Acids Bases Acids vs Bases Acids Taste Sour

Acids & Bases
Acids vs. Bases Acids Taste Sour Are Corrosive Bases Taste Bitter Feel Slippery
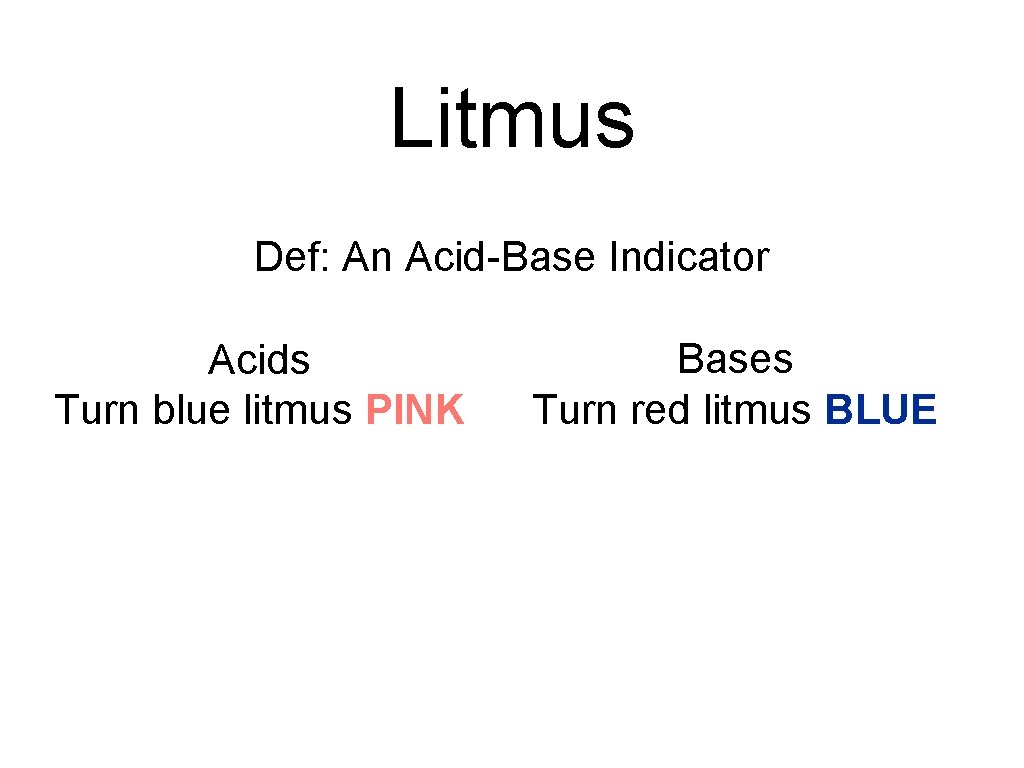
Litmus Def: An Acid-Base Indicator Acids Turn blue litmus PINK Bases Turn red litmus BLUE

Reactions Acids Become less acidic when combined with a base Bases Become less basic when combined with an acid NEUTRALIZATION!
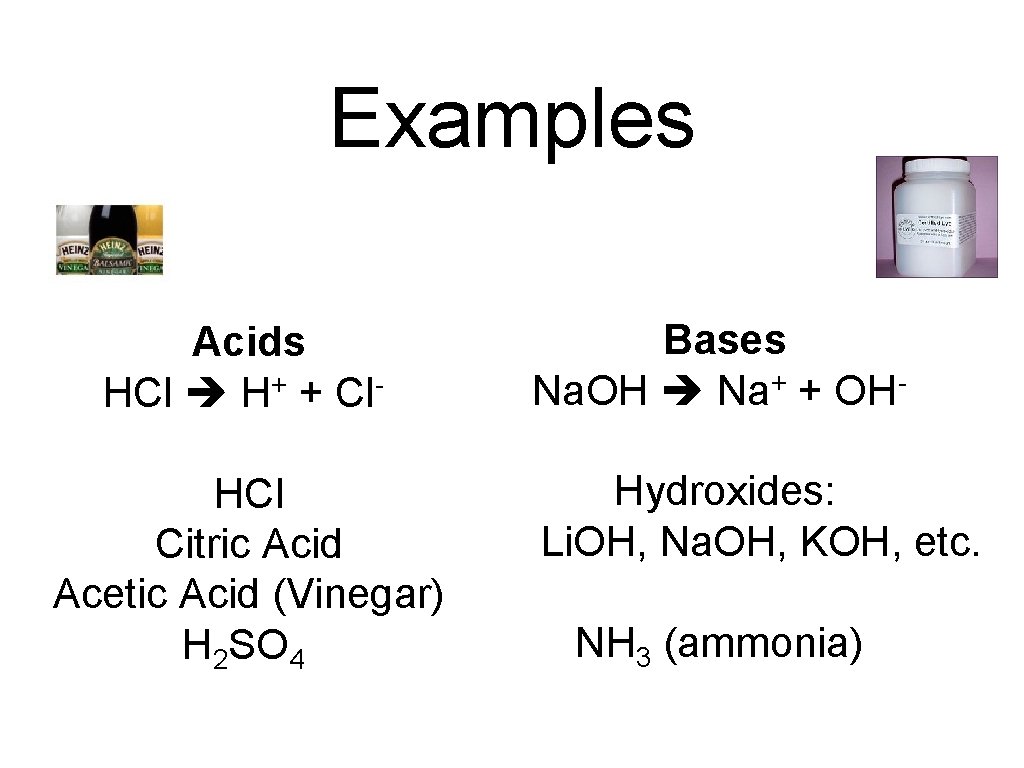
Examples Acids HCl H+ + Cl. HCl Citric Acid Acetic Acid (Vinegar) H 2 SO 4 Bases + Na. OH Na + OH Hydroxides: Li. OH, Na. OH, KOH, etc. NH 3 (ammonia)
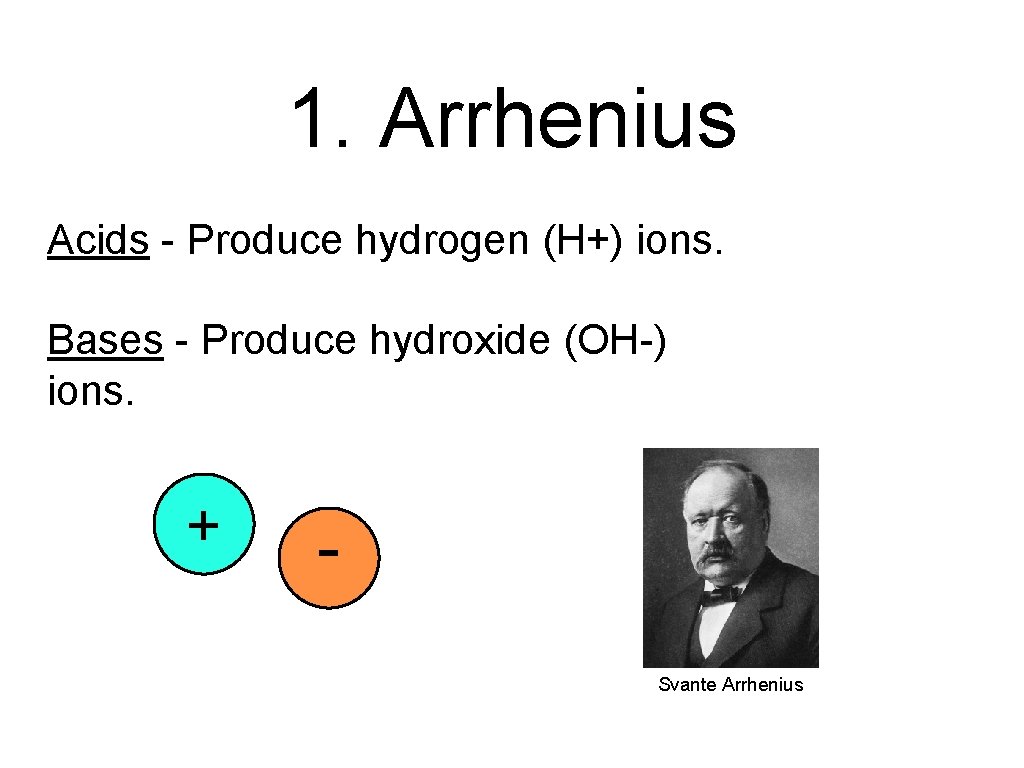
1. Arrhenius Acids - Produce hydrogen (H+) ions. Bases - Produce hydroxide (OH-) ions. + Svante Arrhenius
2. Bronsted-Lowry Acids - Proton (H+) donors. Bases - Proton (H+) acceptors. Proton Thomas Lowry Johannes Bronsted
3. Lewis Acids - Electron pair acceptors. Bases - Electron pair donors. Electron GN Lewis

Conductivity Demo Strong vs. Weak Acids
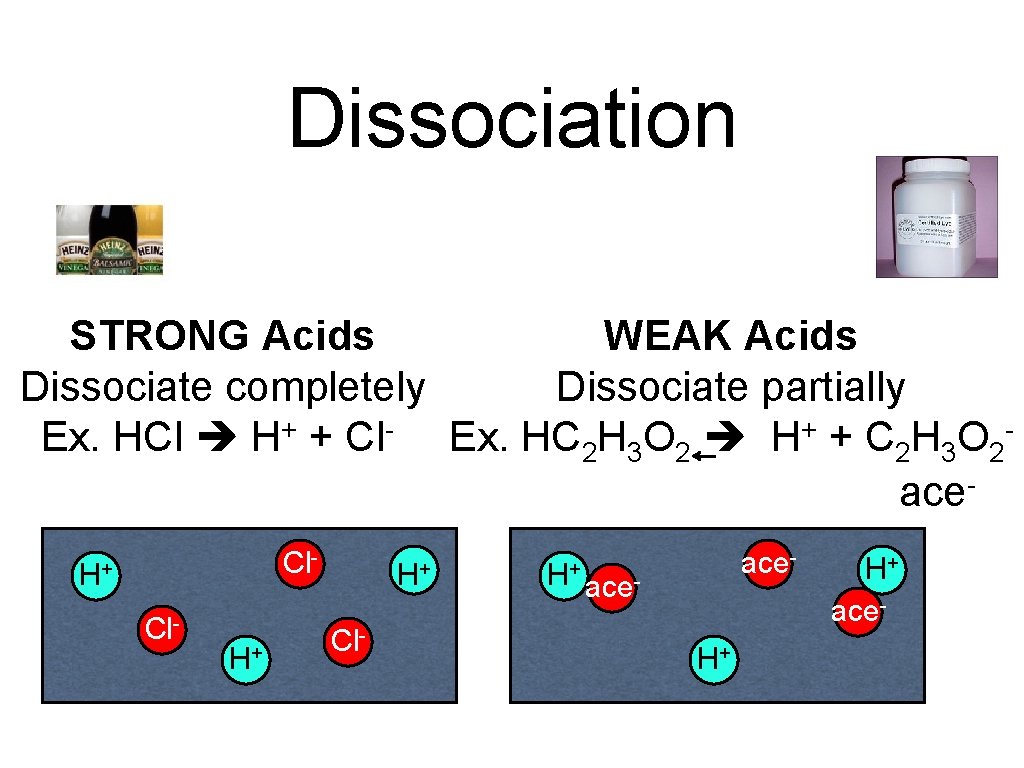
Dissociation STRONG Acids WEAK Acids Dissociate completely Dissociate partially Ex. HCl H+ + Cl- Ex. HC 2 H 3 O 2 H+ + C 2 H 3 O 2 ace. Cl- H+ H+ Cl- H+ ace- ace. H+ H+ ace-
![Terms Amphoteric - can behave as an acid OR a base [ex: water] Conjugate Terms Amphoteric - can behave as an acid OR a base [ex: water] Conjugate](http://slidetodoc.com/presentation_image_h2/47a50c4eb74b4c228b379babc2511cd0/image-11.jpg)
Terms Amphoteric - can behave as an acid OR a base [ex: water] Conjugate Acid - the species formed when a proton is added to a base Conjugate Base - the remaining part of the acid Hydronium - H 3 O+ (hydrated H+) Polyprotic Acids - Contain more than 1 proton [ex: H 2 SO 4, H 3 PO 4] + (H )
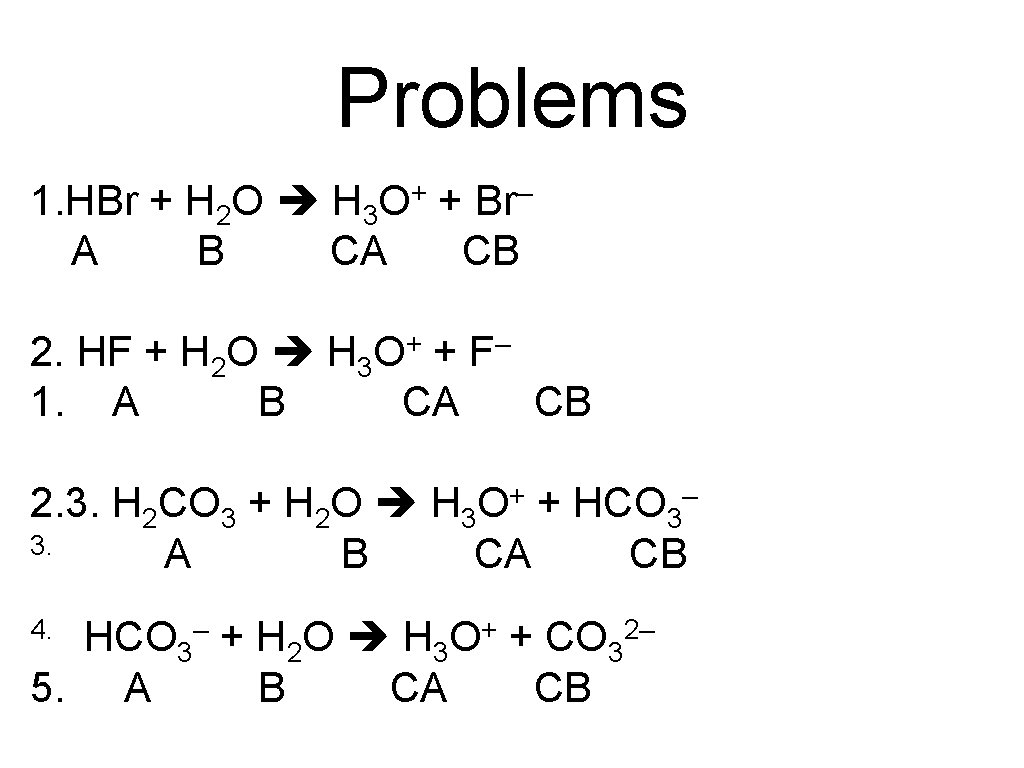
Problems 1. HBr + H 2 O H 3 O+ + Br– A B CA CB 2. HF + H 2 O H 3 O+ + F– 1. A B CA CB 2. 3. H 2 CO 3 + H 2 O H 3 O+ + HCO 3– 3. A B CA CB 4. HCO 3– + H 2 O H 3 O+ + CO 32– 5. A B CA CB
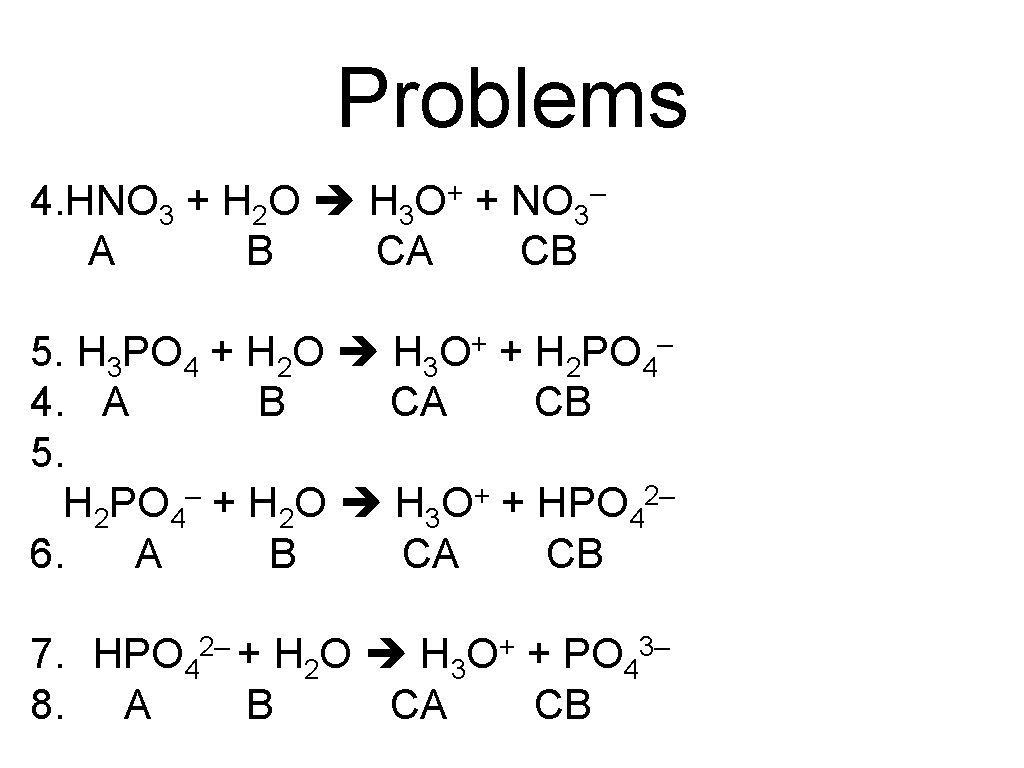
Problems 4. HNO 3 + H 2 O H 3 O+ + NO 3– A B CA CB 5. H 3 PO 4 + H 2 O H 3 O+ + H 2 PO 4– 4. A B CA CB 5. H 2 PO 4– + H 2 O H 3 O+ + HPO 42– 6. A B CA CB 7. HPO 4 8. A 2– + H 2 O H 3 B CA + O + PO 4 CB 3–

Acid and Base Strength p. H scale - measures the strength of an acid or base – 1 0 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Strong Acid | Weak Acid | N | Weak Base | Strong Base Strong Acids & Bases are considered dangerous (burn skin, eat away at materials Weak Acids & Bases are generally safer (used in foods and baking products)
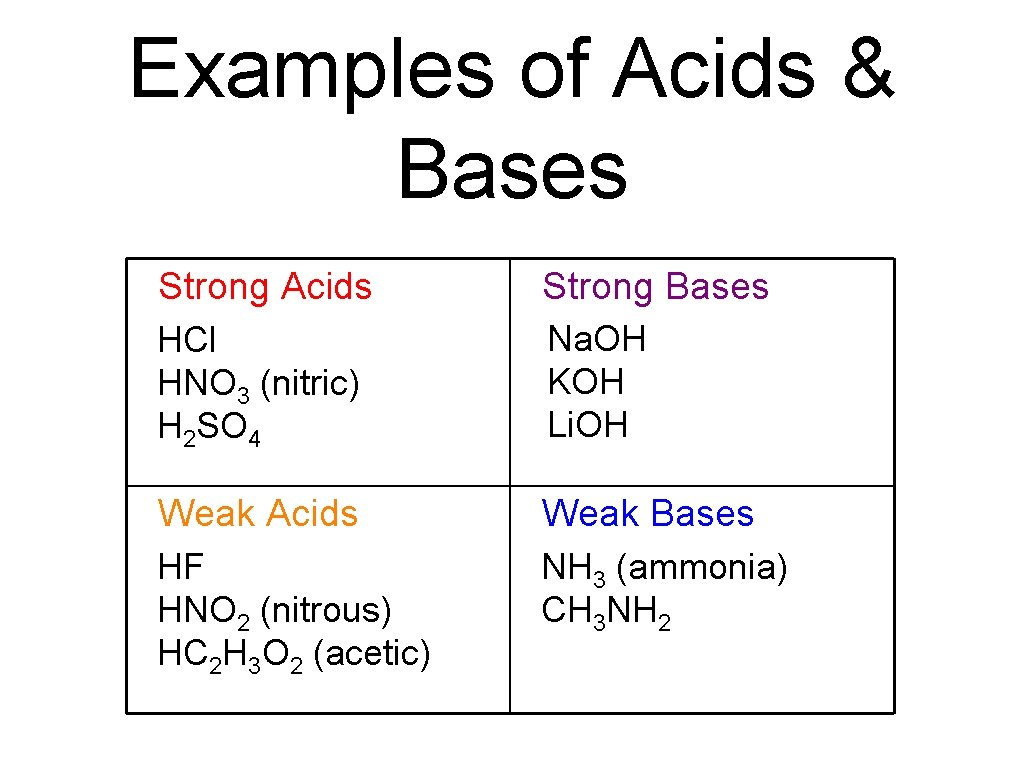
Examples of Acids & Bases Strong Acids Strong Bases HCl HNO 3 (nitric) H 2 SO 4 Na. OH KOH Li. OH Weak Acids Weak Bases HF HNO 2 (nitrous) HC 2 H 3 O 2 (acetic) NH 3 (ammonia) CH 3 NH 2
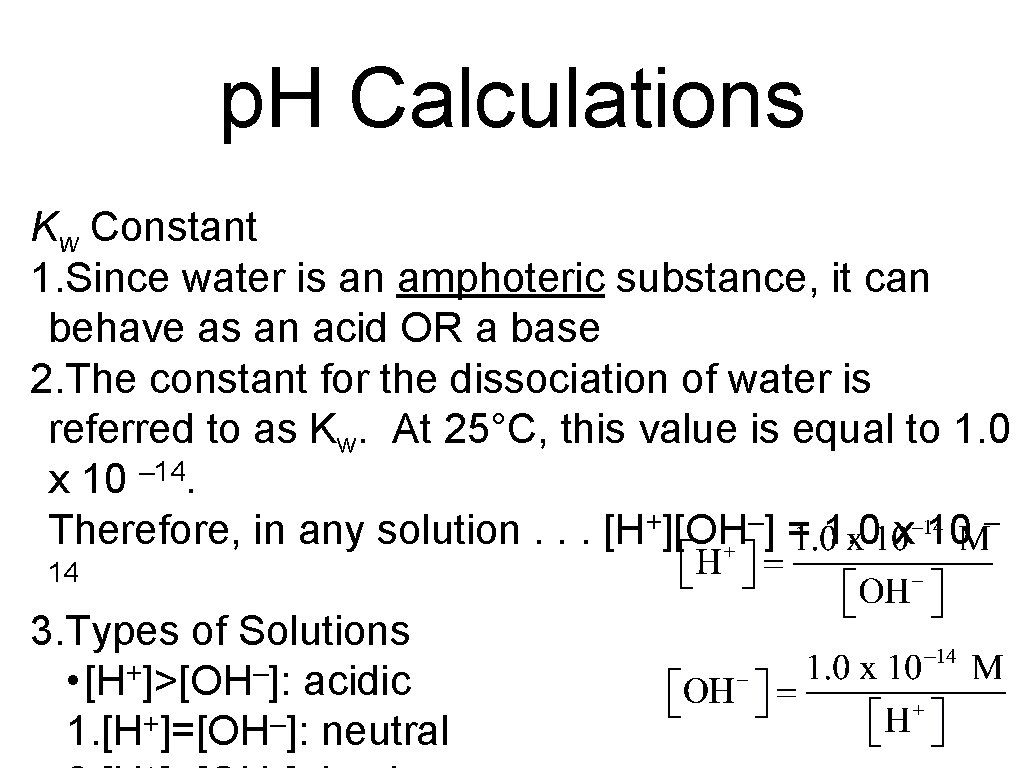
p. H Calculations Kw Constant 1. Since water is an amphoteric substance, it can behave as an acid OR a base 2. The constant for the dissociation of water is referred to as Kw. At 25°C, this value is equal to 1. 0 x 10 – 14. Therefore, in any solution. . . [H+][OH–] = 1. 0 x 10 – 14 3. Types of Solutions • [H+]>[OH–]: acidic 1. [H+]=[OH–]: neutral
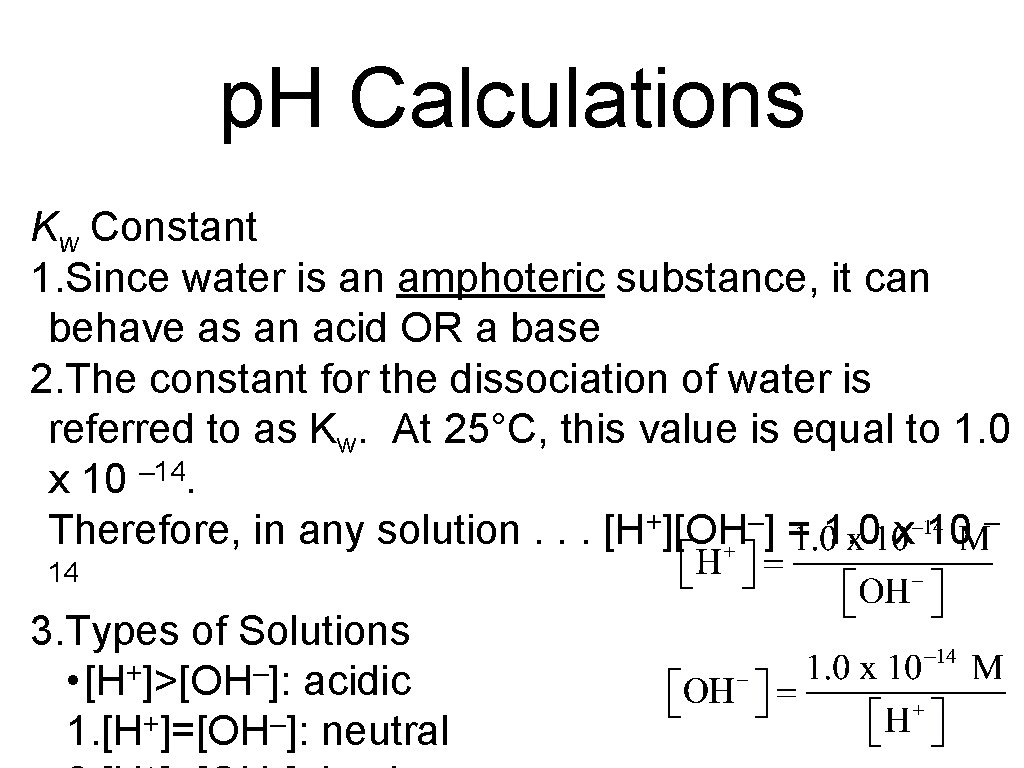
p. H Calculations Kw Constant 1. Since water is an amphoteric substance, it can behave as an acid OR a base 2. The constant for the dissociation of water is referred to as Kw. At 25°C, this value is equal to 1. 0 x 10 – 14. Therefore, in any solution. . . [H+][OH–] = 1. 0 x 10 – 14 3. Types of Solutions • [H+]>[OH–]: acidic 1. [H+]=[OH–]: neutral
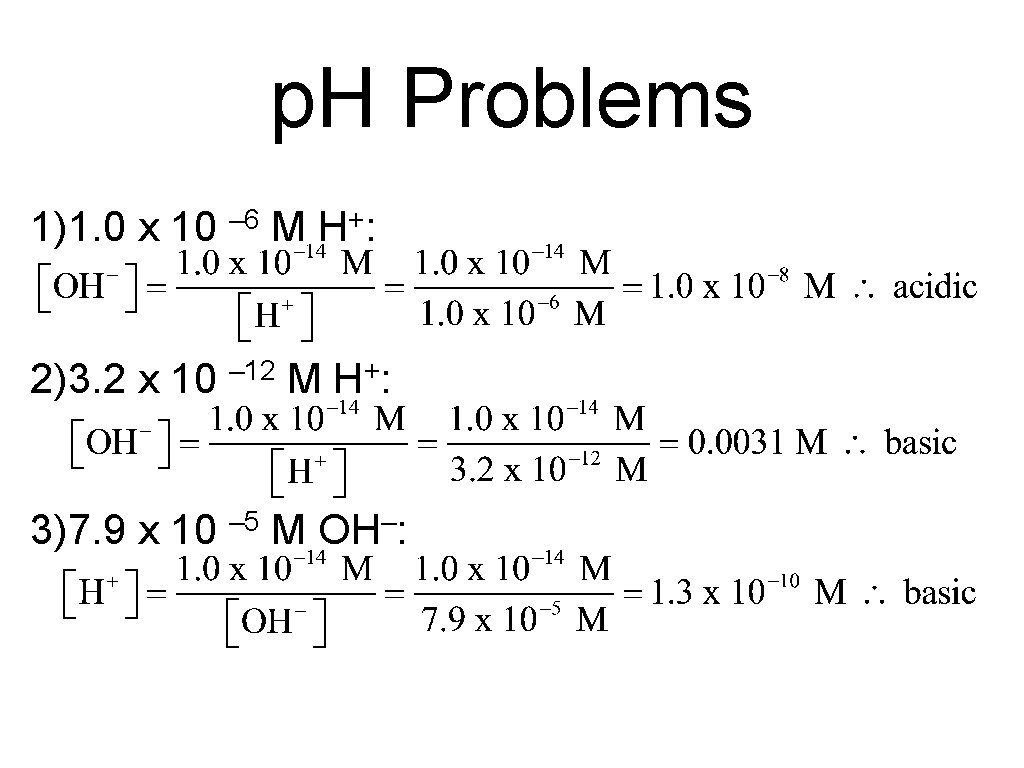
p. H Problems 1)1. 0 x 10 – 6 M H+: 2)3. 2 x 10 – 12 M + H: 3)7. 9 x 10 – 5 M OH–:
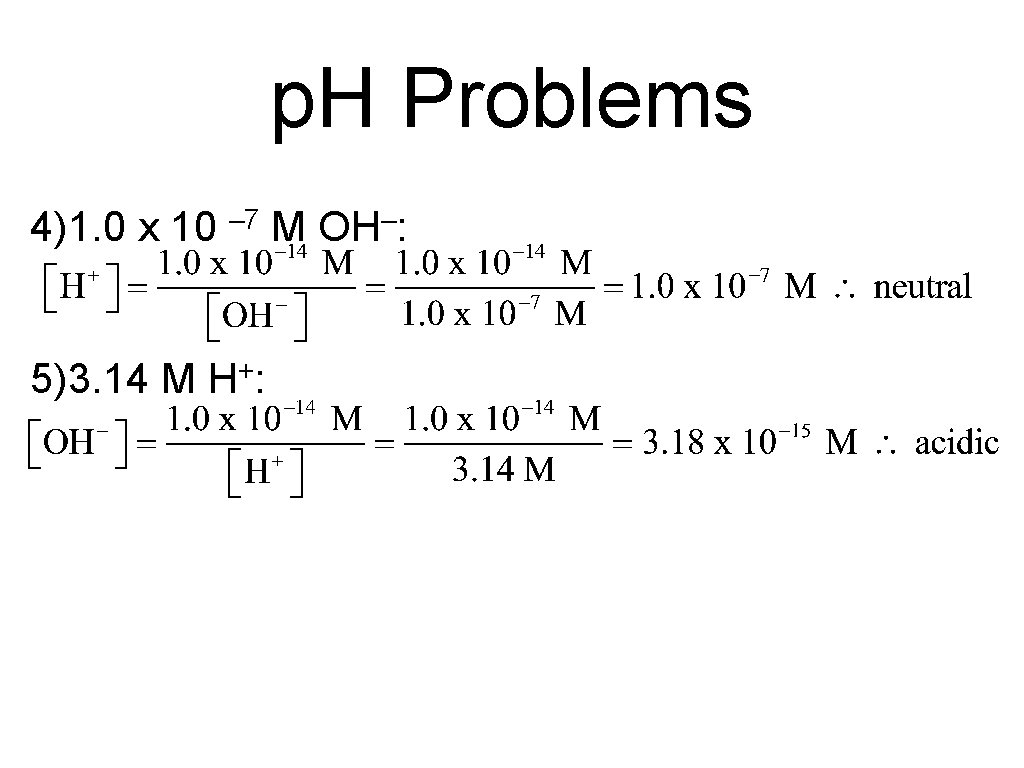
p. H Problems 4)1. 0 x 10 – 7 M OH–: 5)3. 14 M + H:
![p. H Calculations Equations [H+][OH–] = 1. 0 x 10 – 14 p. H p. H Calculations Equations [H+][OH–] = 1. 0 x 10 – 14 p. H](http://slidetodoc.com/presentation_image_h2/47a50c4eb74b4c228b379babc2511cd0/image-20.jpg)
p. H Calculations Equations [H+][OH–] = 1. 0 x 10 – 14 p. H = –log [H+] p. OH = –log [OH–] [H+] = 10 –p. H [OH–] = 10 –p. OH p. H + p. OH = 14 Variables + [H ]: Hydronium Ion Molarity Concentration [OH–]: Hydroxide Ion Molarity Concentration p. H; p. OH
![p. H Calculations a)p. H = –log [H+] p. H = –log (9. 18 p. H Calculations a)p. H = –log [H+] p. H = –log (9. 18](http://slidetodoc.com/presentation_image_h2/47a50c4eb74b4c228b379babc2511cd0/image-21.jpg)
p. H Calculations a)p. H = –log [H+] p. H = –log (9. 18 x 10 – 11 M H+) p. H = 10. 0; basic – –p. OH b)[OH ] = 10 [OH–] = 10 – 7. 8 [OH–] = 1. 6 x 10 – 8 M OH–; acidic c)p. H + p. OH = 14 - p. H p. OH = 14 - 4. 56 p. OH = 9. 44; acidic
![p. H Calculations d)p. OH = –log [OH–] p. H = –log (3. 18 p. H Calculations d)p. OH = –log [OH–] p. H = –log (3. 18](http://slidetodoc.com/presentation_image_h2/47a50c4eb74b4c228b379babc2511cd0/image-22.jpg)
p. H Calculations d)p. OH = –log [OH–] p. H = –log (3. 18 x 10 – 5 M OH–) p. OH = 4. 5 p. H + p. OH = 14 p. H = 14 - p. OH p. H = 14 - 4. 5 p. H = 9. 5; basic e)p. OH = –log [OH–] – 10 – p. OH = –log (4. 55 x 10 M OH ) p. OH = 9. 34; acidic (Note: p. H = 4. 66)
![p. H Calculations f)[H+] = 10 –p. H [H+] = 10 – 8. 9 p. H Calculations f)[H+] = 10 –p. H [H+] = 10 – 8. 9](http://slidetodoc.com/presentation_image_h2/47a50c4eb74b4c228b379babc2511cd0/image-23.jpg)
p. H Calculations f)[H+] = 10 –p. H [H+] = 10 – 8. 9 [H+] = 1. 3 x 10 – 9 M H+; basic g)p. H + p. OH = 14 p. H = 14 - p. OH p. H = 14 - 12. 5 p. H = 1. 5; acidic
![p. H Calculations h)p. H = –log [H+] p. H = –log (5. 8 p. H Calculations h)p. H = –log [H+] p. H = –log (5. 8](http://slidetodoc.com/presentation_image_h2/47a50c4eb74b4c228b379babc2511cd0/image-24.jpg)
p. H Calculations h)p. H = –log [H+] p. H = –log (5. 8 x 10 – 8 M H+) p. H = 7. 2; slightly basic p. H + p. OH = 14 - p. H p. OH = 14 - 7. 2 p. OH = 6. 8
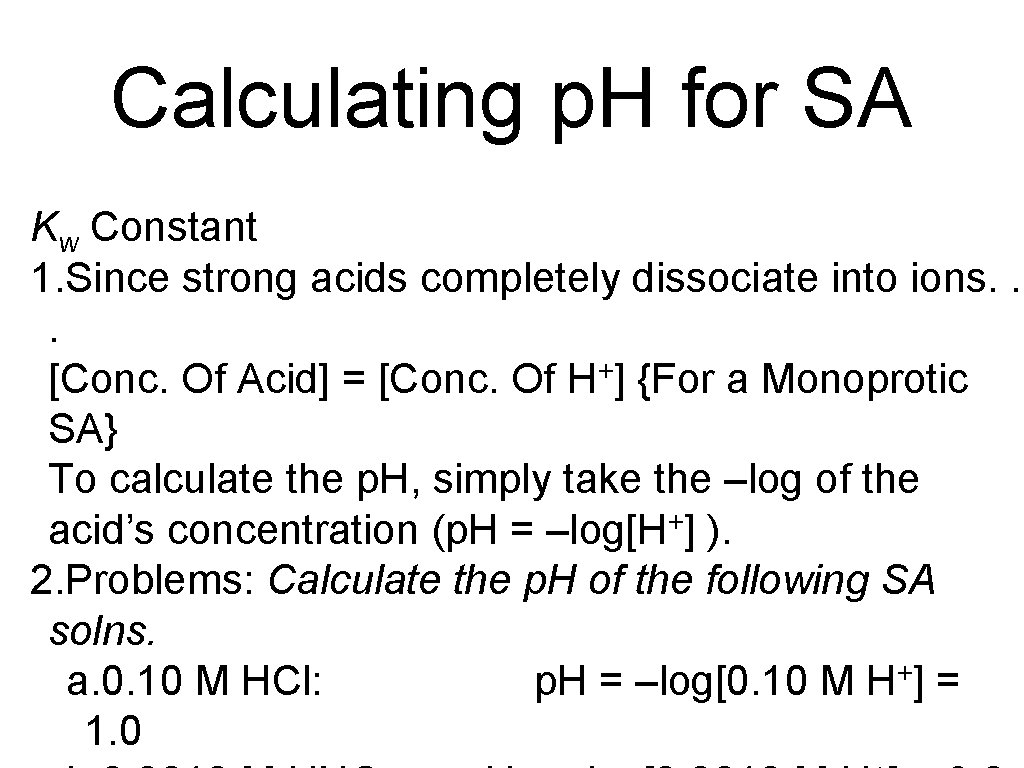
Calculating p. H for SA Kw Constant 1. Since strong acids completely dissociate into ions. . . + [Conc. Of Acid] = [Conc. Of H ] {For a Monoprotic SA} To calculate the p. H, simply take the –log of the acid’s concentration (p. H = –log[H+] ). 2. Problems: Calculate the p. H of the following SA solns. a. 0. 10 M HCl: p. H = –log[0. 10 M H+] = 1. 0

Neutralization Reactions Lakeside Town: Acid from a factory leaks into the lake How can they fix this problem? A. What are Neutralization Reactions? 1. Definition: An acid and a base combine to form water and a salt. (HA + BOH H 2 O + BA) 2. The salt is formed from the cation of the base and the anion of the acid. 3. The p. H “neutralizes”.
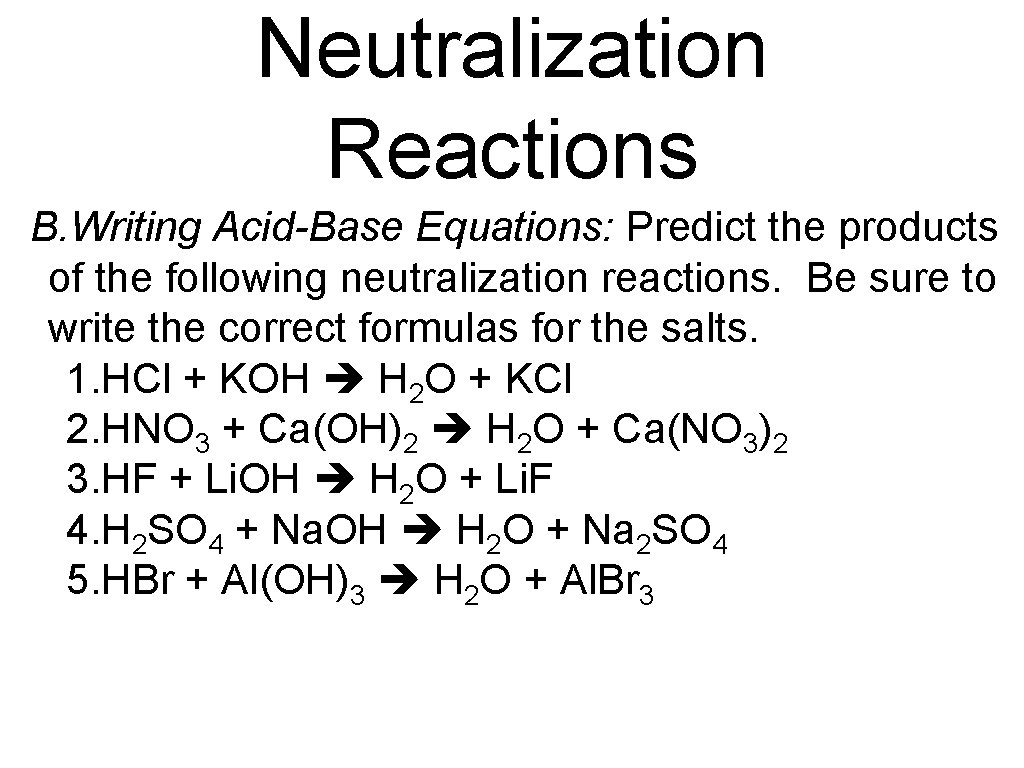
Neutralization Reactions B. Writing Acid-Base Equations: Predict the products of the following neutralization reactions. Be sure to write the correct formulas for the salts. 1. HCl + KOH H 2 O + KCl 2. HNO 3 + Ca(OH)2 H 2 O + Ca(NO 3)2 3. HF + Li. OH H 2 O + Li. F 4. H 2 SO 4 + Na. OH H 2 O + Na 2 SO 4 5. HBr + Al(OH)3 H 2 O + Al. Br 3

Neutralization Reactions C. Titrations & Calculations: Predict the products of the following neutralization reactions. Be sure to write the correct formulas for the salts. 1. Definition: The slow addition of one substance to another in order to determine an unknown concentration. 2. Equipment: Buret (Titrant) & Flask (Analyte) 3. Common Titration Indicators a. Phenolphthalein: Clear (acid) / Dark Pink (base) b. Bromothymol Blue: Yellow (acid) / Blue (base)
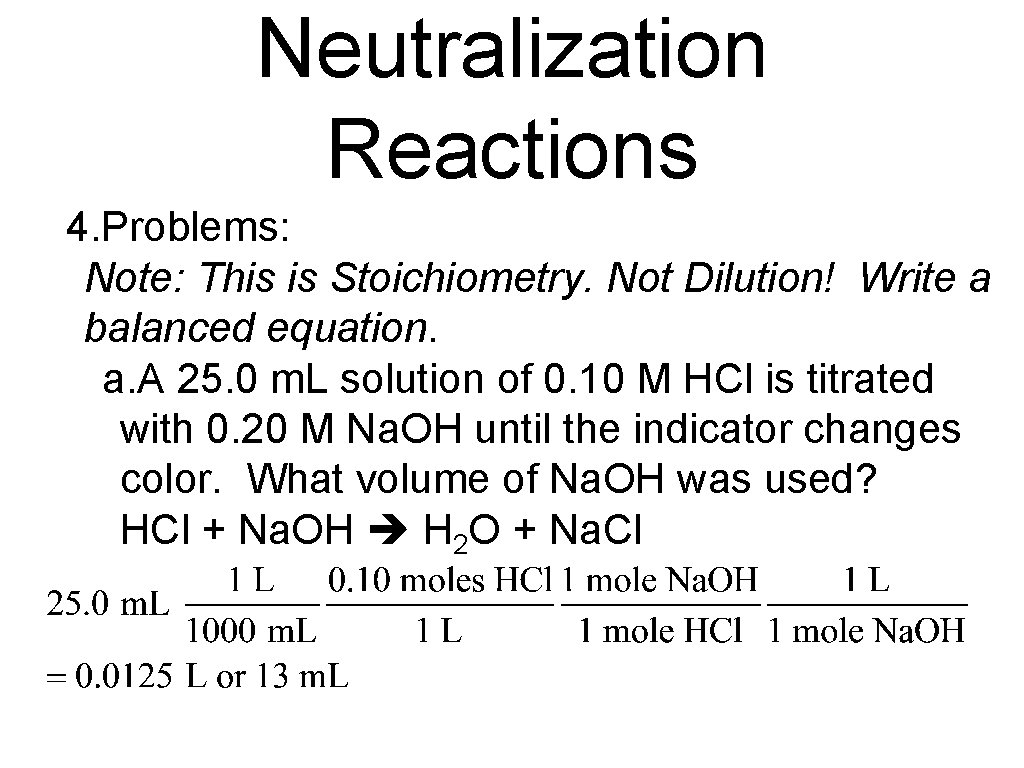
Neutralization Reactions 4. Problems: Note: This is Stoichiometry. Not Dilution! Write a balanced equation. a. A 25. 0 m. L solution of 0. 10 M HCl is titrated with 0. 20 M Na. OH until the indicator changes color. What volume of Na. OH was used? HCl + Na. OH H 2 O + Na. Cl
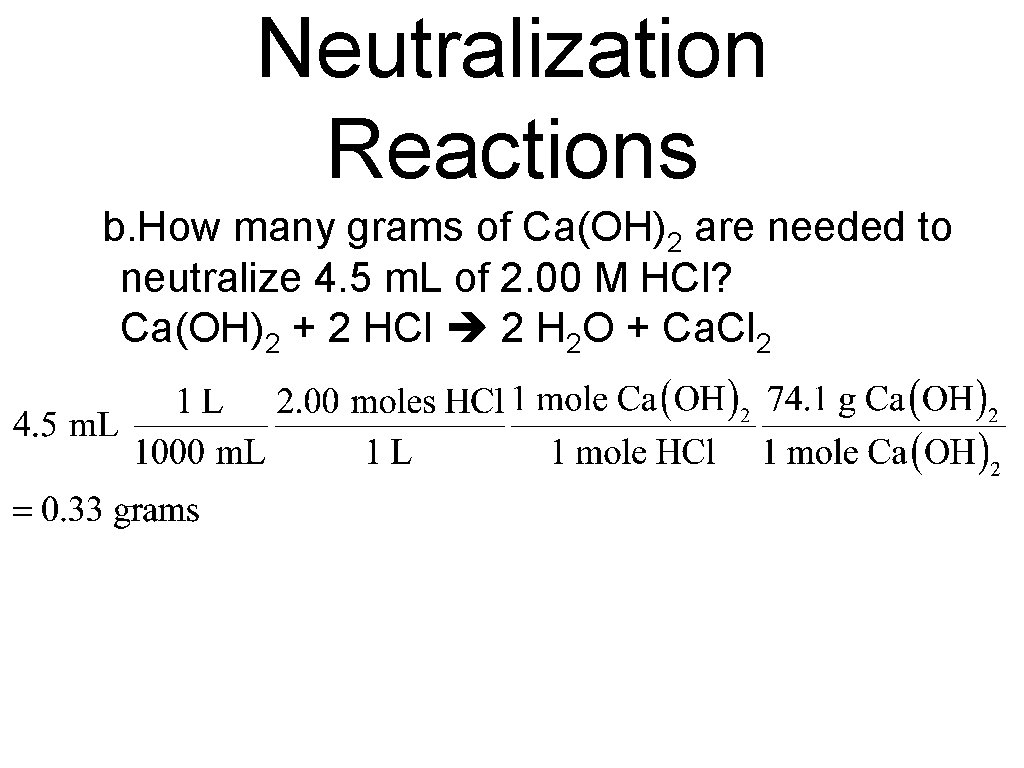
Neutralization Reactions b. How many grams of Ca(OH)2 are needed to neutralize 4. 5 m. L of 2. 00 M HCl? Ca(OH)2 + 2 HCl 2 H 2 O + Ca. Cl 2
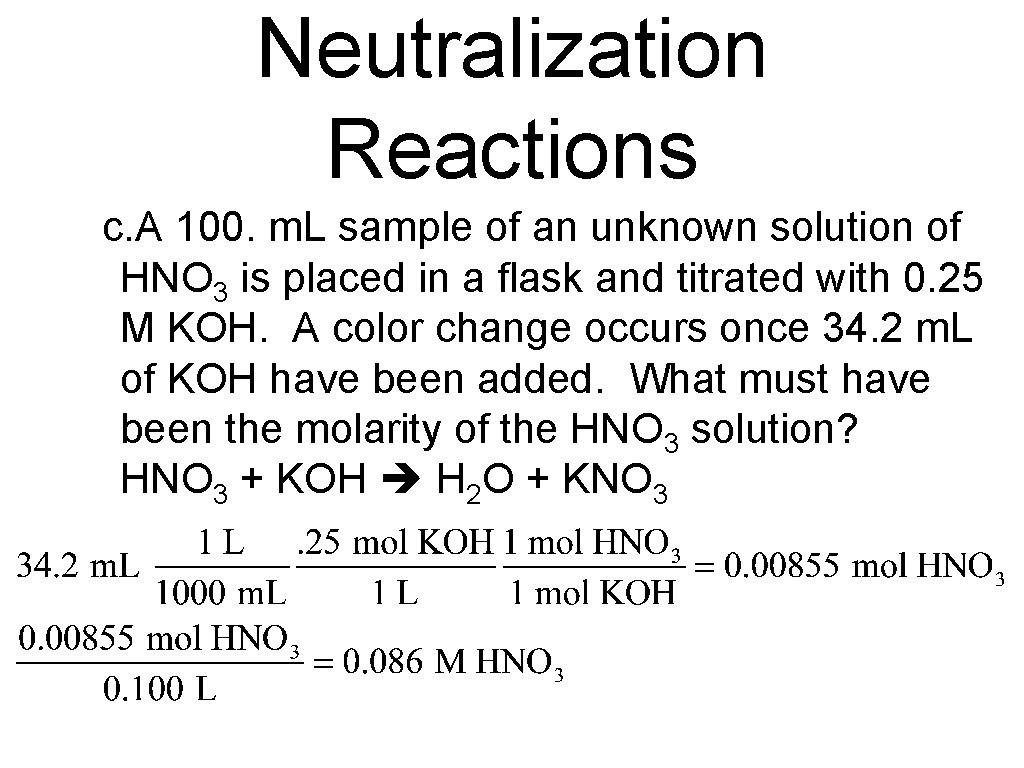
Neutralization Reactions c. A 100. m. L sample of an unknown solution of HNO 3 is placed in a flask and titrated with 0. 25 M KOH. A color change occurs once 34. 2 m. L of KOH have been added. What must have been the molarity of the HNO 3 solution? HNO 3 + KOH H 2 O + KNO 3
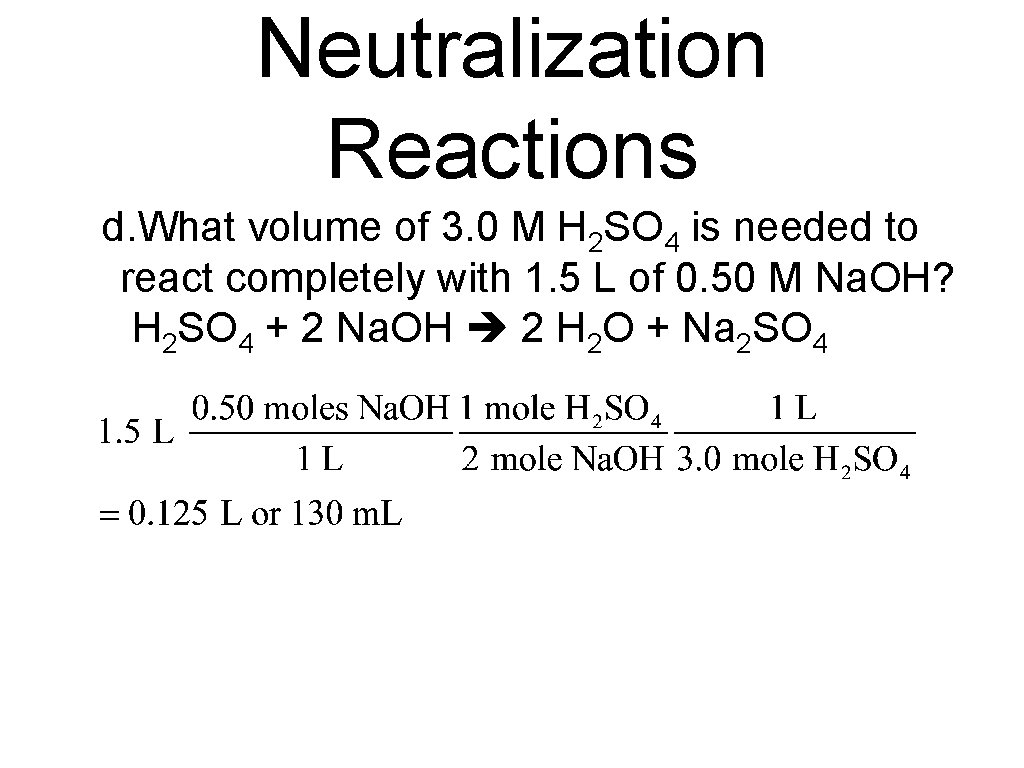
Neutralization Reactions d. What volume of 3. 0 M H 2 SO 4 is needed to react completely with 1. 5 L of 0. 50 M Na. OH? H 2 SO 4 + 2 Na. OH 2 H 2 O + Na 2 SO 4
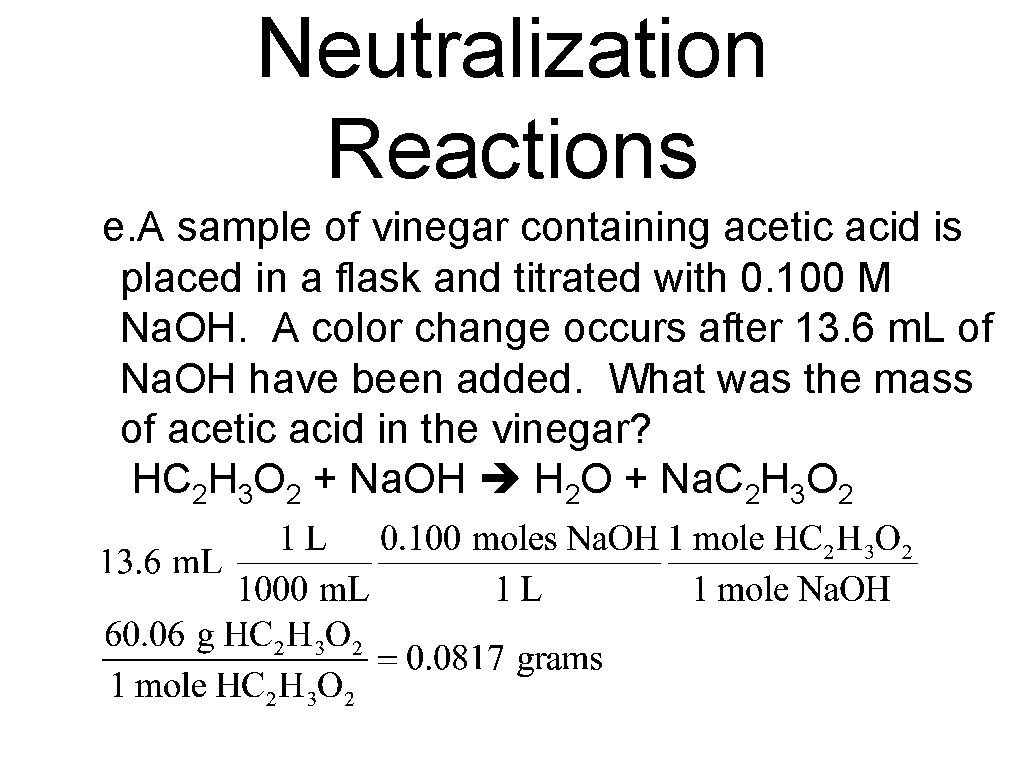
Neutralization Reactions e. A sample of vinegar containing acetic acid is placed in a flask and titrated with 0. 100 M Na. OH. A color change occurs after 13. 6 m. L of Na. OH have been added. What was the mass of acetic acid in the vinegar? HC 2 H 3 O 2 + Na. OH H 2 O + Na. C 2 H 3 O 2

Neutralization Reactions Book Problems: Page 507: 69 - 71, 73 b, 74 b Write balanced equations for each.
- Slides: 34