Why is Earth called the Blue Planet Its

Why is Earth called the Blue Planet? • It’s covered mostly by water! Water is the most abundant compound in most living things.

The Importance of Water • Water has unique properties that allow life to exist on Earth.

Properties of Water

A Molecule of Water • Written as H 2 O 1 atom of Oxygen (O) 2 atoms of Hydrogen (H)

What’s happening here?
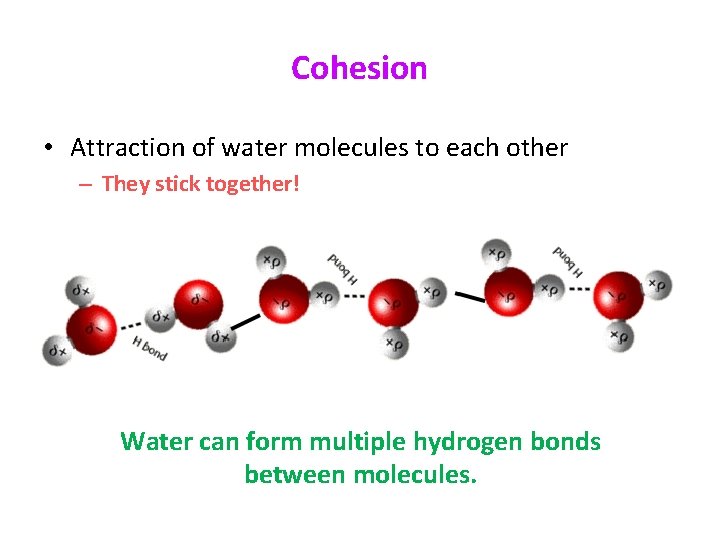
Cohesion • Attraction of water molecules to each other – They stick together! Water can form multiple hydrogen bonds between molecules.

Adhesion • Attraction of water molecules to other things – They stick to other things! This is why a meniscus forms (water molecules stick to the side of the graduated cylinder).

A. Adhesion B. Cohesion Adhesion! The water molecules are holding on to this blade of grass.

A. Adhesion B. Cohesion! Many insects can walk on water, because their weight is not large enough to break the bonds between water molecules.
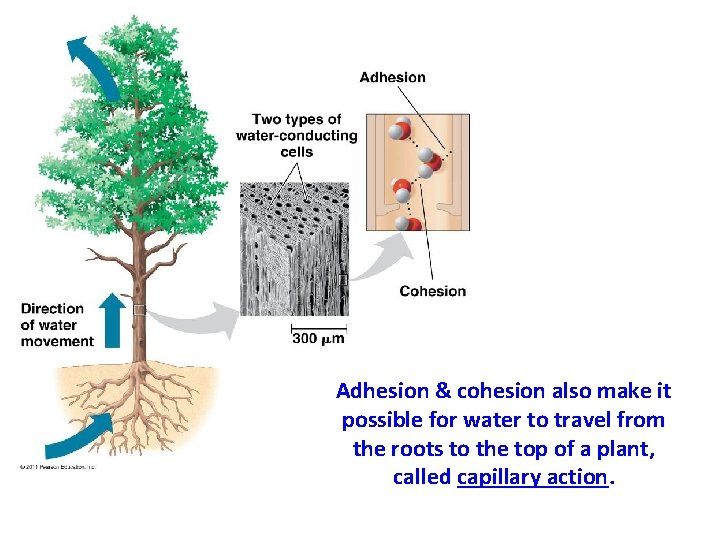
Adhesion & cohesion also make it possible for water to travel from the roots to the top of a plant, called capillary action.

Polarity of Water • Although water is electrically neutral, it does exhibit polarity. – This means that a water molecule has 1 slightly negative end & 1 slightly positive end.
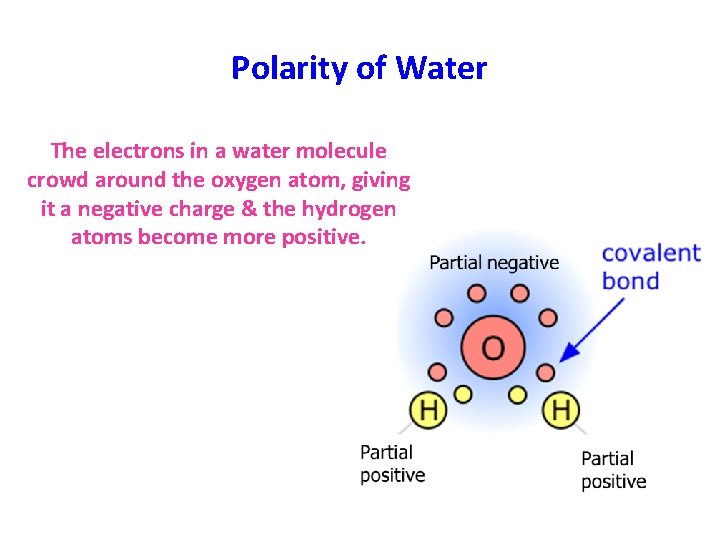
Polarity of Water The electrons in a water molecule crowd around the oxygen atom, giving it a negative charge & the hydrogen atoms become more positive.
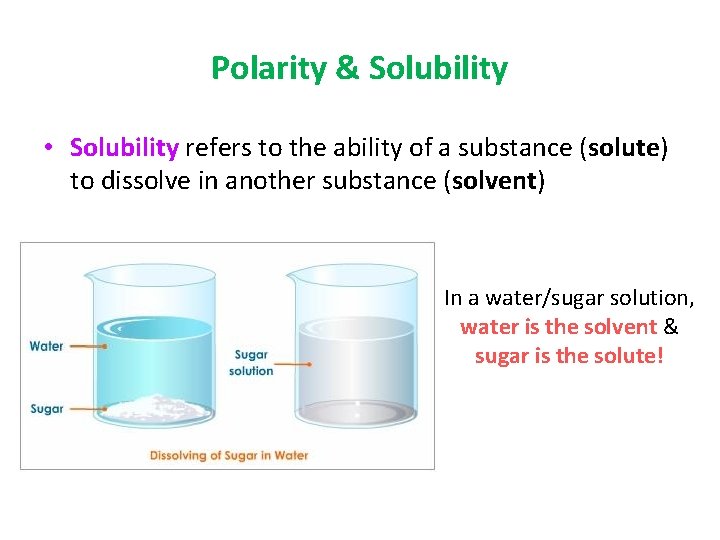
Polarity & Solubility • Solubility refers to the ability of a substance (solute) to dissolve in another substance (solvent) In a water/sugar solution, water is the solvent & sugar is the solute!

You like your tea very sweet, so you add 2 packets of sugar. What’s the solvent? The tea What’s the solute? The sugar
Solutions • The dissolved material (solute) becomes evenly distributed through the entire liquid (solvent)

Suspensions • Mixture of water & a non-dissolved material Mud is a suspension, because the dirt is not fully dissolved in the water!
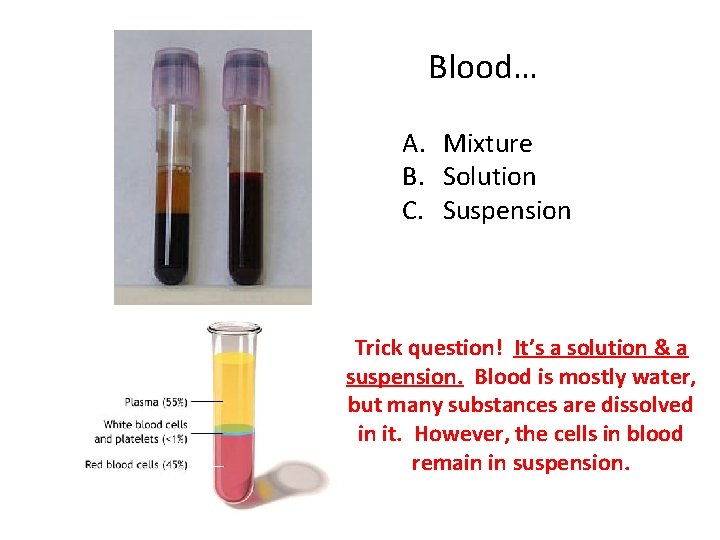
Blood… A. Mixture B. Solution C. Suspension Trick question! It’s a solution & a suspension. Blood is mostly water, but many substances are dissolved in it. However, the cells in blood remain in suspension.

Polarity & Solubility • “Like dissolves like” – Polar substances dissolve other polar substances. – Nonpolar substances dissolve other nonpolar substances. – Polar & nonpolar substances do not mix! What would happen if you poured oil into a glass of water? Is water polar or nonpolar? WATER IS A POLAR MOLECULE!

This is what happens when oil & water get together. Is oil polar or nonpolar? A. Polar B. Nonpolar C. Not enough info. Nonpolar! If we know water & oil don’t mix & that water is POLAR, oil must be NONPOLAR!

Some people add sugar to their coffee. Is sugar polar or nonpolar? A. Polar B. Nonpolar C. Not enough info. Polar! We know sugar will dissolve in water, so sugar must be polar, just like water.

I like cinnamon in my coffee, but it doesn’t dissolve. When added to coffee, the cinnamon clumps together. Is cinnamon polar or nonpolar? A. Polar B. Nonpolar C. Not enough info. Nonpolar! Coffee is polar, so cinnamon must be nonpolar.

Why did the white bear dissolve in water? Because it was polar!
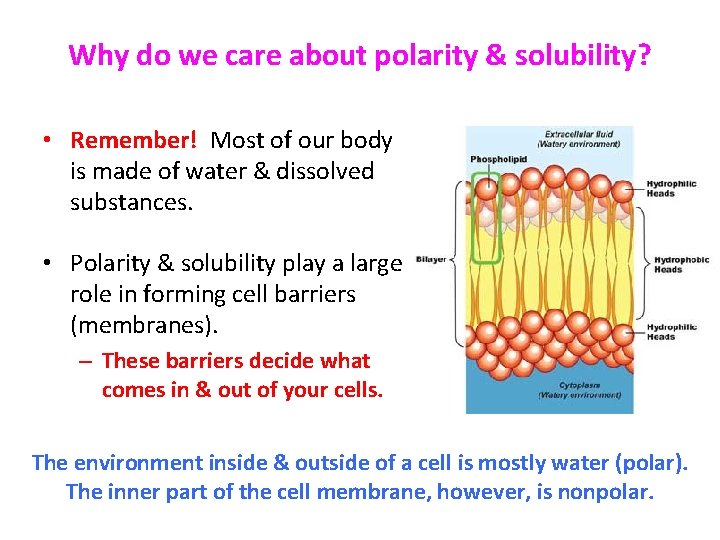
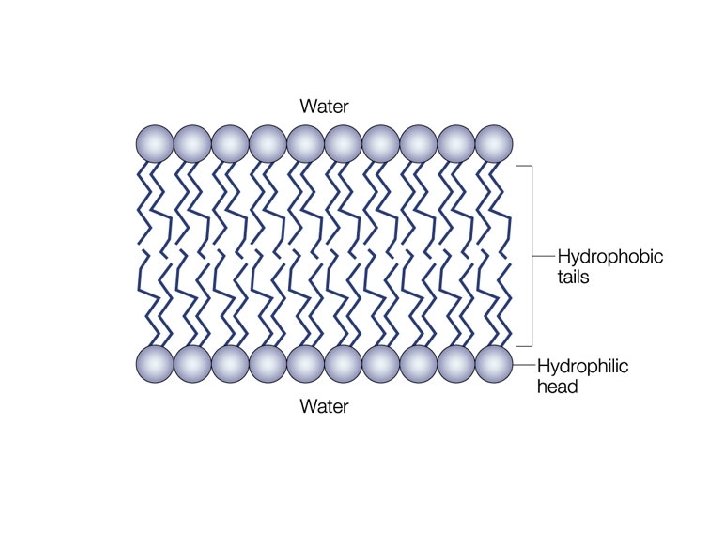
Why do we care about polarity & solubility? • Remember! Most of our body is made of water & dissolved substances. • Polarity & solubility play a large role in forming cell barriers (membranes). – These barriers decide what comes in & out of your cells. The environment inside & outside of a cell is mostly water (polar). The inner part of the cell membrane, however, is nonpolar.
- Slides: 24