Sweet and Sour Dirt A little understanding of



- Slides: 14

Sweet and Sour Dirt A little understanding of p. H to help you use biochar better Contains animation. View in Slide Show mode

Soils Have Flavors! It’s possible to taste a difference between different types of soil • Acidic soils taste sour We don’t recommend this! • Basic (alkaline) soils taste slightly sweet • The flavor of a soil affects… Mike Lieberman of Urban. Organic. Gardener. com – How well different plants grow – What kind of microbes thrive in the soil – How well the solid holds on to various minerals • We talk about soil’s “flavor” as p. H ~2~

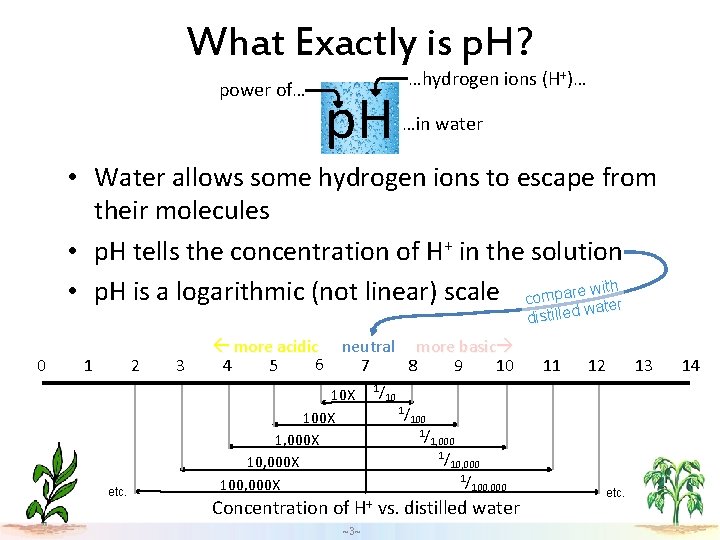
What Exactly is p. H? power of… p. H …hydrogen ions (H+)… …in water • Water allows some hydrogen ions to escape from their molecules • p. H tells the concentration of H+ in the solution • p. H is a logarithmic (not linear) scale compare waittehr w distilled 0 1 2 etc. 3 more acidic neutral more basic 6 4 5 7 8 9 10 10 X 100 X 1, 000 X 100, 000 X Concentration of H+ ~3~ 1/ 11 12 13 10 1/ 100 1/ 1, 000 1/ 100, 000 vs. distilled water etc. 14

Finding the p. H (without tasting) Litmus paper colors shows p. H Hydrangeas’ colors reflect soil p. H Do-it-yourself home test kits Meters read p. H directly 4

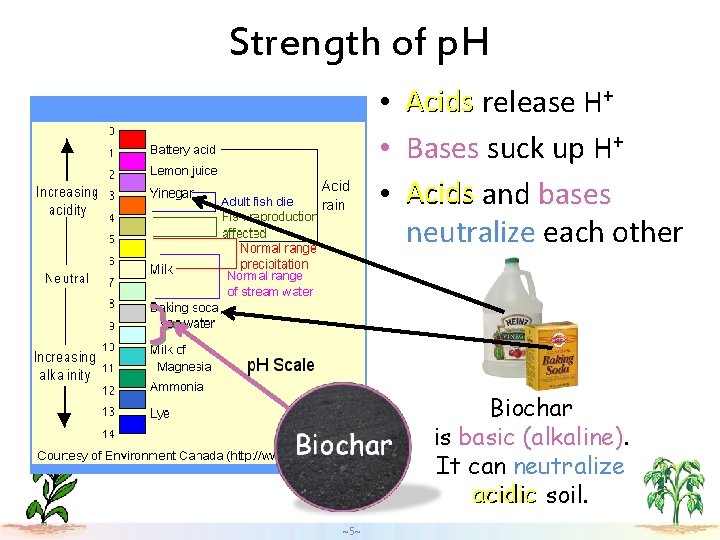
Strength of p. H • • • Acids release H+ Bases suck up H+ Acids and bases neutralize each other Biochar is basic (alkaline). It can neutralize acidic soil. ~5~

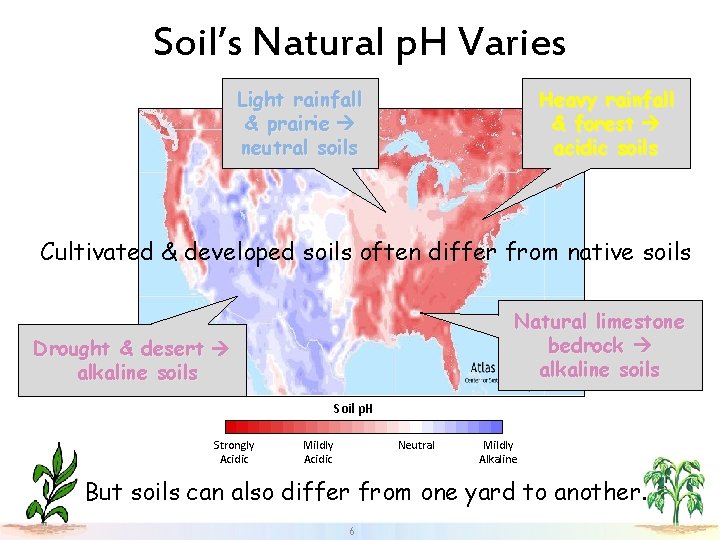
Soil’s Natural p. H Varies Light rainfall & prairie neutral soils Heavy rainfall & forest acidic soils Cultivated & developed soils often differ from native soils Natural limestone bedrock alkaline soils Drought & desert alkaline soils Soil p. H Strongly Acidic Mildly Acidic Neutral Mildly Alkaline But soils can also differ from one yard to another. 6

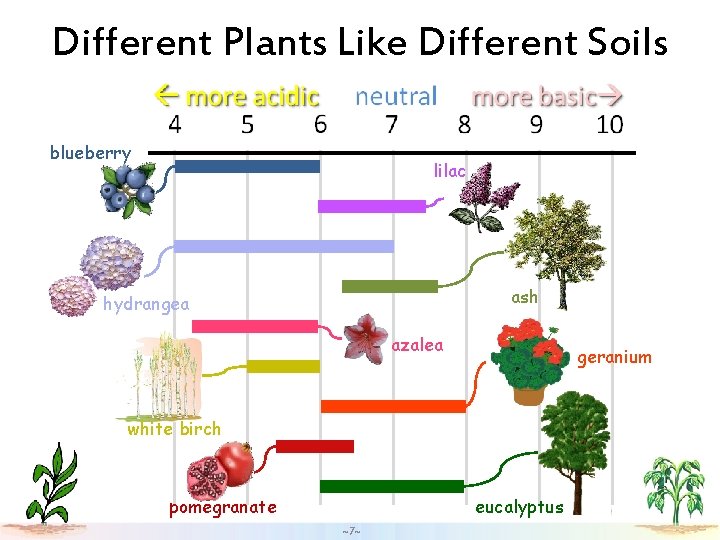
Different Plants Like Different Soils blueberry lilac ash hydrangea azalea geranium white birch eucalyptus pomegranate ~7~

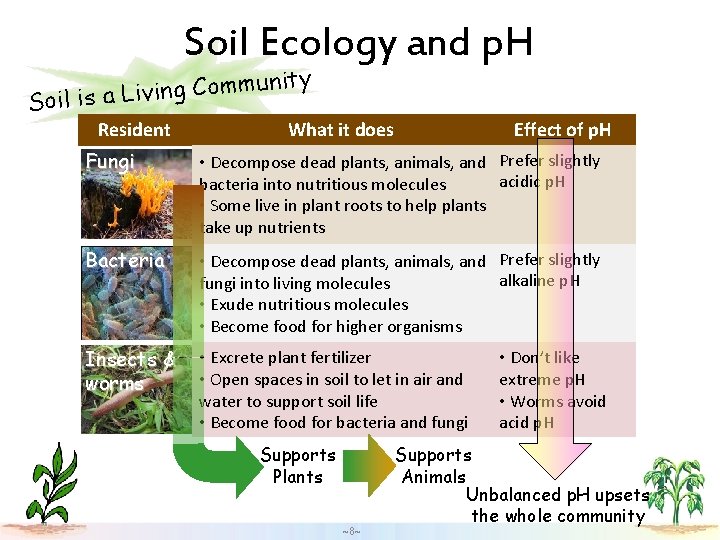
Soil Ecology and p. H ity n u m m o C g n i Soil is a Liv Resident What it does Effect of p. H Fungi • Decompose dead plants, animals, and Prefer slightly acidic p. H bacteria into nutritious molecules • Some live in plant roots to help plants take up nutrients Bacteria • Decompose dead plants, animals, and Prefer slightly alkaline p. H fungi into living molecules • Exude nutritious molecules • Become food for higher organisms Insects & worms • Excrete plant fertilizer • Open spaces in soil to let in air and water to support soil life • Become food for bacteria and fungi Supports Plants ~8~ • Don’t like extreme p. H • Worms avoid acid p. H Supports Animals Unbalanced p. H upsets the whole community

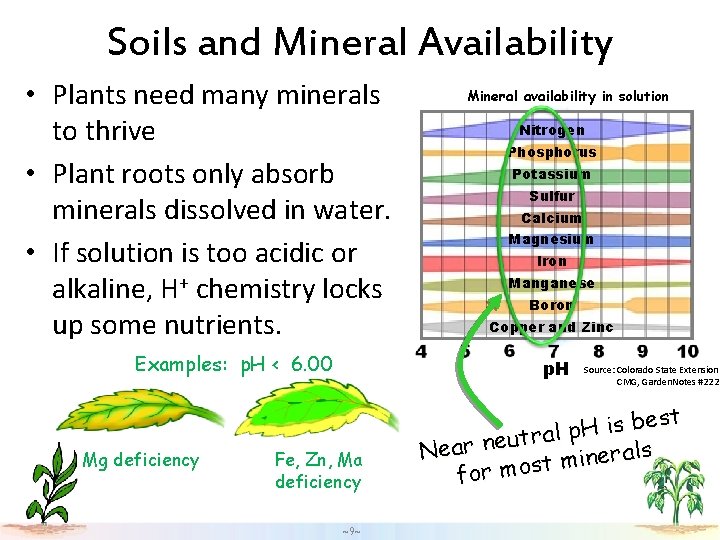
Soils and Mineral Availability • Plants need many minerals to thrive • Plant roots only absorb minerals dissolved in water. • If solution is too acidic or alkaline, H+ chemistry locks up some nutrients. Examples: p. H < 6. 00 Mg deficiency Mineral availability in solution Nitrogen Phosphorus Potassium Sulfur Calcium Magnesium Iron Manganese Boron Copper and Zinc p. H Fe, Zn, Ma deficiency ~9~ Source: Colorado State Extension CMG, Garden. Notes #222 est b s i H p ral t u e n r a Ne als r e n i m t for mos

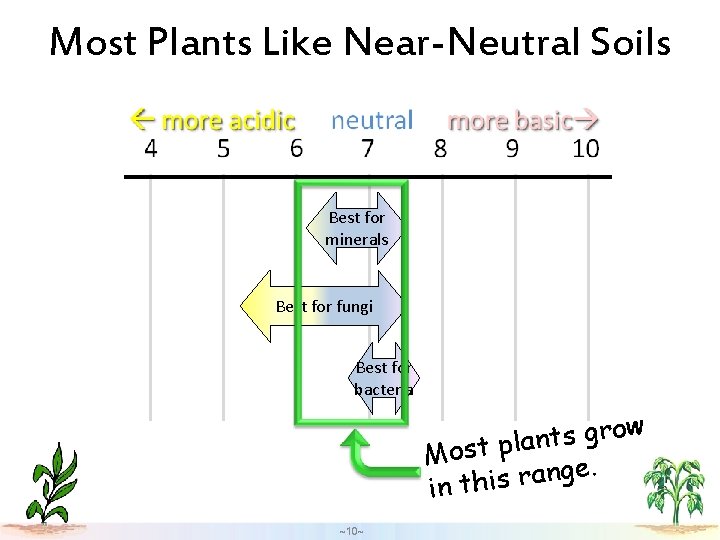
Most Plants Like Near-Neutral Soils Best for minerals Best for fungi Best for bacteria w o r g s t n a Most pl. e g n a r in this ~10~

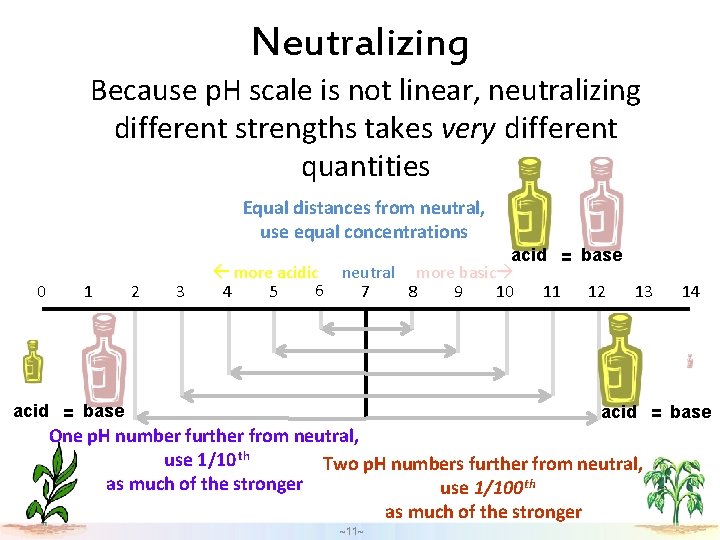
Neutralizing Because p. H scale is not linear, neutralizing different strengths takes very different quantities Equal distances from neutral, use equal concentrations 0 1 2 3 acid = base more acidic neutral more basic 6 4 5 7 8 9 10 11 12 13 acid = base 14 acid = base One p. H number further from neutral, use 1/10 th Two p. H numbers further from neutral, as much of the stronger use 1/100 th as much of the stronger ~11~

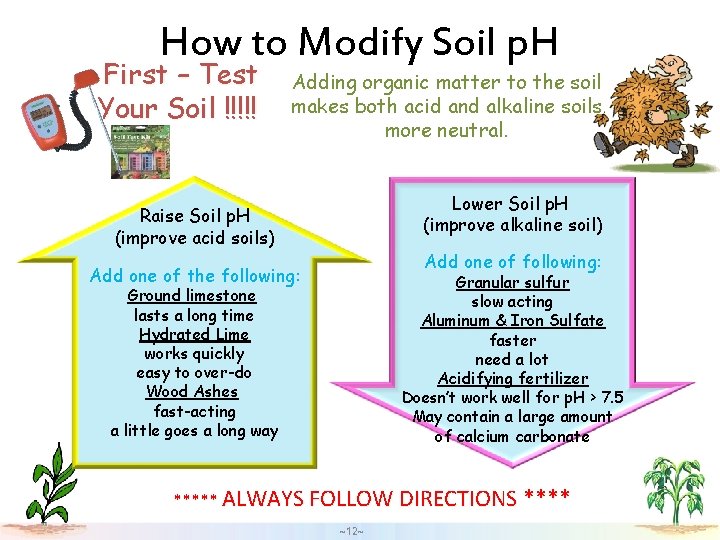
How to Modify Soil p. H First – Test Your Soil !!!!! Adding organic matter to the soil makes both acid and alkaline soils more neutral. Lower Soil p. H (improve alkaline soil) Raise Soil p. H (improve acid soils) Add one of following: Add one of the following: Granular sulfur slow acting Aluminum & Iron Sulfate faster need a lot Acidifying fertilizer Doesn’t work well for p. H > 7. 5 May contain a large amount of calcium carbonate Ground limestone lasts a long time Hydrated Lime works quickly easy to over-do Wood Ashes fast-acting a little goes a long way ***** ALWAYS FOLLOW DIRECTIONS **** ~12~

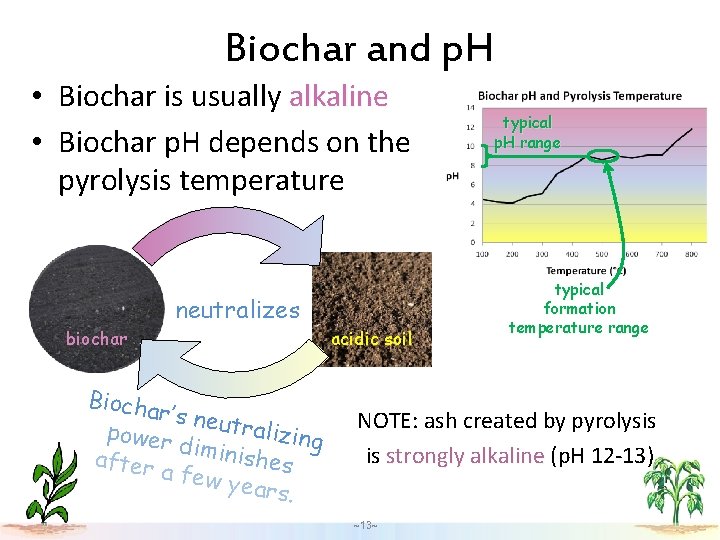
Biochar and p. H • Biochar is usually alkaline • Biochar p. H depends on the pyrolysis temperature biochar neutralizes Biocha r’s neu tralizi power ng diminis after hes a few years. acidic soil typical p. H range typical formation temperature range NOTE: ash created by pyrolysis is strongly alkaline (p. H 12 -13) ~13~

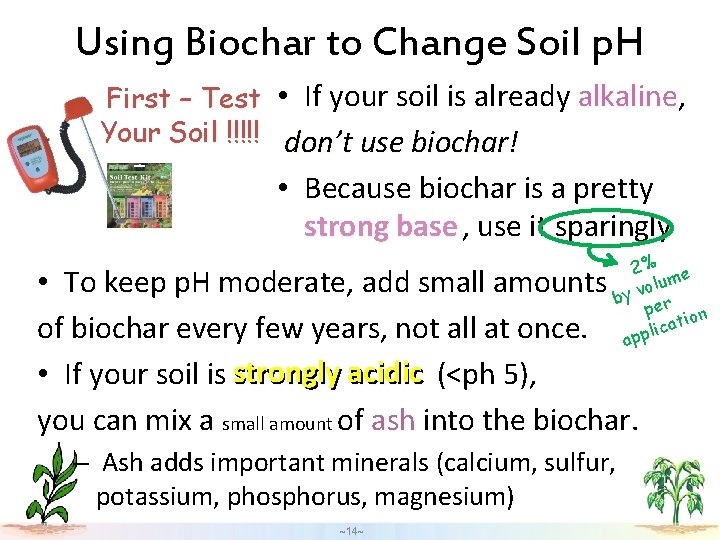
Using Biochar to Change Soil p. H alkaline First – Test • If your soil is already alkaline, Your Soil !!!!! don’t use biochar! • Because biochar is a pretty strong base , use it sparingly 2% e lum o v by er p tion a c i l app • To keep p. H moderate, add small amounts of biochar every few years, not all at once. • If your soil is strongly acidic (<ph 5), you can mix a small amount of ash into the biochar. – Ash adds important minerals (calcium, sulfur, potassium, phosphorus, magnesium) ~14~
 Sour dirt
Sour dirt Sometimes sweet sometimes sour
Sometimes sweet sometimes sour There's a sweet, sweet spirit in this place
There's a sweet, sweet spirit in this place Ten little indian boys poem
Ten little indian boys poem 1 little 2 little 3 little indian
1 little 2 little 3 little indian Freddie mercury
Freddie mercury Taste of basic substances
Taste of basic substances Bases taste sour
Bases taste sour Bitter vs sour
Bitter vs sour Rationalization defense mechanism
Rationalization defense mechanism All of their time and space are foggy slum figure of speech
All of their time and space are foggy slum figure of speech Source text and target text
Source text and target text The candy was sour is that qualitative or quantitative
The candy was sour is that qualitative or quantitative Types of unleavened bread
Types of unleavened bread Fluently 비교급
Fluently 비교급


























