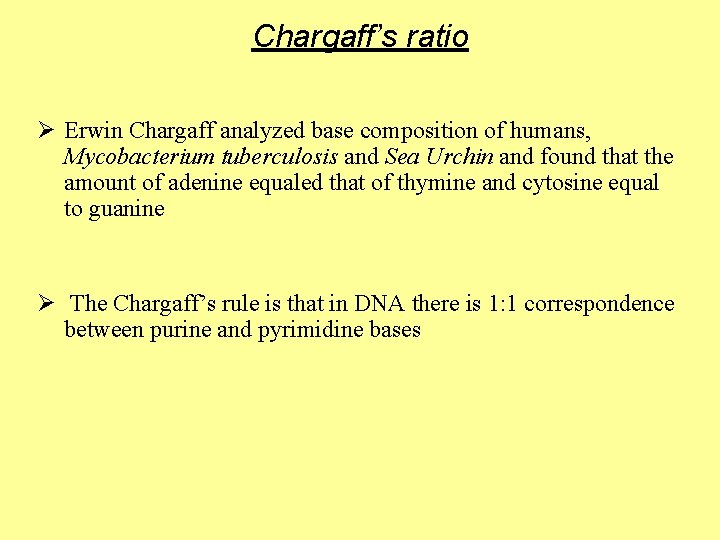
Chargaff’s ratio Ø Erwin Chargaff analyzed base composition of humans, Mycobacterium tuberculosis and Sea Urchin and found that the amount of adenine equaled that of thymine and cytosine equal to guanine Ø The Chargaff’s rule is that in DNA there is 1: 1 correspondence between purine and pyrimidine bases

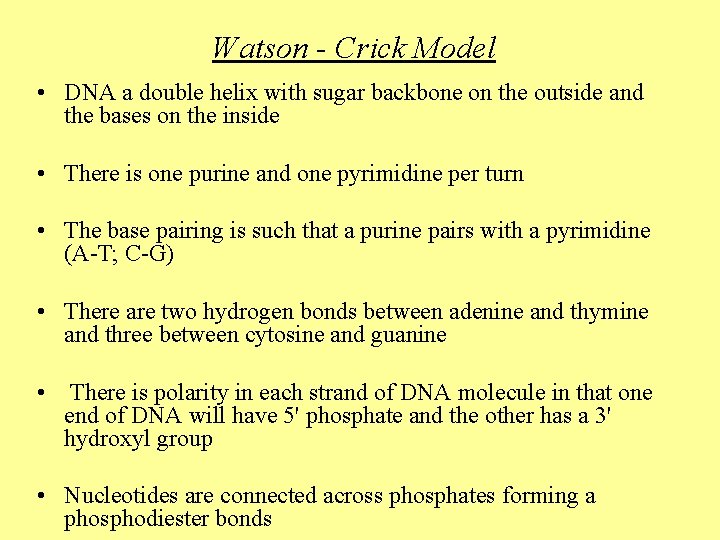
Watson - Crick Model • DNA a double helix with sugar backbone on the outside and the bases on the inside • There is one purine and one pyrimidine per turn • The base pairing is such that a purine pairs with a pyrimidine (A-T; C-G) • There are two hydrogen bonds between adenine and thymine and three between cytosine and guanine • There is polarity in each strand of DNA molecule in that one end of DNA will have 5' phosphate and the other has a 3' hydroxyl group • Nucleotides are connected across phosphates forming a phosphodiester bonds

Figure 3. Double helical structure of DNA

DNA denaturation Ø Hydrogen bonding can be broken and DNA strands separated by heating Ø Since cytosine-guanine pair has three hydrogen bonds to two of adenine-thymine, the former will require more heat to denature

Requirements of Genetic material Ø For something to be classified as a genetic material it needs to meet the following requirements: - Control of protein synthesis - Later lectures will show that DNA has the complexity to direct protein synthesis - Self-replication – DNA is capable of self replication as explained in the next lecture - Location in the nucleus – In prokaryote the majority of the cell’s DNA is found in the chromosome

Alternative forms of DNA Ø The form of DNA we have described above is a right handed double helix and it is called B DNA and its turns clockwise when viewed at its axis and bases are stacked perpendicular to the main axis Ø If water content increases to about 75% bases are tilted in regard to the axis and there are more bases per turn and this DNA is called A DNA. Ø In 1979 Alexander Rich discovered Z DNA which is a left handed helix. This DNA requires high salts to be stable but it can be stabilized in physiologically normal conditions if methyl groups are added to cytosine. This DNA may be involved in regulating gene expression in eukaryotes