Module 7 Home Planet the Earth Activity 2































- Slides: 49

Module 7: Home Planet – the Earth Activity 2: The Earth’s Atmosphere & Magnetic Field

Summary In this Activity, we will investigate (a) the composition of the Earth’s atmosphere, (b) layers & temperatures in the Earth’s atmosphere, (c) the effects of sunlight, (d) the greenhouse effect, (e) the ozone layer, (f) atmospheric circulation, and (g) the structure of the Earth’s magnetic field.

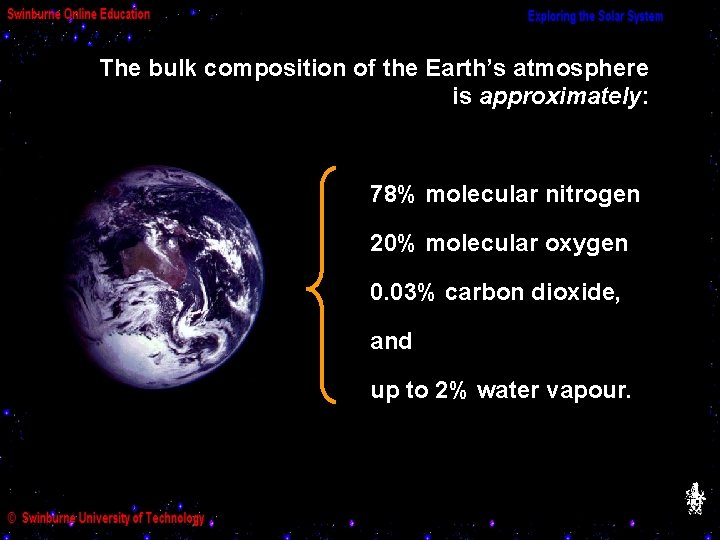
(a) The composition of the Earth’s atmosphere The Earth’s atmosphere has played a pivotal part in our evolution, and as we shall see in the next Module, it too has evolved dramatically with time. Other planets have atmospheres too, though none quite like ours! We’ll be comparing their atmospheres to ours in later Modules. We can study an atmosphere in various ways. The easiest way to start is with its “bulk” (i. e. average) properties:

The bulk composition of the Earth’s atmosphere is approximately: 78% molecular nitrogen 20% molecular oxygen 0. 03% carbon dioxide, and up to 2% water vapour.

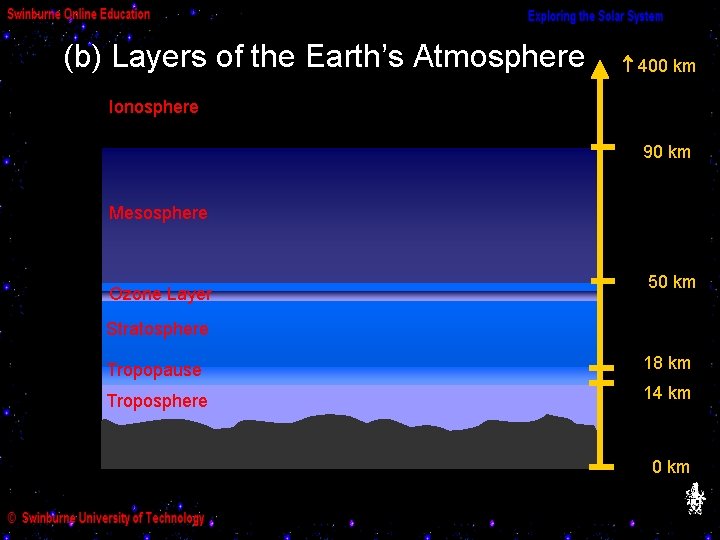
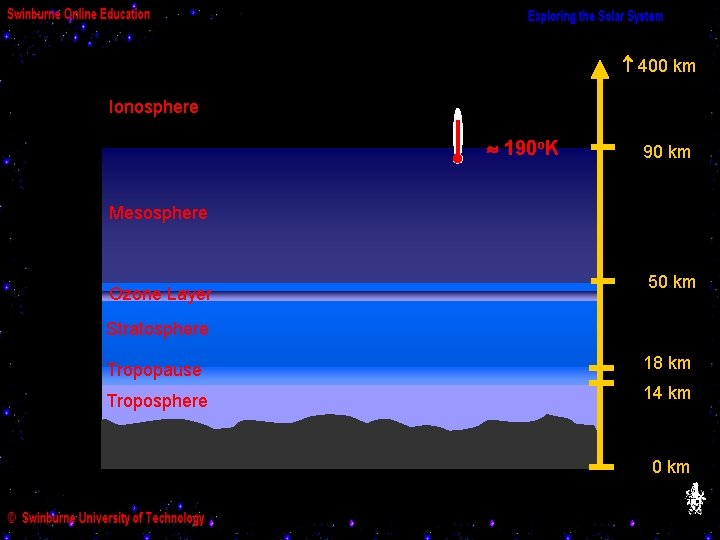
(b) Layers of the Earth’s Atmosphere 400 km Ionosphere 90 km Mesosphere Ozone Layer 50 km Stratosphere Tropopause 18 km Troposphere 14 km 0 km

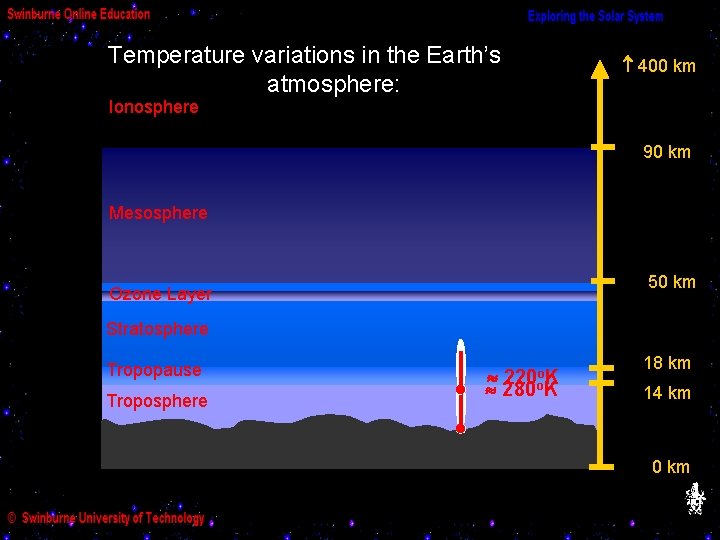
Temperature variations in the Earth’s atmosphere: 400 km Ionosphere 90 km Mesosphere 50 km Ozone Layer Stratosphere Tropopause Troposphere 220 oo. K 280 K 18 km 14 km 0 km

The troposphere is mainly heated by infrared radiation re-emitted by the ground, so the temperature in the troposphere decreases with altitude.

400 km Ionosphere 90 km Mesosphere Ozone Layer 260 o. K 50 km Stratosphere Tropopause 18 km Troposphere 14 km 0 km

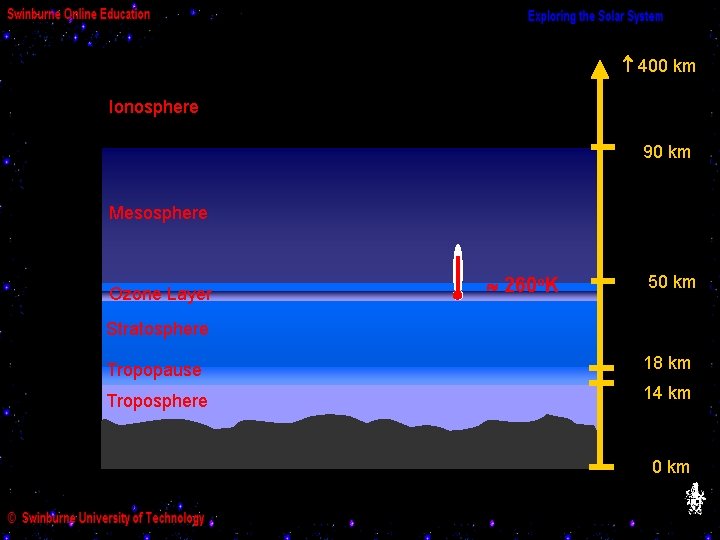
The ozone layer is in the upper stratosphere and lower mesosphere. The ozone absorbs ultraviolet radiation from the Sun, and this process heats up the neighbouring layers - which are therefore warmer than the upper troposphere.

400 km Ionosphere 190 o. K 90 km Mesosphere Ozone Layer 50 km Stratosphere Tropopause 18 km Troposphere 14 km 0 km

The temperature gradually drops again as we go up in altitude through the mesosphere,

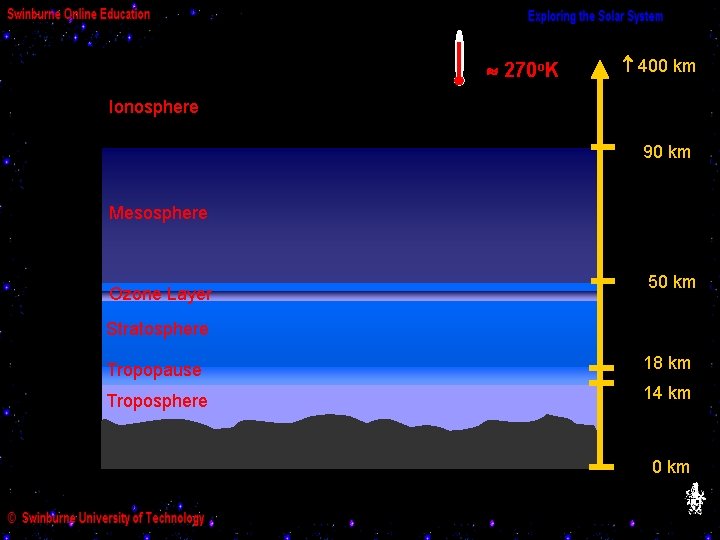
270 o. K 400 km Ionosphere 90 km Mesosphere Ozone Layer 50 km Stratosphere Tropopause 18 km Troposphere 14 km 0 km

… until we reach the ionosphere (sometimes called the “thermosphere”) which is heated by absorbing energy from energetic X-rays from the Sun, so the temperature there can be quite high - but the density is extremely low.

(c) The Effects of Sunlight The Earth’s atmosphere is profoundly affected by another member of our Solar System …. … the Sun.

1. 37 k. J of energy per square metre arrives at the Earth’s orbit every second from the Sun! The solar energy incident on the daytime side of the Earth is eventually reflected or absorbed. The absorbed part heats the Earth’s atmosphere and surface. Without its atmosphere, the Earth’s surface temperature would vary more widely, and its average would be well below freezing.

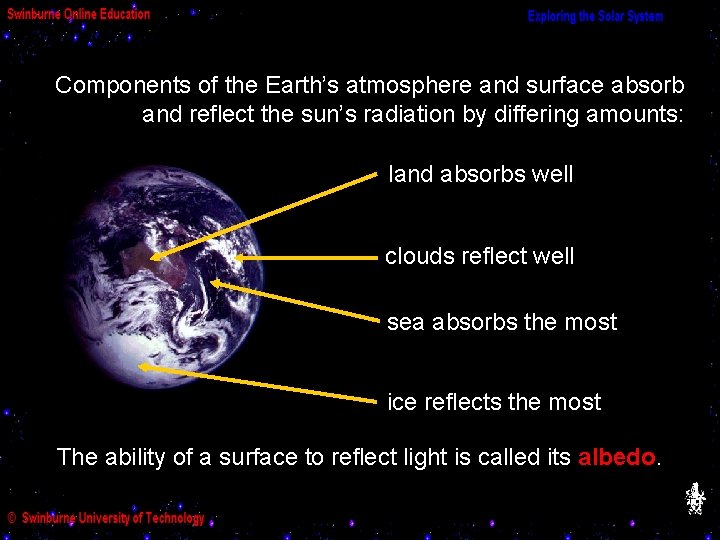
Components of the Earth’s atmosphere and surface absorb and reflect the sun’s radiation by differing amounts: land absorbs well clouds reflect well sea absorbs the most ice reflects the most The ability of a surface to reflect light is called its albedo.

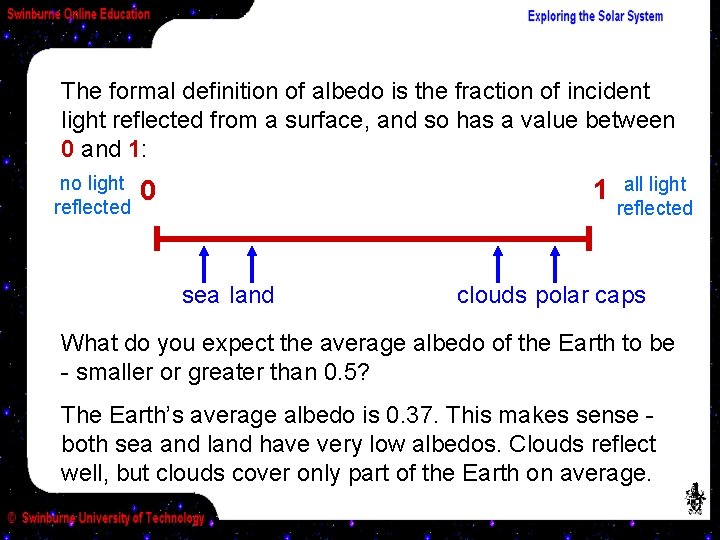
The formal definition of albedo is the fraction of incident light reflected from a surface, and so has a value between 0 and 1: no light reflected 1 0 sea land all light reflected clouds polar caps What do you expect the average albedo of the Earth to be - smaller or greater than 0. 5? The Earth’s average albedo is 0. 37. This makes sense both sea and land have very low albedos. Clouds reflect well, but clouds cover only part of the Earth on average.

We will see in later Modules that Mercury, a planet with no cloud, has a very low albedo (0. 12) - whereas Venus, which is permanently shrouded in cloud, has a very high albedo (0. 76). There actually several different types of albedo, including: • monochromatic albedo, which is simply the ratio of incident energy to reflected energy at any particular wavelength (e. g. in the optical) • Bond albedo is the ratio of the total radiation reflected from a surface to the total light incident from the Sun averaged over all wavelengths

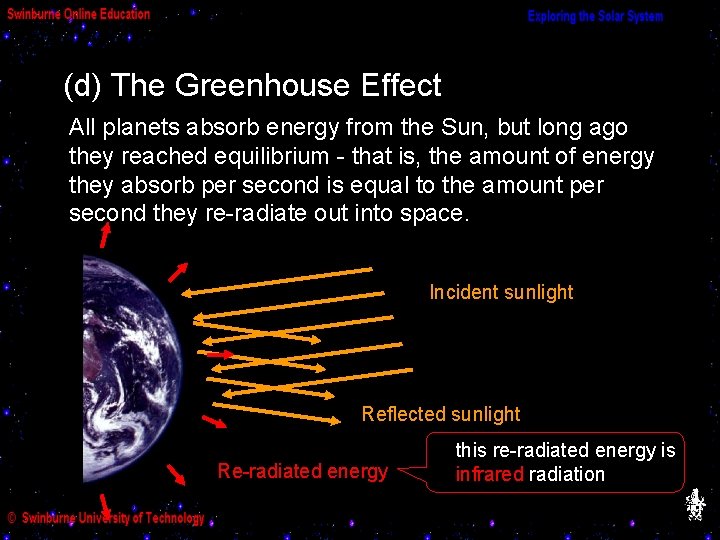
(d) The Greenhouse Effect All planets absorb energy from the Sun, but long ago they reached equilibrium - that is, the amount of energy they absorb per second is equal to the amount per second they re-radiate out into space. Incident sunlight Reflected sunlight Re-radiated energy this re-radiated energy is infrared radiation

Water vapour, methane and (to a lesser extent) carbon dioxide and certain other gases in our atmosphere are good absorbers of infrared radiation, so they trap much of the reradiated energy inside the atmosphere. Most of the re-radiated infrared radiation is trapped within the atmosphere The result is that the Earth is significantly warmer than it would be without an atmosphere. Water vapour, carbon dioxide and methane are examples of “greenhouse gases”. To find out why the term greenhouse is (mis)used, click here.

Water vapour is the main greenhouse gas. Its levels in the Earth’s atmosphere vary from time to time, but remain roughly constant on average. The current scientific debate about the greenhouse effect centres on the rising levels of carbon dioxide and methane in the Earth’s atmosphere, due to sources such as the burning of fossil fuels and effects such as deforestation and increased agricultural activities.

The average atmospheric temperature of the Earth appears to be rising somewhat - is this due to increased levels of greenhouse gases due to human activities, or other natural effects such as long-term fluctuations in the Earth’s weather? This is a continuing (and important) scientific & political debate. From whatever cause, geological evidence suggests that the Earth is now as warm as it has ever been in the last 150, 000 years, and the Earth’s global temperature increased by about 0. 6°C in the 20 th Century. Unfortunately we can’t afford to watch for the few hundred years it would take to establish a firm trend, and the cause and effects, conclusively either way!

As we will see, there is one place in the Solar System where we can see the effects of a runaway greenhouse effect: Venus.

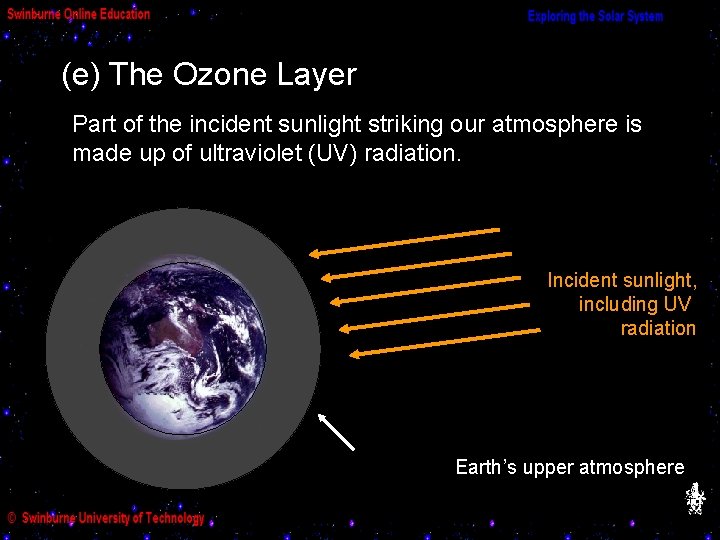
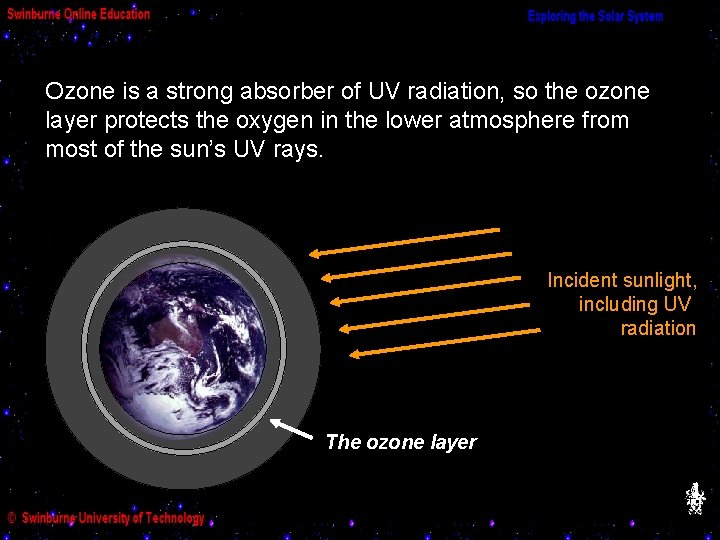
(e) The Ozone Layer Part of the incident sunlight striking our atmosphere is made up of ultraviolet (UV) radiation. Incident sunlight, including UV radiation Earth’s upper atmosphere

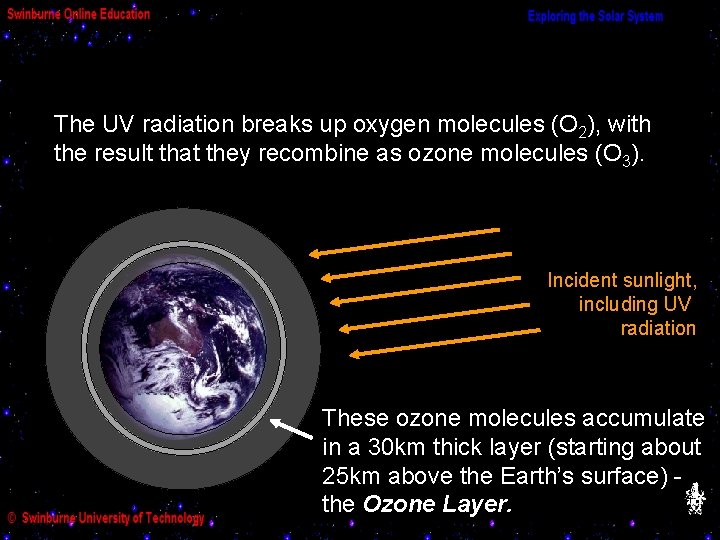
The UV radiation breaks up oxygen molecules (O 2), with the result that they recombine as ozone molecules (O 3). Incident sunlight, including UV radiation These ozone molecules accumulate in a 30 km thick layer (starting about 25 km above the Earth’s surface) the Ozone Layer.

Ozone is a strong absorber of UV radiation, so the ozone layer protects the oxygen in the lower atmosphere from most of the sun’s UV rays. Incident sunlight, including UV radiation The ozone layer

In recent times the ozone layer appears to be thinning out. For example, the ozone concentration over the Antarctic dropped by a factor of two from the 1950 s to the 1980 s. The prime suspects are “chlorofluorocarbons” (CFCs), released from old-style refrigerant systems & spray cans. Each chlorine atom is capable of breaking up approximately 100, 000 ozone molecules.

The Antarctic and Arctic regions are particularly at risk, because, in the polar winters, the stratosphere in those regions becomes cold enough to form water ice and nitric acid ice particles, which act as catalysts to accelerate the production of chlorine molecules. Once summer returns to these regions, sunlight breaks (‘photodissociates’) the chlorine molecules up into chlorine atoms, which then in turn attack the ozone layer. International efforts are now taking place to reverse this trend. With prompt action, the levels of ozone in the ozone layer can be built up again.

(f) Atmospheric Circulation As we who live here well know, the Earth’s atmosphere is not static. Winds & storms are regular features on this and other planets.

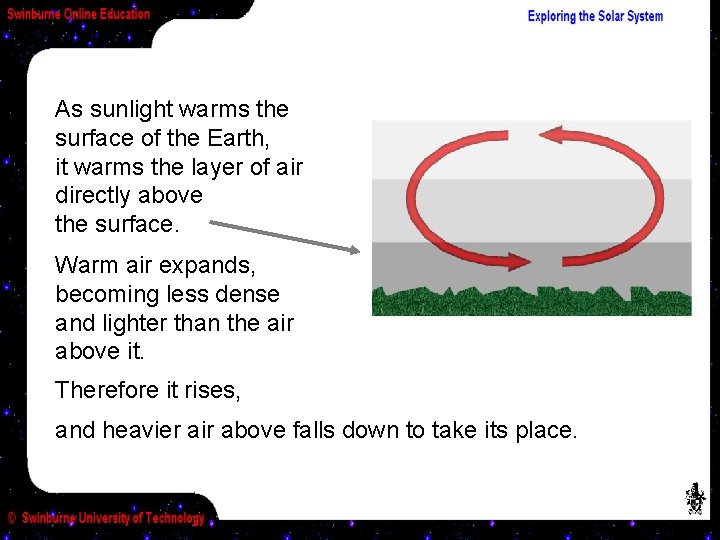
As sunlight warms the surface of the Earth, it warms the layer of air directly above the surface. Warm air expands, becoming less dense and lighter than the air above it. Therefore it rises, and heavier air above falls down to take its place.

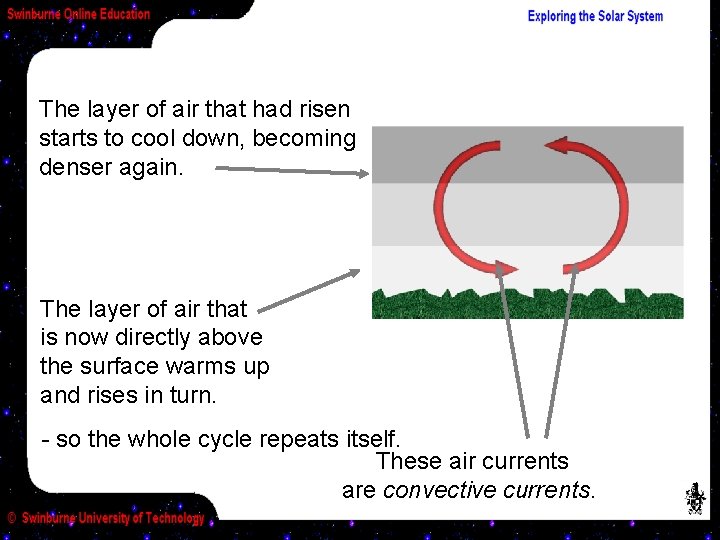
The layer of air that had risen starts to cool down, becoming denser again. The layer of air that is now directly above the surface warms up and rises in turn. - so the whole cycle repeats itself. These air currents are convective currents.

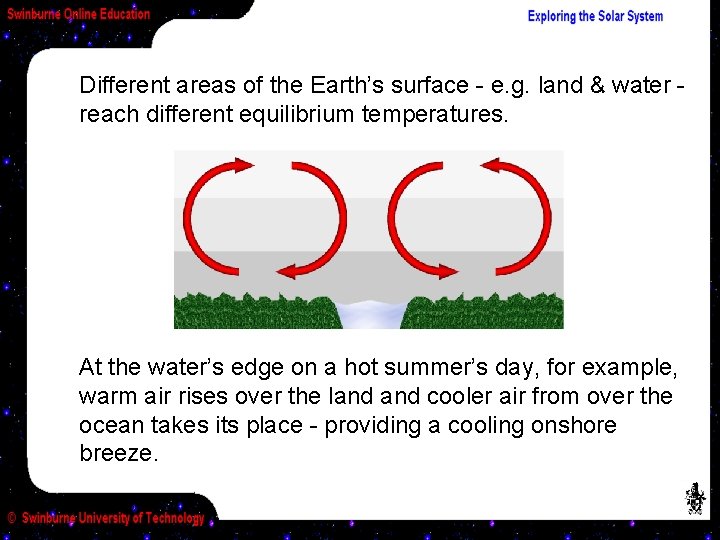
Different areas of the Earth’s surface - e. g. land & water reach different equilibrium temperatures. At the water’s edge on a hot summer’s day, for example, warm air rises over the land cooler air from over the ocean takes its place - providing a cooling onshore breeze.

In these ways convective currents are set up in the Earth’s lower atmosphere. The Earth’s rotation twists the convective currents to establish global atmospheric circulation patterns. In the Activities*on the Jovian planets, we will compare their atmospheric circulation to that of the Earth. * Jupiter, the Dominant Gas Giant Planet, and The Other Jovians

The Earth’s atmospheric circulation patterns are traced by its cloud patterns.

The Earth’s atmospheric circulation patterns are complicated by the presence of significant amounts of water vapour. Water vapour is the only gas in the Earth’s atmosphere which can change to a liquid (in clouds) and fall to the surface (as rain). When water vapour turns to rain, the local air pressure drops somewhat, providing local variations in the air currents and making the atmospheric circulation more complex.

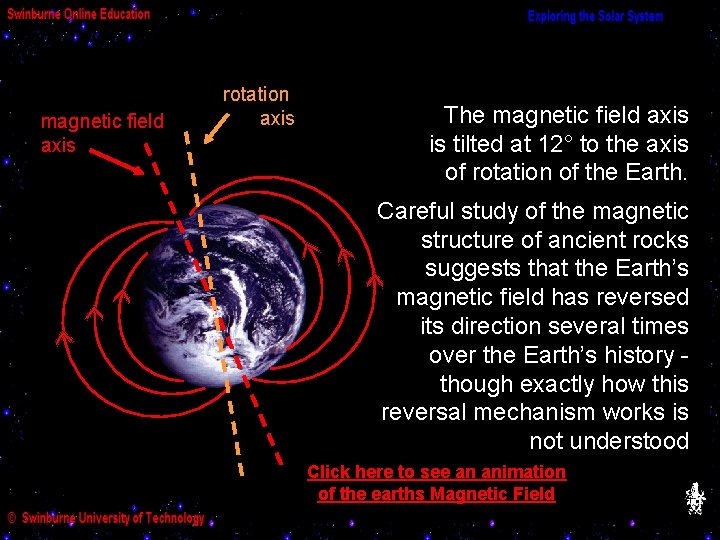
(g) The Structure of the Earth’s Magnetic Field The Earth acts much like a bar magnet, possessing a magnetic field which deflects compasses on the Earth’s surface to point northwards. We represent the magnetic field at any point on or above the Earth’s surface by a line pointing in the direction a compass would point.

magnetic field axis rotation axis The magnetic field axis is tilted at 12° to the axis of rotation of the Earth. Careful study of the magnetic structure of ancient rocks suggests that the Earth’s magnetic field has reversed its direction several times over the Earth’s history though exactly how this reversal mechanism works is not understood Click here to see an animation of the earths Magnetic Field

It is known, however, that the magnetic poles are constantly on the move. The location of the magnetic north pole has been recorded for over 170 years and has been steadily moving north by an average 10 km per year. The magnetic pole’s northward journey The global magnetic field strength has also weakened by about 10% since the 19 th century. The jury is still out as to whether this means we are due for another field reversal or not.

To understand magnetic reversal, we first need to understand what actually drives the Earth’s magnetic field. Magnetic fields are created by moving electric charges. The Earth’s magnetic field is thought to be produced by the motion of charged particles in the convective currents of the metallic liquid outer core The theory of the Earth’s self-generating magnetic field is called the “dynamo effect”, though the exact details are not fully understood.

For a planet to have a magnetic field, it needs a region where charged particles are moving in convective currents. The planet’s rotation is also important in helping generate its magnetic field.

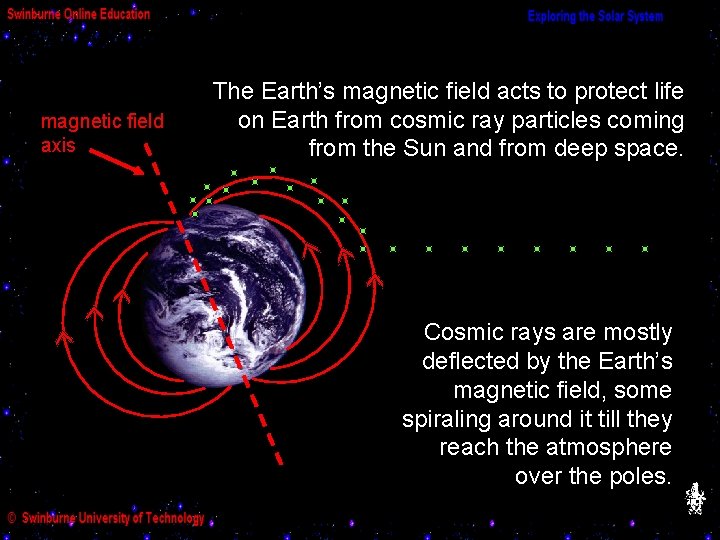
magnetic field axis The Earth’s magnetic field acts to protect life on Earth from cosmic ray particles coming from the Sun and from deep space. Cosmic rays are mostly deflected by the Earth’s magnetic field, some spiraling around it till they reach the atmosphere over the poles.

When the number of cosmic rays is high, the energy they release when striking the atmosphere is seen at the polar regions as the northern and southern lights, or aurorae. Aurora are rapidly varying colourful displays that shimmer across large regions of the sky. The different colours are mainly due to excited oxygen (green and red) and nitrogen (blue) atoms and molecules in the upper atmosphere. We will discuss aurorae again in the Activity on High Energy Astronomy.

Summary In this Activity, we have looked at the average properties of the Earth’s atmosphere, including its composition and structure. The effects of the Sun on our atmosphere and atmospheric circulation were also investigated, and we introduced the Earth’s magnetic field. In the next Module, we will investigate how the Earth has evolved since its formation over 4. 5 billion years ago.

Image Credits NASA: View of Australia http: //nssdc. gsfc. nasa. gov/image/planetary/earth/gal_australia. jpg NASA: Monsoon over India http: //earth. jsc. nasa. gov/lores. cgi? PHOTO=STS 51 F-31 -0069 NASA: View of the Mid-Pacific Ocean http: //nssdc. gsfc. nasa. gov/image/planetary/earth/gal_mid-pacific. jpg NASA: The Northern Lights http: //www. athena. ivv. nasa. gov/curric/space/solterr/aurora. html NASA: World Cloud Cover Pattern http: //www. hq. nasa. gov/office/ese/gallery/Originals/cloud. jpg Natural Resources Canada: Movement of Earth’s north magnetic pole http: //www. geolab. nrcan. gc. ca/geomag/images/nmppath 2001. gif

Now return to the Module home page, and read more about the Earth’s atmosphere and magnetic field in the Textbook Readings. Hit the Esc key (escape) to return to the Module 7 Home Page


The Greenhouse Effect Greenhouses maintain a higher temperature than their surroundings - which is why delicate plants are kept in them in cold winters. They achieve this due to their glass (or plastic) walls, which let light in which is largely absorbed by the plants and surfaces inside the greenhouse. These reradiate infrared radiation, which warms up the air in the greenhouse. This sounds pretty similar to the situation of the Earth and its atmosphere, which is why the term greenhouse effect is used.

There is an important difference though. Although the air and walls of a greenhouse do absorb infrared radiation, the main reason that a greenhouse stays warmer than its surroundings in winter is that its walls trap the warm air, preventing cooling drafts. So the Earth’s atmosphere is not exactly like a greenhouse: it has no walls. Our atmosphere is relatively warm because it traps re-radiated infrared radiation by absorbing most of it before it reaches space. Back to the Activity!
