Unit 2 Solid Earth Introduction to Planet Earth




























- Slides: 51

Unit 2 -Solid Earth Introduction to Planet “Earth”

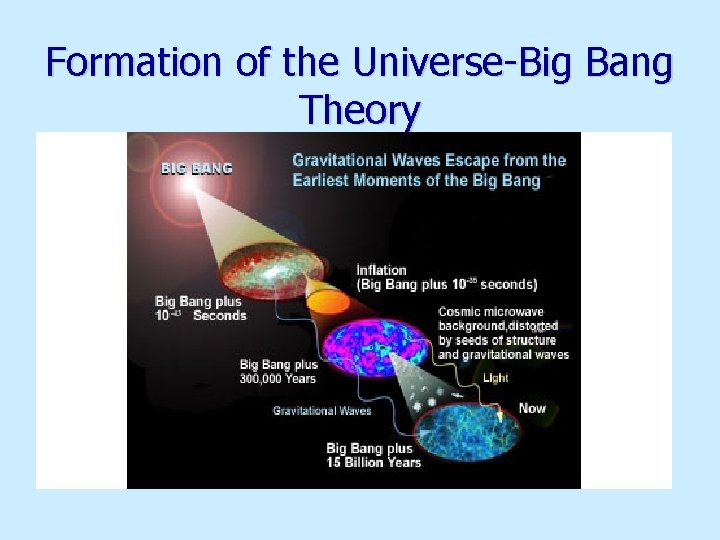
Formation of the Universe-Big Bang Theory

Big Bang

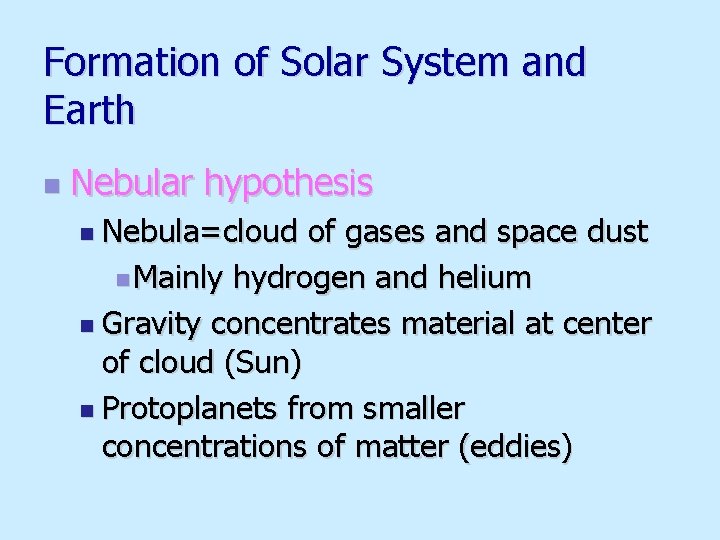
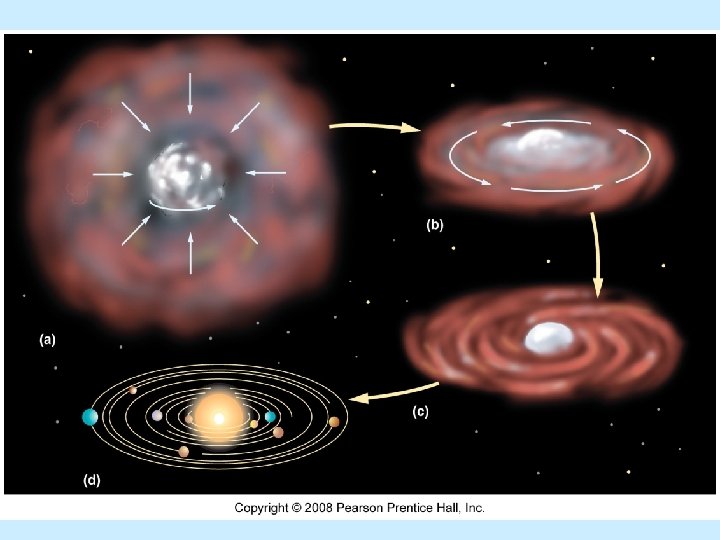
Formation of Solar System and Earth n Nebular hypothesis n Nebula=cloud of gases and space dust n Mainly hydrogen and helium n Gravity concentrates material at center of cloud (Sun) n Protoplanets from smaller concentrations of matter (eddies)





Protoearth Larger than Earth today n Homogeneous composition n Bombarded by meteorites n Moon formed from collision with large asteroid n Heat from solar radiation n Initial atmosphere boiled away n Ionized particles (solar wind) swept away nebular gases n


Protoearth Radioactive heat n Spontaneous disintegration of atoms n Heat from contraction (protoplanet shrinks due to gravity) n Protoearth partially melts n Density stratification (layered Earth) n

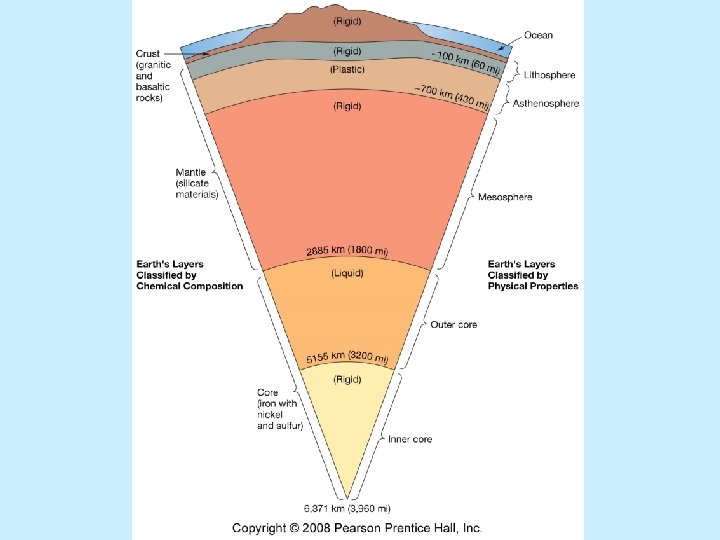
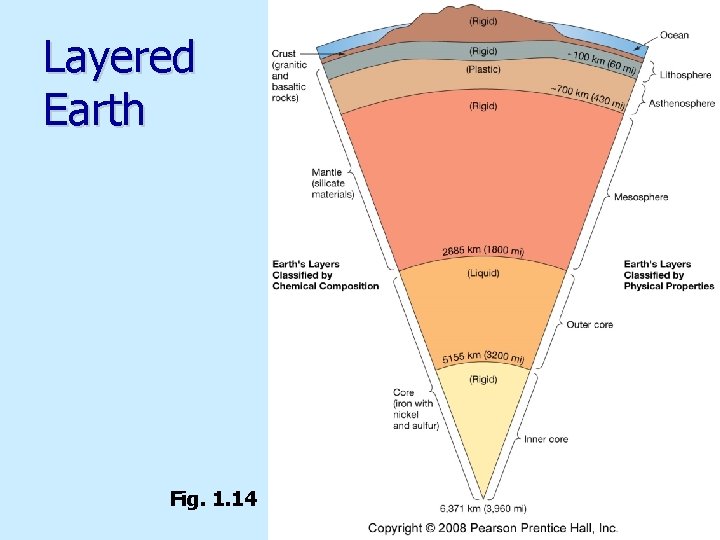
Earth’s internal structure Highest density material at center (core) n Lowest density material at surface (crust) n Earth layered n Chemical composition n Physical properties n


Chemical composition Crust n Low-density, mainly silicate minerals n Mantle n Mainly Fe and Mg silicate minerals n Core n High-density, mainly Fe and Ni n

Layered Earth Fig. 1. 14

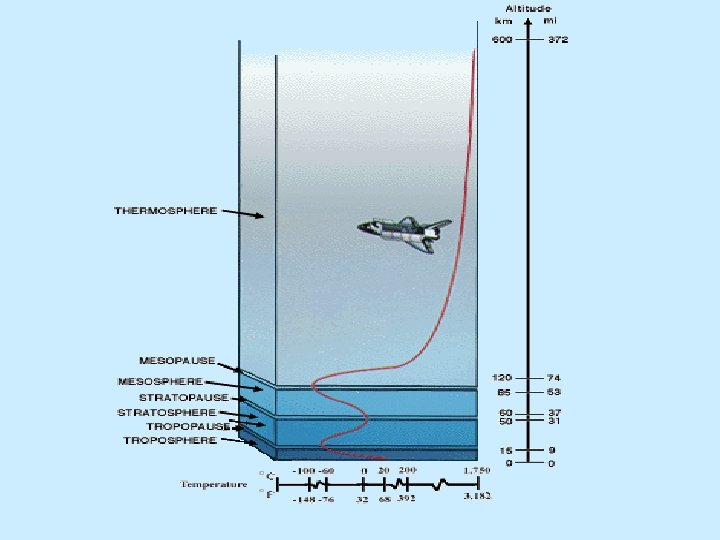
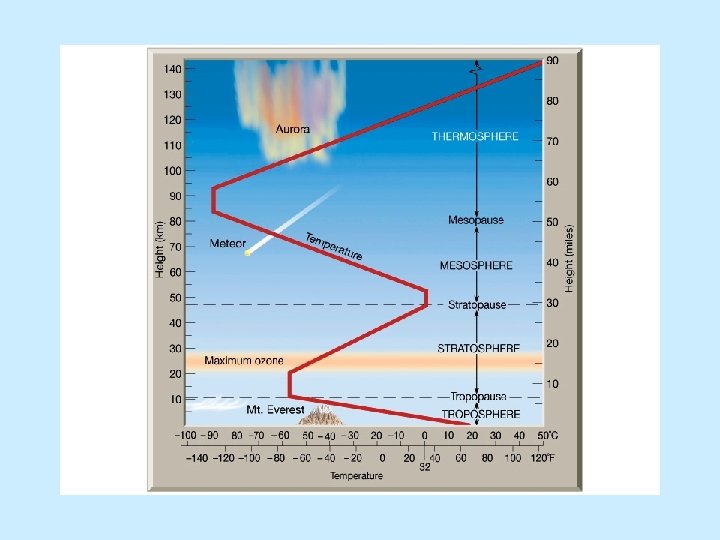
Physical properties n Lithosphere n Asthenosphere n Mesosphere n Outer core n Inner core

Physical properties n Lithosphere n Cool, rigid, brittle n Surface to about 100 km (62 miles) n Asthenosphere n Warm, plastic, able to flow n From 100 km to 700 km (430 miles)

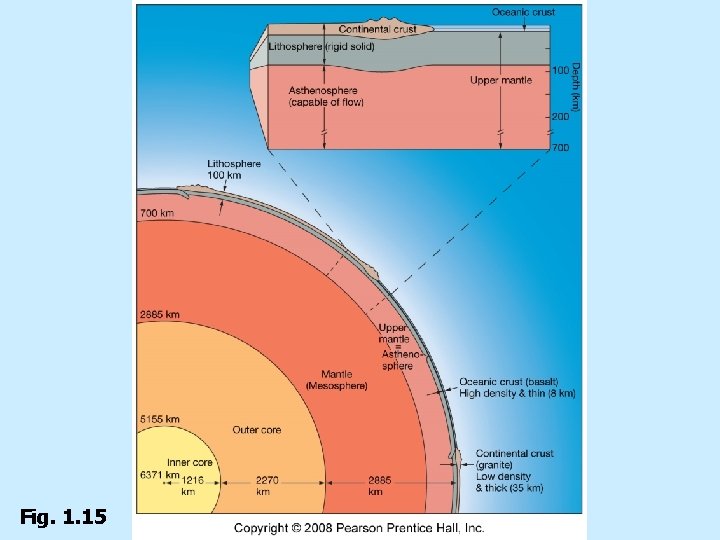
Fig. 1. 15

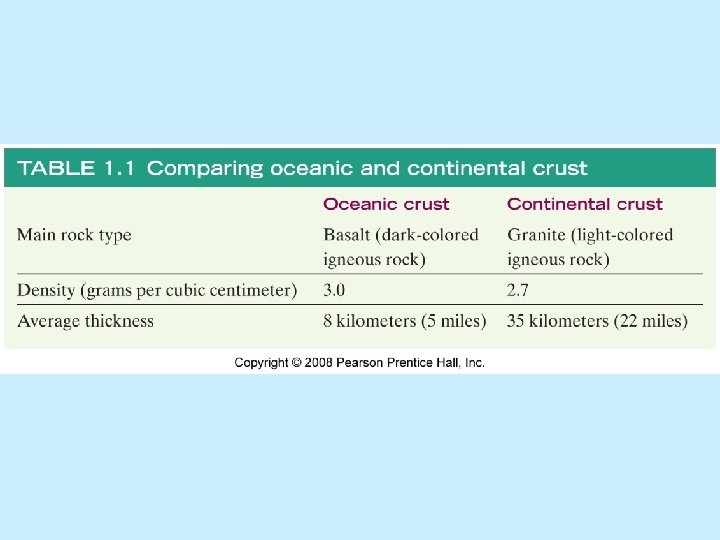
Lithosphere n Oceanic crust n Underlies ocean basins n Igneous rock basalt n Average thickness 8 km (5 miles) n Relatively high density n 3. 0 g/cm 3

Lithosphere- Crust and Uppermost mantle fused together. n Continental crust n Underlies continents n Igneous rock granite n Average thickness 35 km (22 miles) n Lower density n 2. 7 g/cm 3


Asthenosphere n Upper mantle n Plastic—deforms by flowing n High viscosity—flows slowly

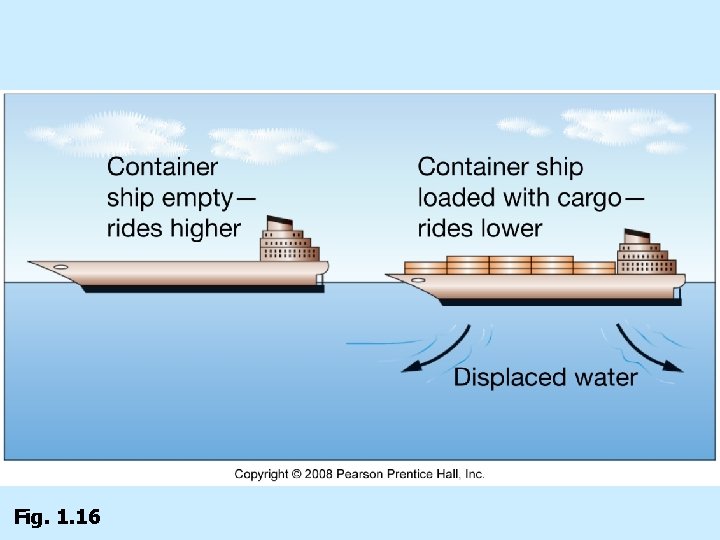
Isostatic adjustment n Buoyancy n n n Less dense “floats” higher than more dense Continental crust “floats” higher than oceanic crust on plastic asthenosphere Questions: n n Based on Isostatic adjustment, what is the impact of removing a large percentage of groundwater from California? Based on geologic response, what is the impact of removing a large percentage of groundwater from California?

Fig. 1. 16

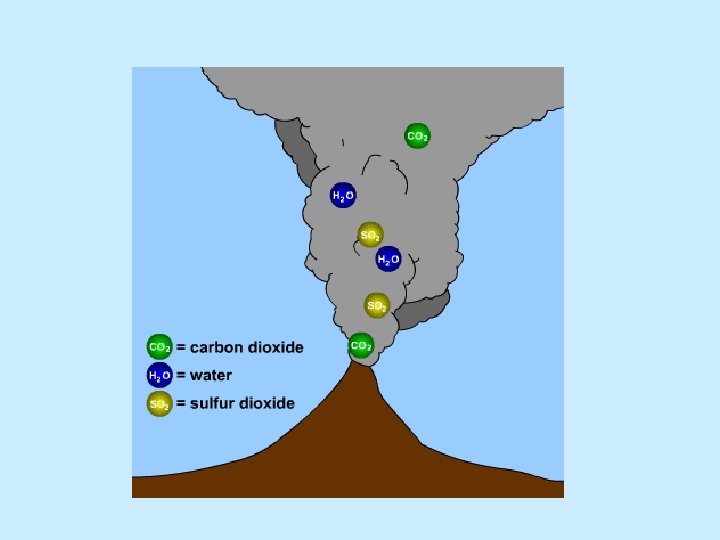
Origin of Earth’s atmosphere n Partial melting resulted in outgassing about 4 billion years ago n Similar to gases emitted from volcanoes n Mainly water vapor n Carbon dioxide, hydrogen n Other gases such as methane and ammonia

Evolution of Earth’s Atmosphere Some scientists describe three stages in the evolution of Earth’s atmosphere as it is today

Just formed Earth: Like Earth, the hydrogen (H 2) and helium (He) were very warm. These molecules of gas moved so fast they escaped Earth's gravity and eventually all drifted off into space. 1. Earth’s original atmosphere was probably just hydrogen and helium, because these were the main gases in the dusty, gassy disk around the Sun from which the planets formed. The Earth and its atmosphere were very hot. Molecules of hydrogen and helium move really fast, especially when warm. Actually, they moved so fast they eventually all escaped Earth's gravity and drifted off into space.

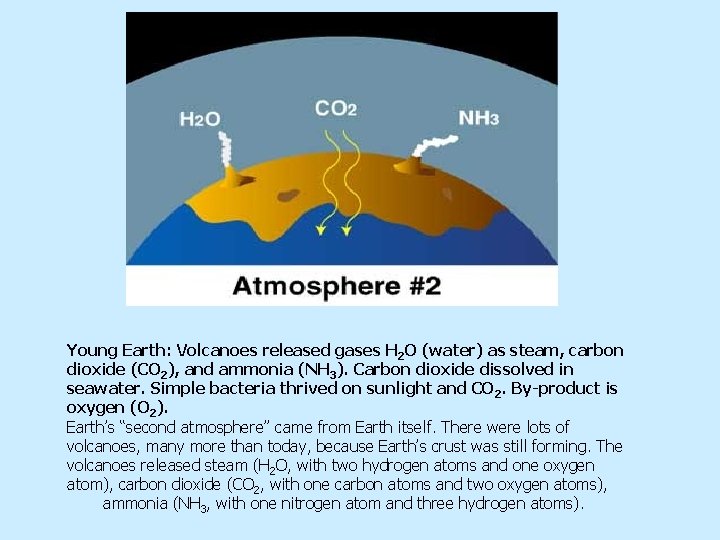
Young Earth: Volcanoes released gases H 2 O (water) as steam, carbon dioxide (CO 2), and ammonia (NH 3). Carbon dioxide dissolved in seawater. Simple bacteria thrived on sunlight and CO 2. By-product is oxygen (O 2). Earth’s “second atmosphere” came from Earth itself. There were lots of volcanoes, many more than today, because Earth’s crust was still forming. The volcanoes released steam (H 2 O, with two hydrogen atoms and one oxygen atom), carbon dioxide (CO 2, with one carbon atoms and two oxygen atoms), ammonia (NH 3, with one nitrogen atom and three hydrogen atoms).


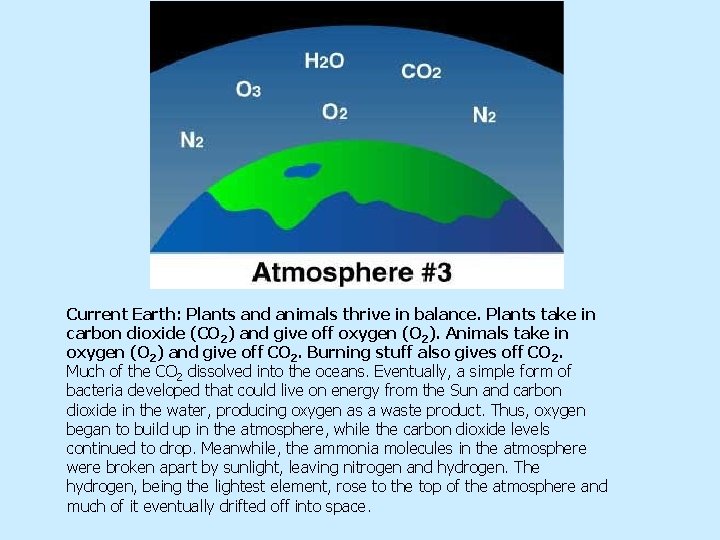
Current Earth: Plants and animals thrive in balance. Plants take in carbon dioxide (CO 2) and give off oxygen (O 2). Animals take in oxygen (O 2) and give off CO 2. Burning stuff also gives off CO 2. Much of the CO 2 dissolved into the oceans. Eventually, a simple form of bacteria developed that could live on energy from the Sun and carbon dioxide in the water, producing oxygen as a waste product. Thus, oxygen began to build up in the atmosphere, while the carbon dioxide levels continued to drop. Meanwhile, the ammonia molecules in the atmosphere were broken apart by sunlight, leaving nitrogen and hydrogen. The hydrogen, being the lightest element, rose to the top of the atmosphere and much of it eventually drifted off into space.

Now we have Earth’s “third atmosphere, ” the one we all know and love—an atmosphere containing enough oxygen for animals, including ourselves, to evolve. So plants and some bacteria use carbon dioxide and give off oxygen, and animals use oxygen and give off carbon-dioxide—how convenient! The atmosphere upon which life depends was created by life itself.




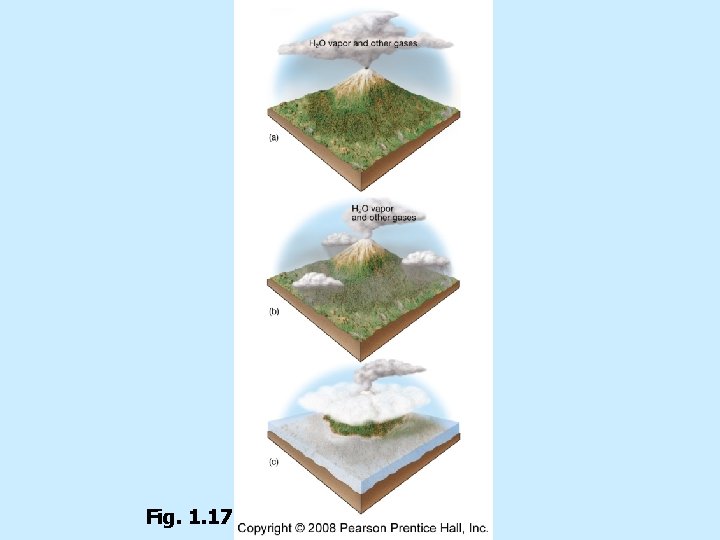
Origin of Earth’s oceans Water vapor released by outgassing n Condensed as rain n Accumulated in ocean basins n About 4 billion years ago n n Ice Comets were also important to adding water to the Earth system

Fig. 1. 17

Volcanic outgassing of Kilauea volcano on Hawai’i

Ocean salinity n Rain dissolves rocks n Dissolved compounds (ions) accumulate in ocean basins n Ocean salinity based on balance between input and output of ions n Ocean salinity nearly constant over past 4 billion years

Life in oceans n Earliest life forms fossilized bacteria in rocks about 3. 5 billion years old n Marine rocks n Life originated in oceans?


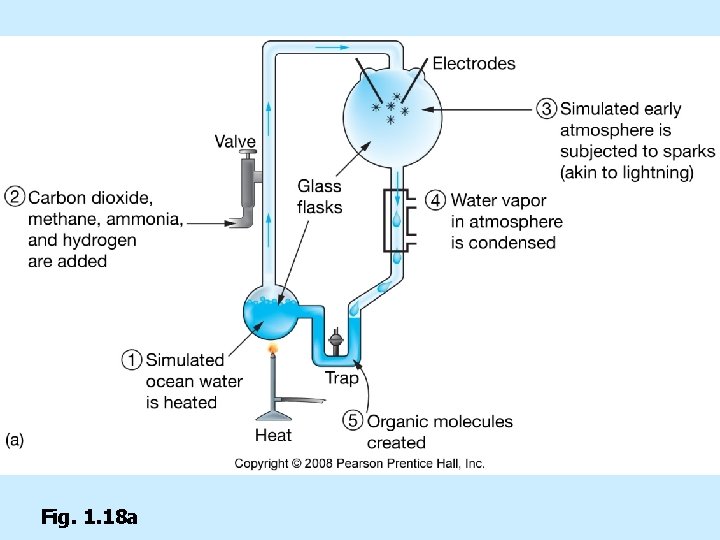
Stanley Miller’s experiment n Organic molecules formed by ultraviolet light, electrical spark (lightning), and mixture of water, carbon dioxide, hydrogen, methane, and ammonia

Fig. 1. 18 a

Evolution and natural selection Organisms adapt and change through time n Advantageous traits are naturally selected n Traits inherited n Organisms adapt to environments n Organisms change environments n

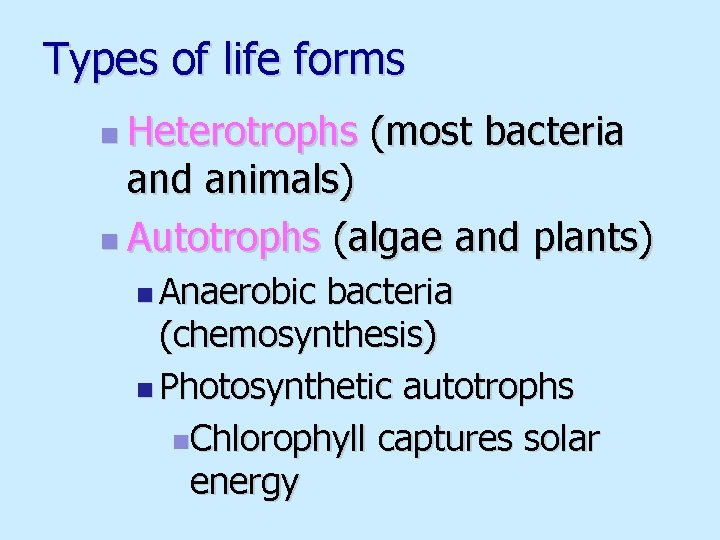
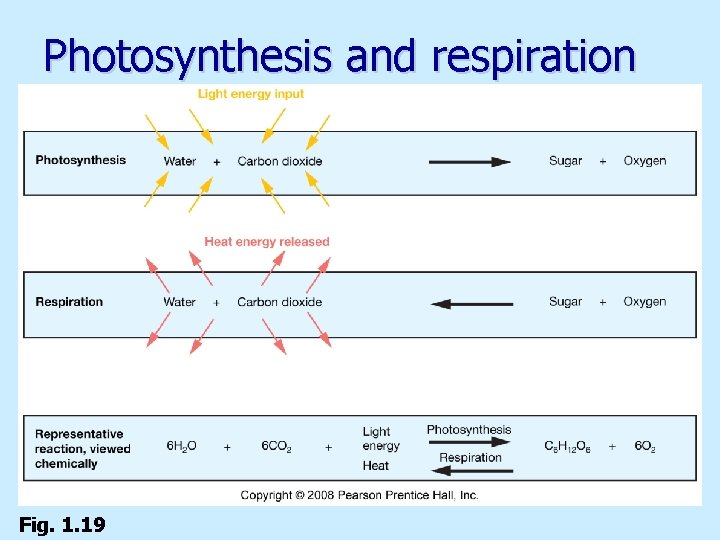
Types of life forms n Heterotrophs (most bacteria and animals) n Autotrophs (algae and plants) n Anaerobic bacteria (chemosynthesis) n Photosynthetic autotrophs n. Chlorophyll captures solar energy

Photosynthesis and respiration Fig. 1. 19

Oxygen crisis Photosynthetic bacteria release oxygen (O 2) to atmosphere n About 2 billion years ago, sufficient O 2 in atmosphere to oxidize (rust) rocks n Ozone (O 3) builds up in atmosphere n n Protects Earth’s surface from ultraviolet solar radiation

Oxygen crisis About 1. 8 billion years ago, most anaerobic bacteria killed off by O 2 -rich atmosphere n Photosynthetic organisms created today’s O 2 -rich atmosphere n n O 2 makes up about 21% of gases in modern atmosphere n Animals thrive

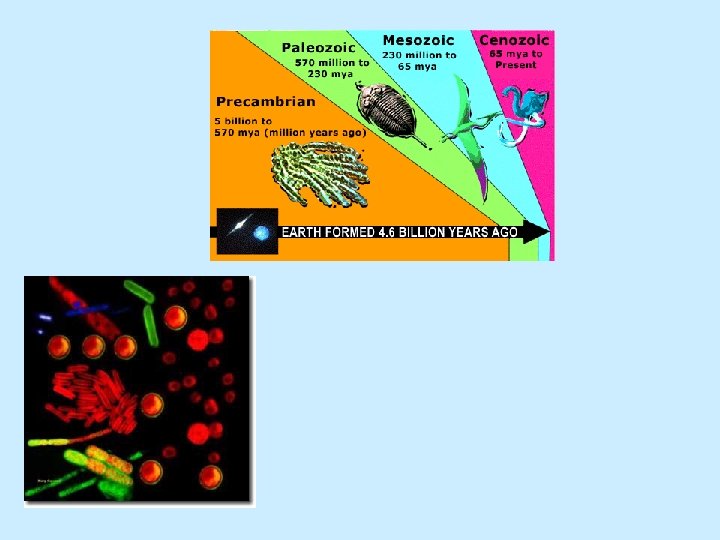
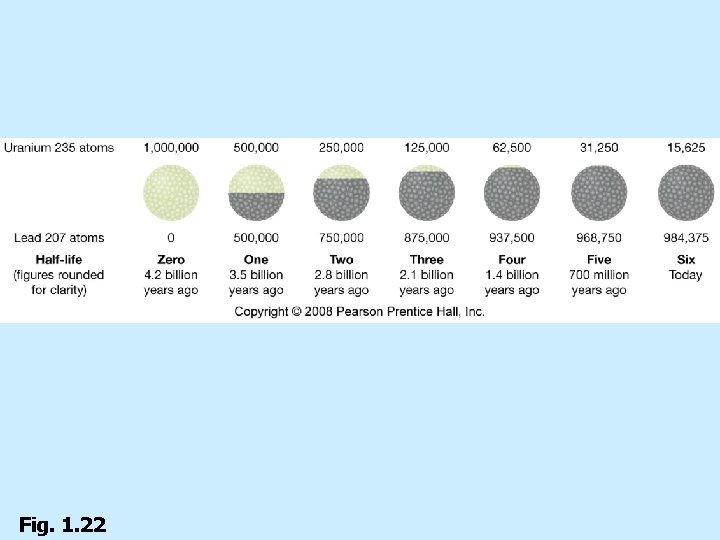
Age of Earth n Radiometric age dating n Spontaneous change/decay n Half-life n Earth is about 4. 6 billion years old

Fig. 1. 22

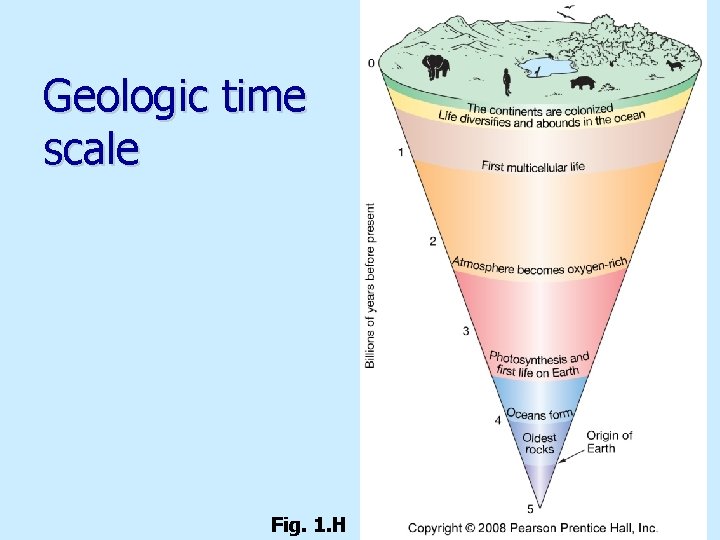
Geologic time scale Fig. 1. H

End of CHAPTER 1 Introduction to Planet “Earth”