n n n Taste sour Form solution that






![Ex : calculate [H+] or [OH-] as required for each of the following at Ex : calculate [H+] or [OH-] as required for each of the following at](https://slidetodoc.com/presentation_image/1e54a6b6d904c678d95ff1e4c0c299ec/image-17.jpg)
![b) 10. 0 M H+ Find: OHSolution: [H+][OH-] = 1 x 10 -14 [OH-] b) 10. 0 M H+ Find: OHSolution: [H+][OH-] = 1 x 10 -14 [OH-]](https://slidetodoc.com/presentation_image/1e54a6b6d904c678d95ff1e4c0c299ec/image-18.jpg)
![In Basic solution, [OH-] can be expressed as p. OH n p. OH = In Basic solution, [OH-] can be expressed as p. OH n p. OH =](https://slidetodoc.com/presentation_image/1e54a6b6d904c678d95ff1e4c0c299ec/image-22.jpg)
- Slides: 36


n n n Taste sour Form solution that conduct electricity React with metals Turn blue litmus paper to red React with bases to form salt and water Ex: fruit juice, vinegar, milk

n n n Form solutions that conduct electricity Slippery or soapy in the skin Taste bitter Turn red litmus paper to blue Reacts with acids to form salt and water Ex: soap, shampoo, cleaning agent

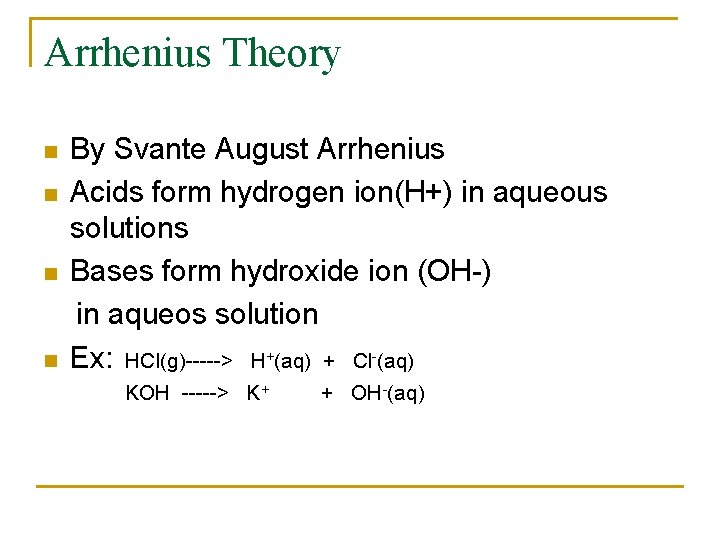
Arrhenius Theory n n By Svante August Arrhenius Acids form hydrogen ion(H+) in aqueous solutions Bases form hydroxide ion (OH-) in aqueos solution Ex: HCl(g)-----> H+(aq) + Cl-(aq) KOH -----> K+ + OH-(aq)

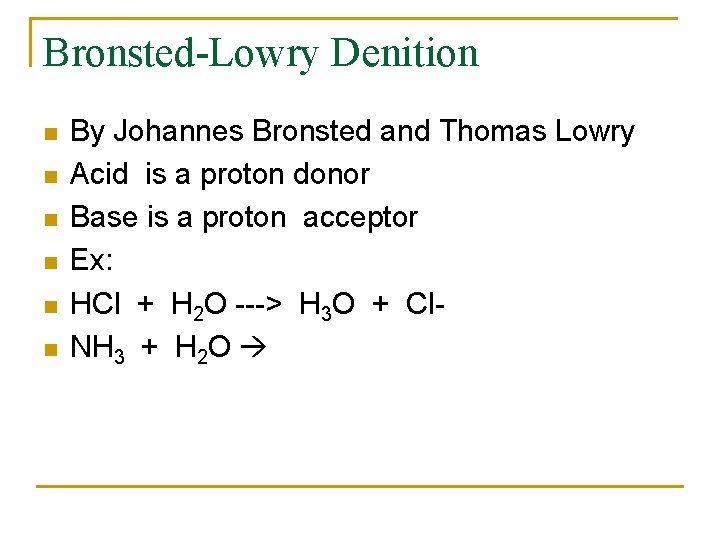
Bronsted-Lowry Denition n n n By Johannes Bronsted and Thomas Lowry Acid is a proton donor Base is a proton acceptor Ex: HCl + H 2 O ---> H 3 O + Cl. NH 3 + H 2 O

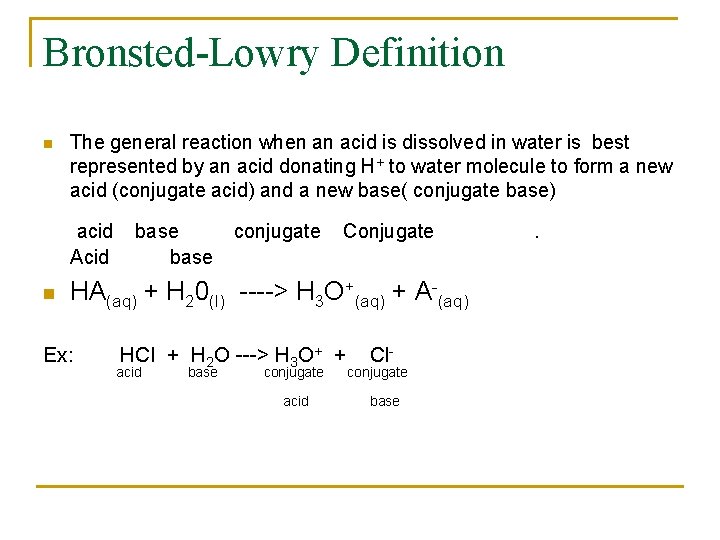
Bronsted-Lowry Definition n The general reaction when an acid is dissolved in water is best represented by an acid donating H+ to water molecule to form a new acid (conjugate acid) and a new base( conjugate base) acid Acid n base conjugate Conjugate HA(aq) + H 20(l) ----> H 3 O+(aq) + A-(aq) Ex: HCl + H 2 O ---> H 3 O+ + acid base conjugate acid Cl- conjugate base .

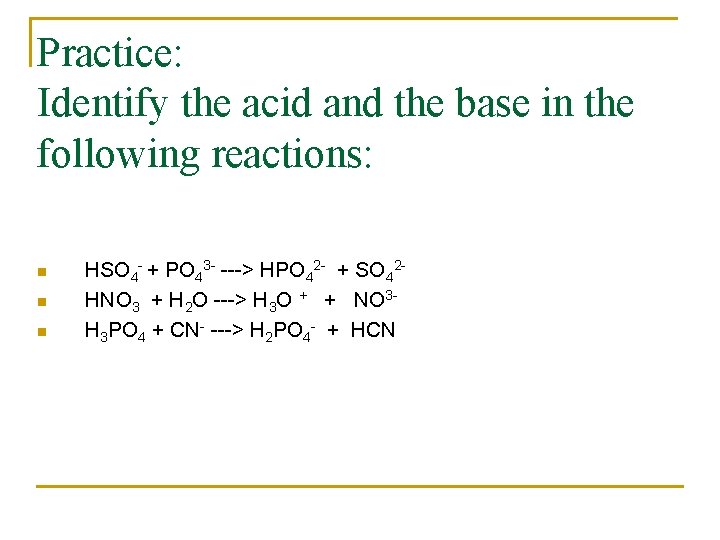
Practice: Identify the acid and the base in the following reactions: n n n HSO 4 - + PO 43 - ---> HPO 42 - + SO 42 HNO 3 + H 2 O ---> H 3 O + + NO 3 H 3 PO 4 + CN- ---> H 2 PO 4 - + HCN

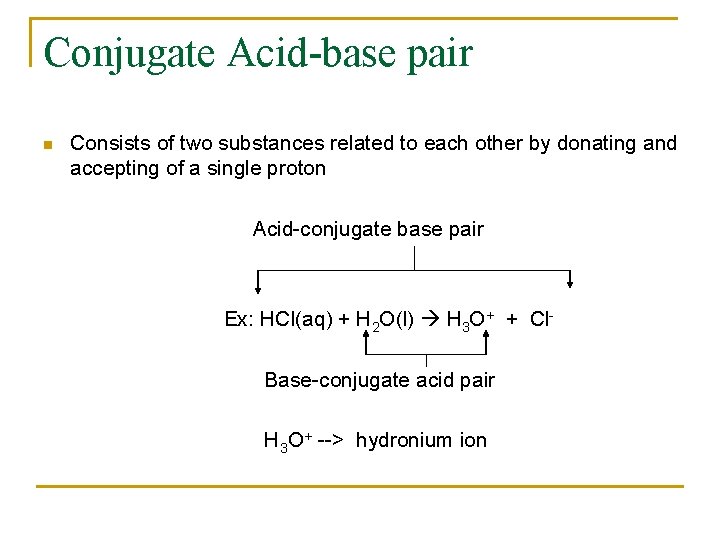
Conjugate Acid-base pair n Consists of two substances related to each other by donating and accepting of a single proton Acid-conjugate base pair Ex: HCl(aq) + H 2 O(l) H 3 O+ + Cl. Base-conjugate acid pair H 3 O+ --> hydronium ion


Strong Acids n n n Bronsted-Lowry definition is useful in describing the strength of an acid Strong acid is one that dissociates or ionizes completely in water Ex: HCl + H 2 O H 3 O+ + Cl. Forward reaction predominates which indicates ionization is complete Strong acid contains a weak conjugate base weaker than water

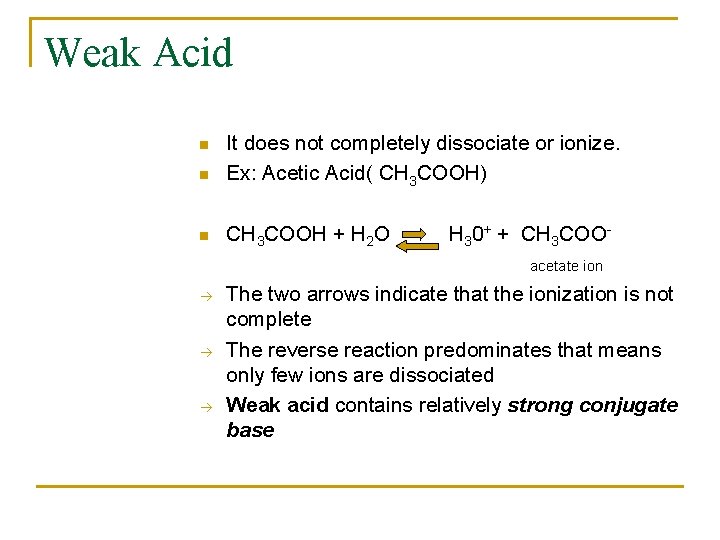
Weak Acid n It does not completely dissociate or ionize. Ex: Acetic Acid( CH 3 COOH) n CH 3 COOH + H 2 O n H 30+ + CH 3 COOacetate ion The two arrows indicate that the ionization is not complete The reverse reaction predominates that means only few ions are dissociated Weak acid contains relatively strong conjugate base

Strong Bases n n Common strong bases are those that contain the hydroxide ion Ex: , Na. OH, KOH Mg(OH)2, Ca(OH)2 Ba(OH)2 n Very soluble in water

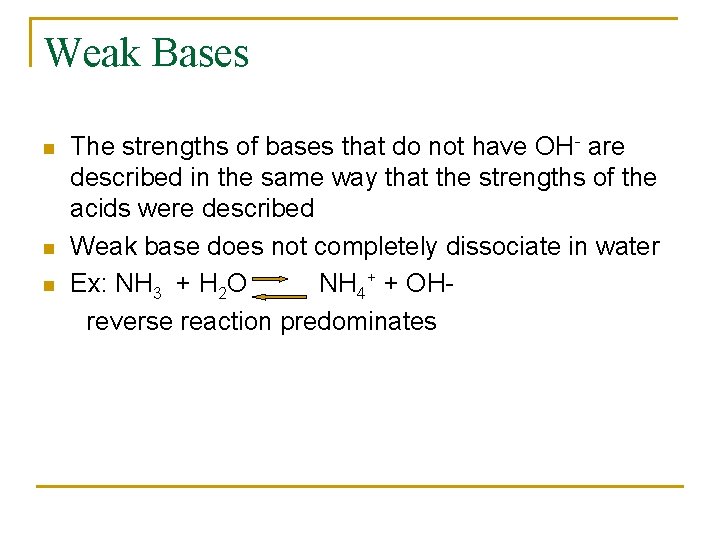
Weak Bases n n n The strengths of bases that do not have OH- are described in the same way that the strengths of the acids were described Weak base does not completely dissociate in water Ex: NH 3 + H 2 O NH 4+ + OHreverse reaction predominates

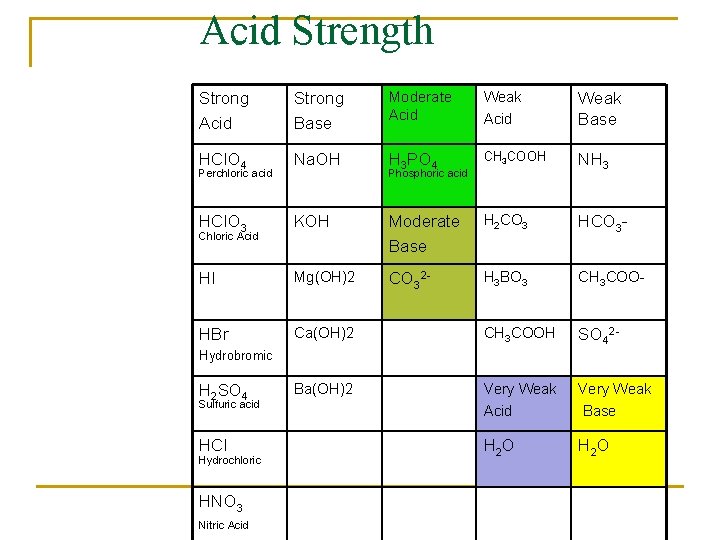
Acid Strength Strong Acid Strong Base Moderate Acid Weak Base HCl. O 4 Na. OH H 3 PO 4 CH 3 COOH NH 3 HCl. O 3 KOH Moderate Base H 2 CO 3 HCO 3 - HI Mg(OH)2 CO 32 - H 3 BO 3 CH 3 COO- HBr Ca(OH)2 CH 3 COOH SO 42 - Ba(OH)2 Sulfuric acid Very Weak Acid Very Weak Base HCl H 2 O Perchloric acid Chloric Acid Phosphoric acid Hydrobromic H 2 SO 4 Hydrochloric HNO 3 Nitric Acid

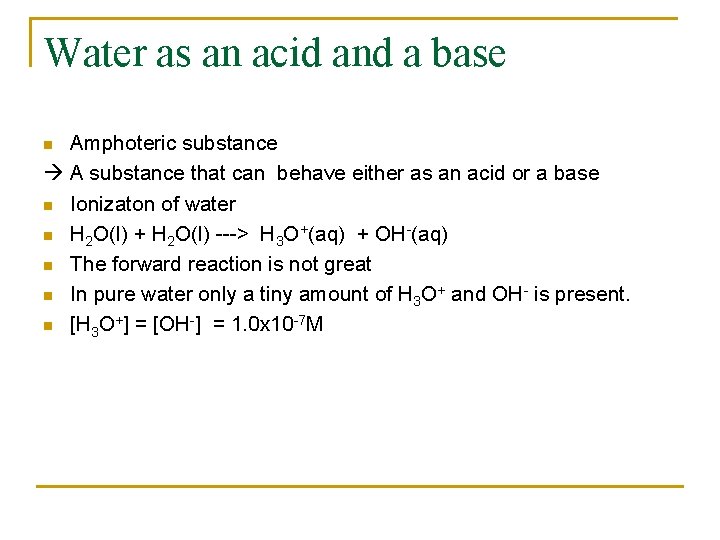
Water as an acid and a base Amphoteric substance A substance that can behave either as an acid or a base n Ionizaton of water n H 2 O(l) + H 2 O(l) ---> H 3 O+(aq) + OH-(aq) n The forward reaction is not great n In pure water only a tiny amount of H 3 O+ and OH- is present. n [H 3 O+] = [OH-] = 1. 0 x 10 -7 M n

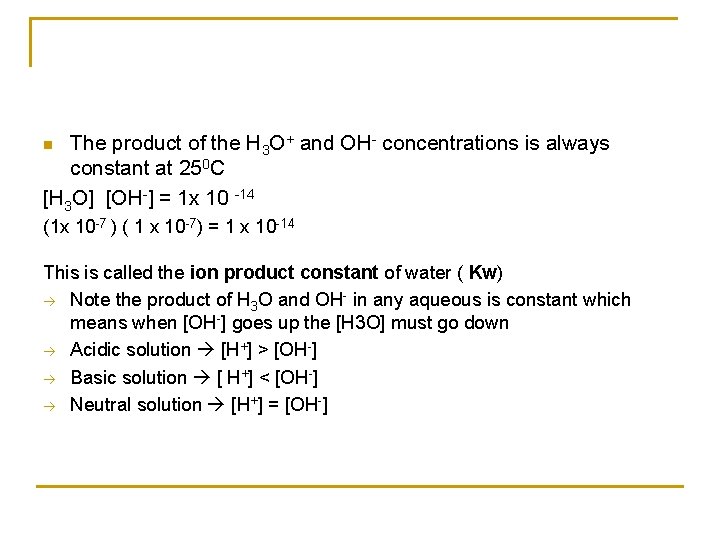
The product of the H 3 O+ and OH- concentrations is always constant at 250 C [H 3 O] [OH-] = 1 x 10 -14 n (1 x 10 -7 ) ( 1 x 10 -7) = 1 x 10 -14 This is called the ion product constant of water ( Kw) Note the product of H 3 O and OH- in any aqueous is constant which means when [OH-] goes up the [H 3 O] must go down Acidic solution [H+] > [OH-] Basic solution [ H+] < [OH-] Neutral solution [H+] = [OH-]
![Ex calculate H or OH as required for each of the following at Ex : calculate [H+] or [OH-] as required for each of the following at](https://slidetodoc.com/presentation_image/1e54a6b6d904c678d95ff1e4c0c299ec/image-17.jpg)
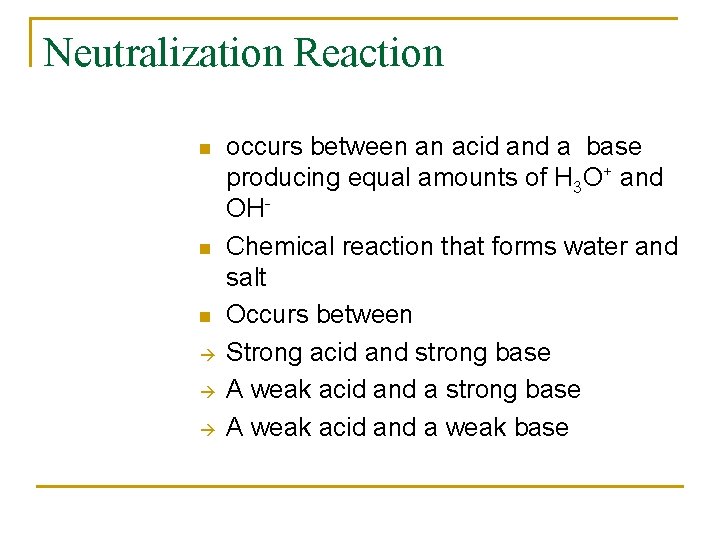
Ex : calculate [H+] or [OH-] as required for each of the following at 250 C and state whether the solution is acidic, basic and neutral A) 1. 0 x 10 -5 M OHGiven: [OH-] = 1 x 10 -5 Find: H+ Solution: [H+] [OH-] = 1 x 10 -14 [H+] = 1 x 10 -14 [OH-] [H+] = 1 x 10 -14 [ 1 x 10 -5] n 1 x 10 -9 M Since OH- = 1 x 10 -5 M H+ = 1 x 10 -9 M The solution is basic since OH- > H+ =
![b 10 0 M H Find OHSolution HOH 1 x 10 14 OH b) 10. 0 M H+ Find: OHSolution: [H+][OH-] = 1 x 10 -14 [OH-]](https://slidetodoc.com/presentation_image/1e54a6b6d904c678d95ff1e4c0c299ec/image-18.jpg)
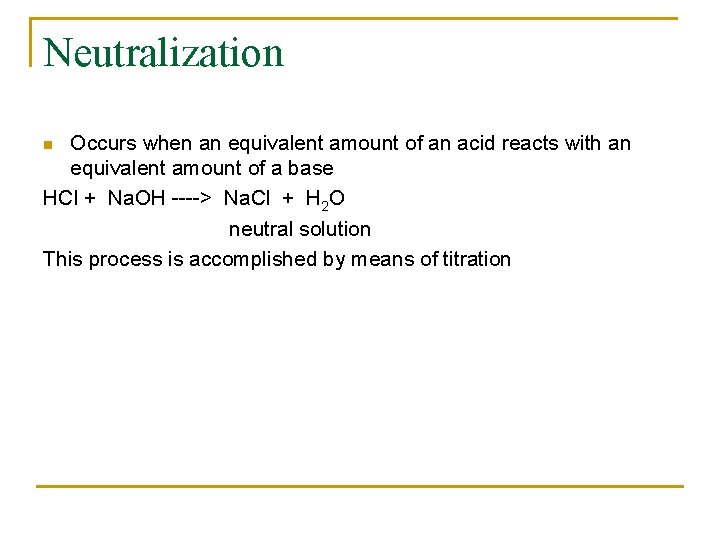
b) 10. 0 M H+ Find: OHSolution: [H+][OH-] = 1 x 10 -14 [OH-] = 1 x 10 -14 10. 0 M = 1 x 10 -15 M [OH-] = 1 x 10 -15 [H+] = 1 x 101 M [H+] > [OH-] = the solution is acidic n

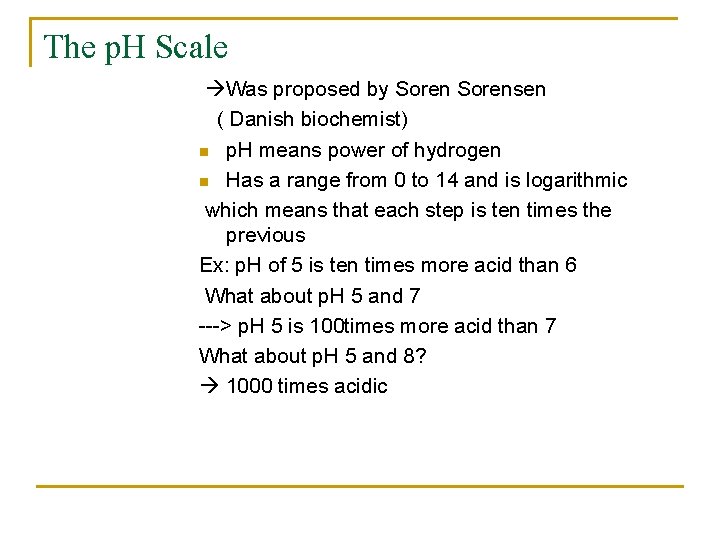
The p. H Scale Was proposed by Sorensen ( Danish biochemist) n p. H means power of hydrogen n Has a range from 0 to 14 and is logarithmic which means that each step is ten times the previous Ex: p. H of 5 is ten times more acid than 6 What about p. H 5 and 7 ---> p. H 5 is 100 times more acid than 7 What about p. H 5 and 8? 1000 times acidic

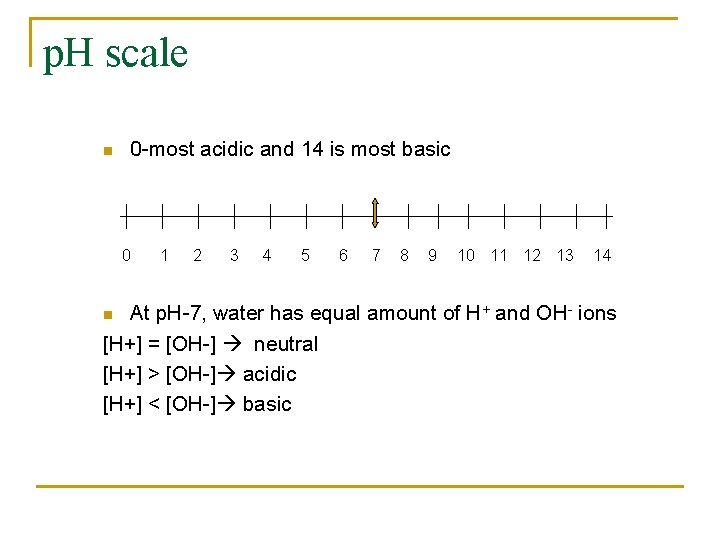
p. H scale n 0 -most acidic and 14 is most basic 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 At p. H-7, water has equal amount of H+ and OH- ions [H+] = [OH-] neutral [H+] > [OH-] acidic [H+] < [OH-] basic n

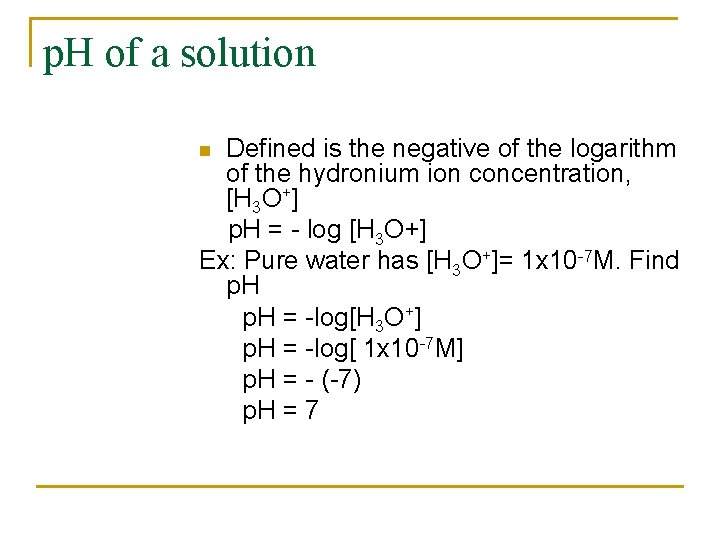
p. H of a solution Defined is the negative of the logarithm of the hydronium ion concentration, [H 3 O+] p. H = - log [H 3 O+] Ex: Pure water has [H 3 O+]= 1 x 10 -7 M. Find p. H = -log[H 3 O+] p. H = -log[ 1 x 10 -7 M] p. H = - (-7) p. H = 7 n
![In Basic solution OH can be expressed as p OH n p OH In Basic solution, [OH-] can be expressed as p. OH n p. OH =](https://slidetodoc.com/presentation_image/1e54a6b6d904c678d95ff1e4c0c299ec/image-22.jpg)
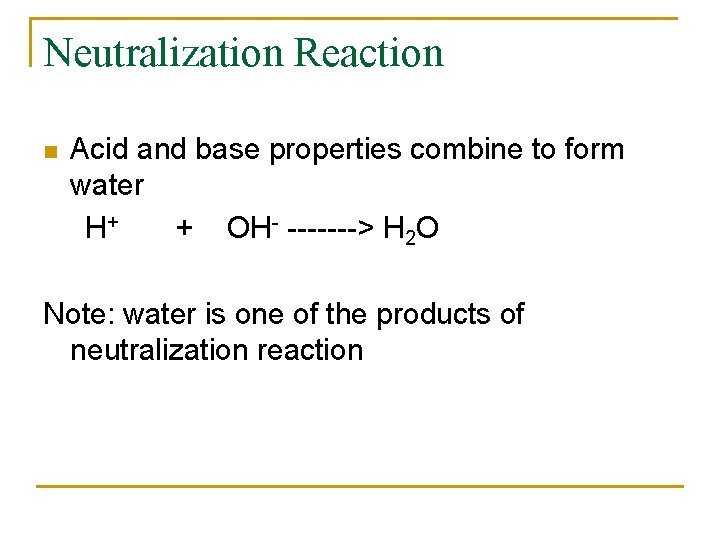
In Basic solution, [OH-] can be expressed as p. OH n p. OH = -log [OH-] Recall that [H 3 O+] [OH-] = 1 x 10 -14 Using the definition of p. H and p. OH the equation can be translated as p. H + p. OH = 14 In summary, p. H= -log[H 3 O+] p. OH = -log [OH-] [H 3 O+][OH-] = 1 x 10 -14 p. H + p. OH = 14 n

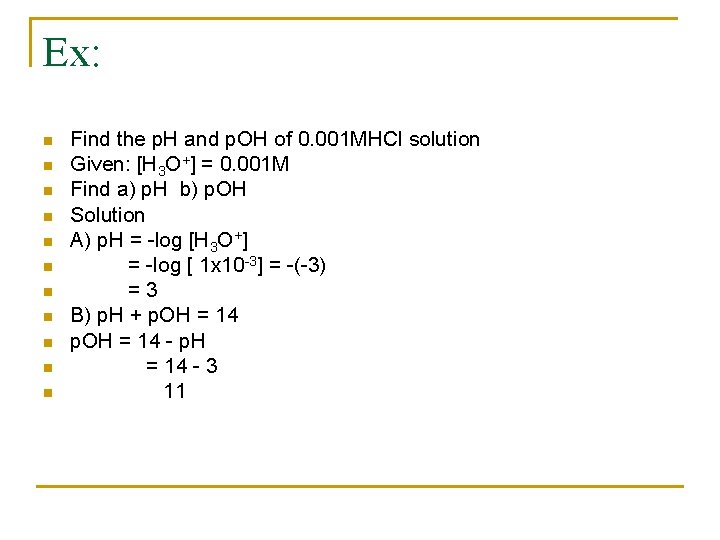
Ex: n n n Find the p. H and p. OH of 0. 001 MHCl solution Given: [H 3 O+] = 0. 001 M Find a) p. H b) p. OH Solution A) p. H = -log [H 3 O+] = -log [ 1 x 10 -3] = -(-3) =3 B) p. H + p. OH = 14 - p. H = 14 - 3 11

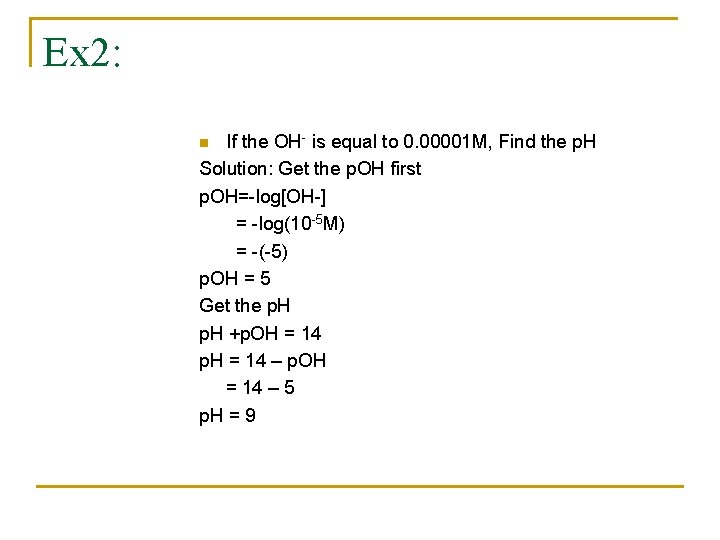
Ex 2: If the OH- is equal to 0. 00001 M, Find the p. H Solution: Get the p. OH first p. OH=-log[OH-] = -log(10 -5 M) = -(-5) p. OH = 5 Get the p. H +p. OH = 14 p. H = 14 – p. OH = 14 – 5 p. H = 9 n

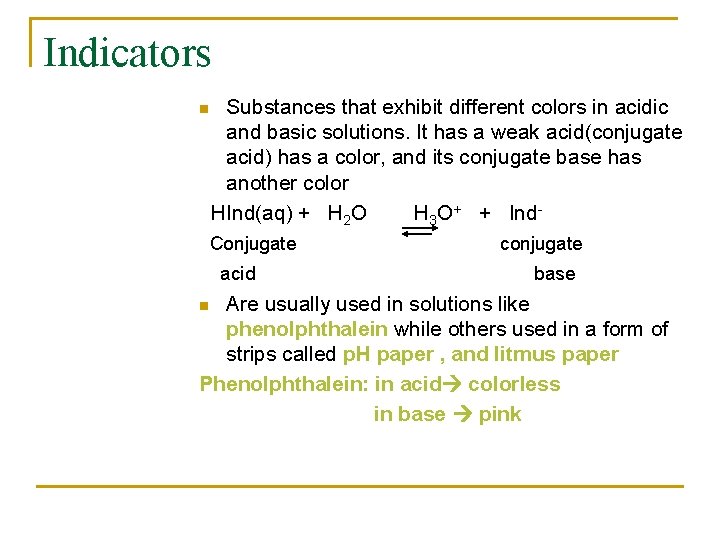
Indicators n Substances that exhibit different colors in acidic and basic solutions. It has a weak acid(conjugate acid) has a color, and its conjugate base has another color HInd(aq) + H 2 O H 3 O+ + Ind- Conjugate acid conjugate base Are usually used in solutions like phenolphthalein while others used in a form of strips called p. H paper , and litmus paper Phenolphthalein: in acid colorless in base pink n

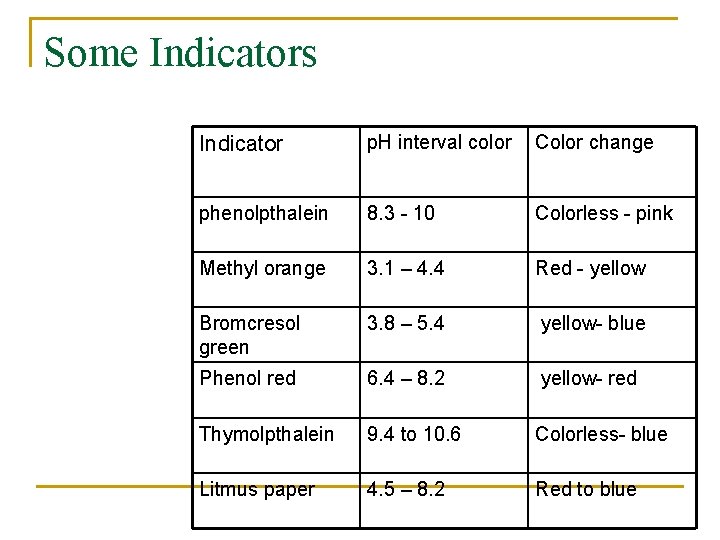
Some Indicators Indicator p. H interval color Color change phenolpthalein 8. 3 - 10 Colorless - pink Methyl orange 3. 1 – 4. 4 Red - yellow Bromcresol green 3. 8 – 5. 4 yellow- blue Phenol red 6. 4 – 8. 2 yellow- red Thymolpthalein 9. 4 to 10. 6 Colorless- blue Litmus paper 4. 5 – 8. 2 Red to blue

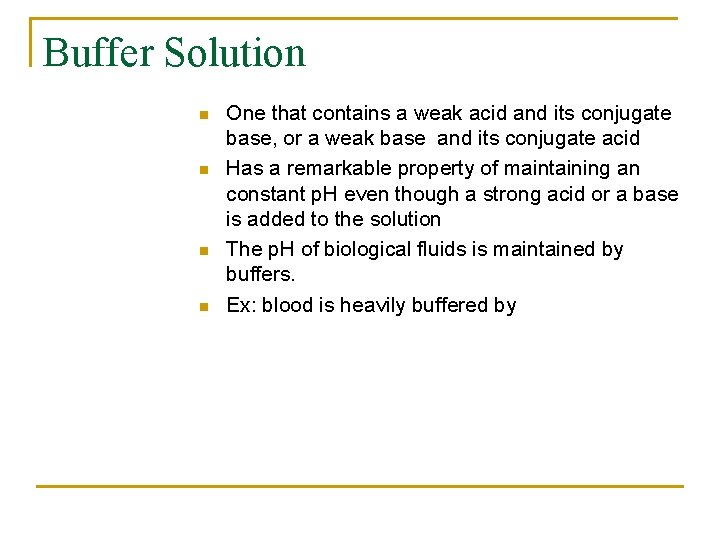
Buffer Solution n n One that contains a weak acid and its conjugate base, or a weak base and its conjugate acid Has a remarkable property of maintaining an constant p. H even though a strong acid or a base is added to the solution The p. H of biological fluids is maintained by buffers. Ex: blood is heavily buffered by

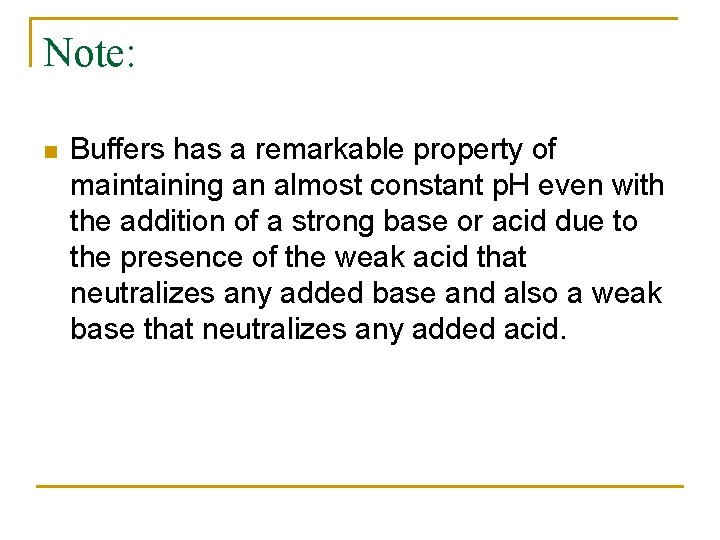
Note: n Buffers has a remarkable property of maintaining an almost constant p. H even with the addition of a strong base or acid due to the presence of the weak acid that neutralizes any added base and also a weak base that neutralizes any added acid.

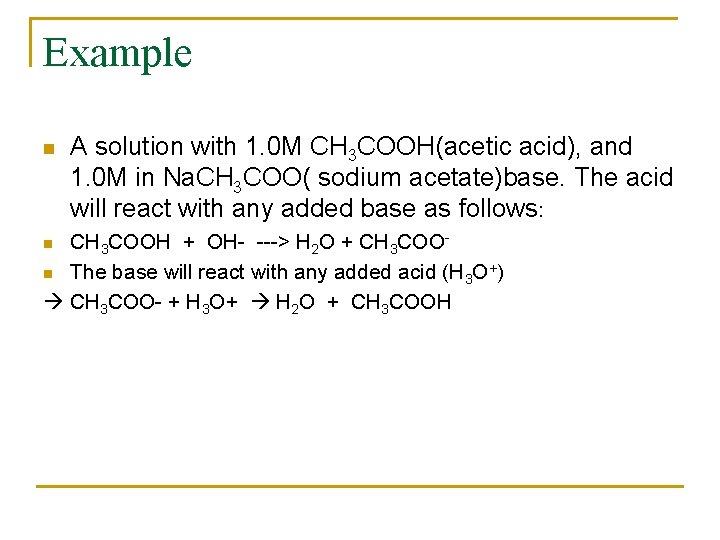
Example n A solution with 1. 0 M CH 3 COOH(acetic acid), and 1. 0 M in Na. CH 3 COO( sodium acetate)base. The acid will react with any added base as follows: CH 3 COOH + OH- ---> H 2 O + CH 3 COOn The base will react with any added acid (H 3 O+) CH 3 COO- + H 3 O+ H 2 O + CH 3 COOH n

Neutralization Reaction n occurs between an acid and a base producing equal amounts of H 3 O+ and OHChemical reaction that forms water and salt Occurs between Strong acid and strong base A weak acid and a weak base

Neutralization Occurs when an equivalent amount of an acid reacts with an equivalent amount of a base HCl + Na. OH ----> Na. Cl + H 2 O neutral solution This process is accomplished by means of titration n

Neutralization Reaction n Acid and base properties combine to form water H+ + OH- -------> H 2 O Note: water is one of the products of neutralization reaction

Acid Base Titration n n Is a useful application of neutralization reaction A process of adding a solution of accurately known concentration, standard solution, ( titrant) to another solution of unknown concentration, ( analyte until the chemical reaction between the two is complete ( the equivalence point) or end point n Equivalence point is the point in titration where the indicator used undergoes a color change n Note; indicator changes color at p. H=7

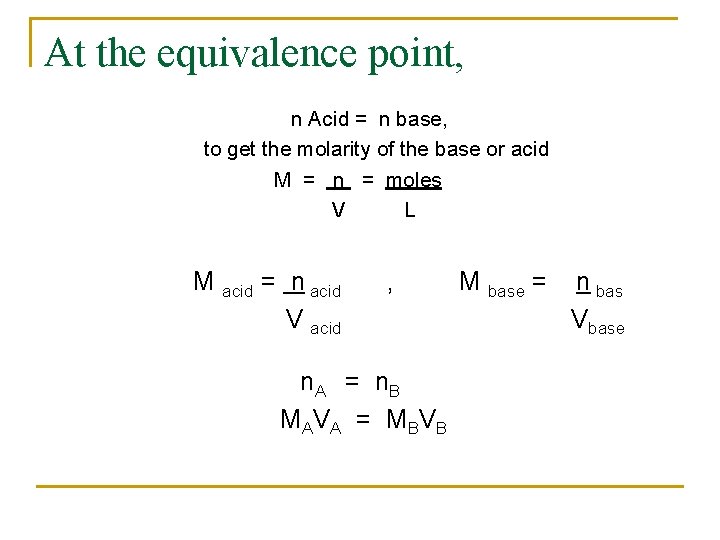
At the equivalence point, n Acid = n base, to get the molarity of the base or acid M = n = moles V L M acid = n acid V acid , n. A = n. B M AV A = M BV B M base = n bas Vbase

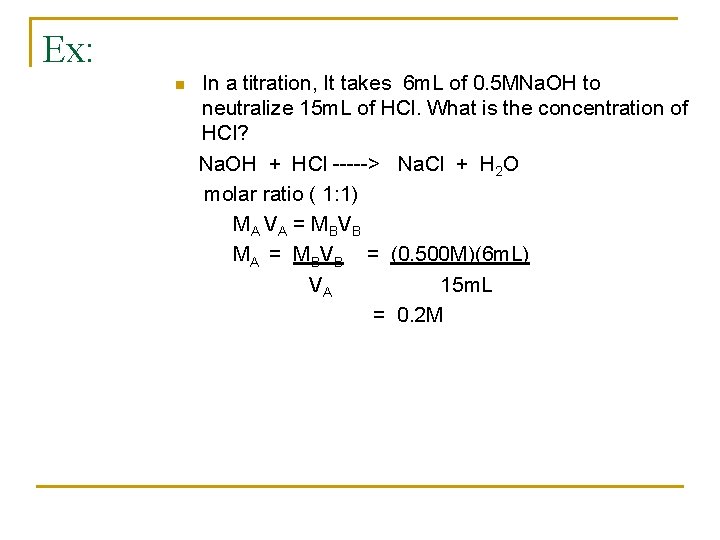
Ex: n In a titration, It takes 6 m. L of 0. 5 MNa. OH to neutralize 15 m. L of HCl. What is the concentration of HCl? Na. OH + HCl -----> Na. Cl + H 2 O molar ratio ( 1: 1) M A V A = M BV B MA = MBVB = (0. 500 M)(6 m. L) VA 15 m. L = 0. 2 M

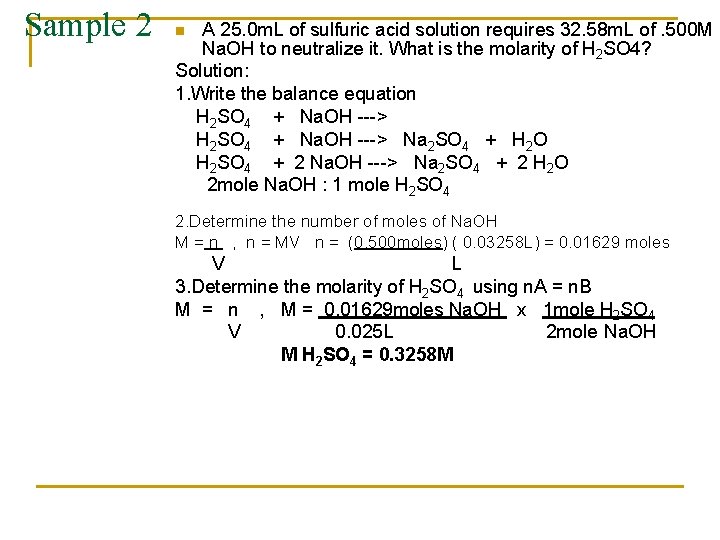
Sample 2 A 25. 0 m. L of sulfuric acid solution requires 32. 58 m. L of. 500 M Na. OH to neutralize it. What is the molarity of H 2 SO 4? Solution: 1. Write the balance equation H 2 SO 4 + Na. OH ---> Na 2 SO 4 + H 2 O H 2 SO 4 + 2 Na. OH ---> Na 2 SO 4 + 2 H 2 O 2 mole Na. OH : 1 mole H 2 SO 4 n 2. Determine the number of moles of Na. OH M = n , n = MV n = (0. 500 moles) ( 0. 03258 L) = 0. 01629 moles V L 3. Determine the molarity of H 2 SO 4 using n. A = n. B M = n , M = 0. 01629 moles Na. OH x 1 mole H 2 SO 4 V 0. 025 L 2 mole Na. OH M H 2 SO 4 = 0. 3258 M