Acids Have a sour taste Vinegar owes its



- Slides: 8


Acids Have a sour taste. ﺭﺍﺋﺤﺔ ﻗﺎﺑﻀﺔ ﺣﻤﻀﻴﺔ ﺍﻟﻄﻌﻢ Vinegar owes its taste to acetic acid. Citrus fruits ﺍﻟﻔﻮﺍﻛﻬﺔ ﺍﻟﺤﻤﻀﻴﺔ contain citric acid. React with certain metals to produce hydrogen gas. React with carbonates and bicarbonates to produce carbon dioxide gas Bases Have a bitter taste. ﻣﺬﻟﻘﻬﺎ ﻻﺫﻉ Feel slippery. ﻣﻠﻤﺴﻪ ﺯﻟﻖ Many soaps contain bases. ﺃﻐﻠﺐ ﺍﻟﺼﻮﺍﺑﻴﻦ ﺗﺤﺘﻮﻱ ﻋﻠﻰ ﻗﻮﺍﻋﺪ

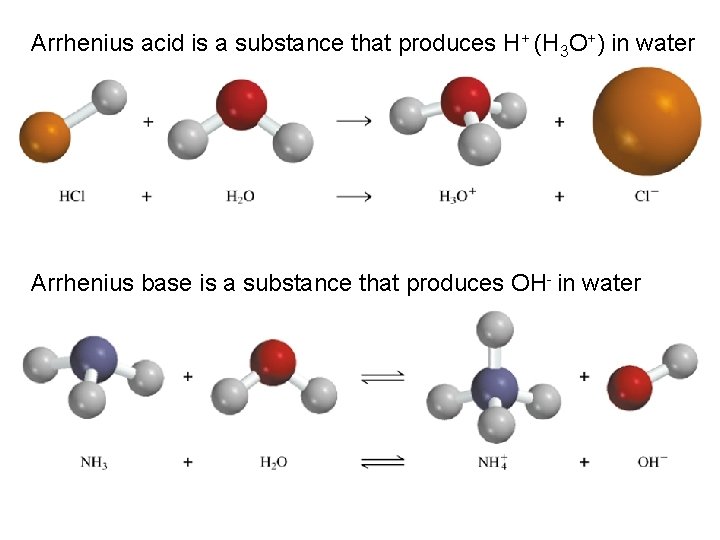
Arrhenius acid is a substance that produces H+ (H 3 O+) in water Arrhenius base is a substance that produces OH- in water

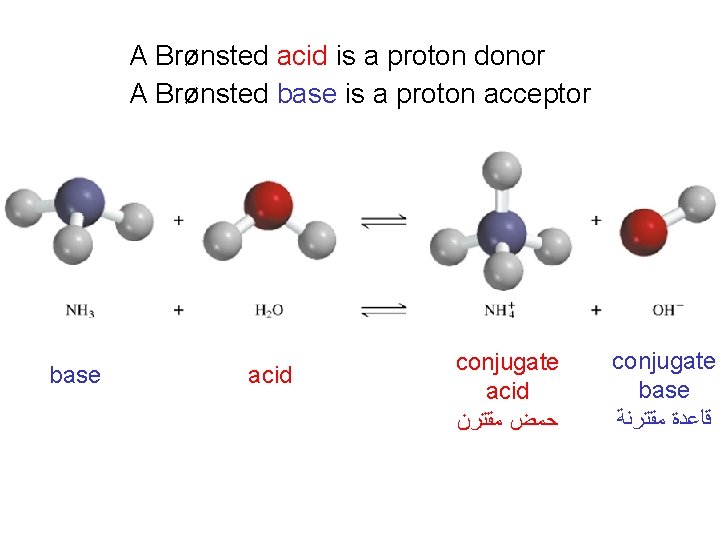
A Brønsted acid is a proton donor A Brønsted base is a proton acceptor base acid conjugate acid ﺣﻤﺾ ﻣﻘﺘﺮﻥ conjugate base ﻗﺎﻋﺪﺓ ﻣﻘﺘﺮﻧﺔ

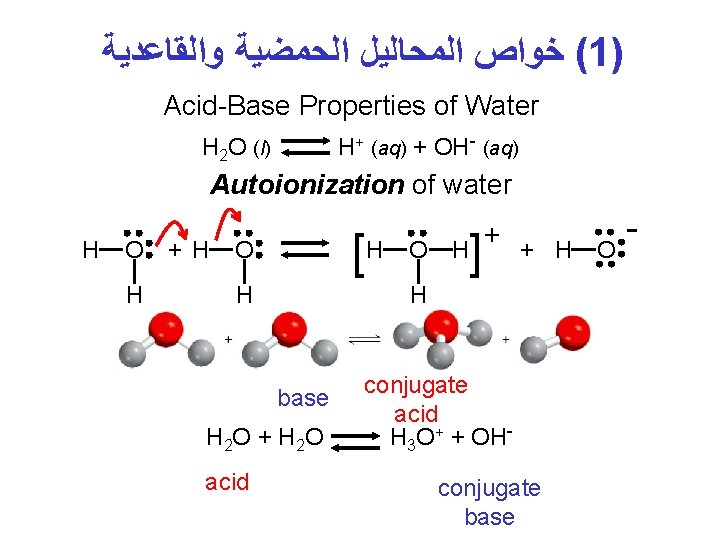
( ﺧﻮﺍﺹ ﺍﻟﻤﺤﺎﻟﻴﻞ ﺍﻟﺤﻤﻀﻴﺔ ﻭﺍﻟﻘﺎﻋﺪﻳﺔ 1) Acid-Base Properties of Water H+ (aq) + OH- (aq) H 2 O (l) Autoionization of water H O H + H [H O H ] H + H H base H 2 O + H 2 O acid O + conjugate acid H 3 O+ + OHconjugate base O -

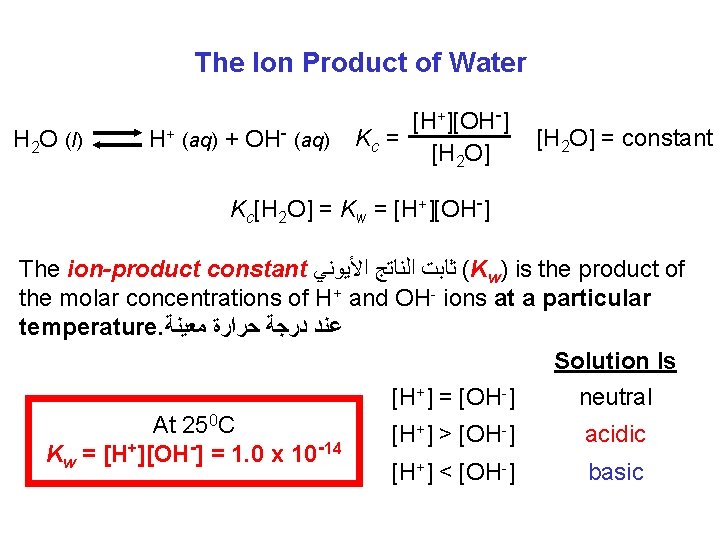
The Ion Product of Water H 2 O (l) H+ (aq) + OH- (aq) [H+][OH-] Kc = [H 2 O] = constant Kc[H 2 O] = Kw = [H+][OH-] The ion-product constant ( ﺛﺎﺑﺖ ﺍﻟﻨﺎﺗﺞ ﺍﻷﻴﻮﻧﻲ Kw) is the product of the molar concentrations of H+ and OH- ions at a particular temperature. ﻋﻨﺪ ﺩﺭﺟﺔ ﺣﺮﺍﺭﺓ ﻣﻌﻴﻨﺔ Solution Is [H+] = [OH-] neutral At 250 C +] > [OH-] [H acidic + 14 Kw = [H ][OH ] = 1. 0 x 10 [H+] < [OH-] basic

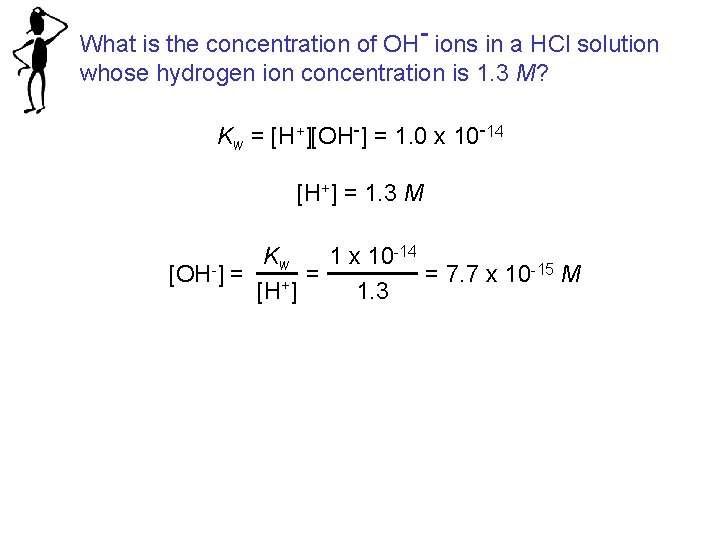
What is the concentration of OH- ions in a HCl solution whose hydrogen ion concentration is 1. 3 M? Kw = [H+][OH-] = 1. 0 x 10 -14 [H+] = 1. 3 M -14 K 1 x 10 w -15 M = = 7. 7 x 10 [OH-] = [H+] 1. 3