Lecture Notes Chem 150 K Marr Chapter 13







































- Slides: 75

Lecture Notes Chem 150 - K. Marr Chapter 13 Properties of Solutions Silberberg 3 ed

Properties of Solutions 13. 1 Types of Solutions: IMF’s and Predicting Solubility 13. 2 Energy Changes in the Solution Process 13. 3 Solubility as an Equilibrium Process 13. 4 Quantitative Ways of Expressing Concentration 13. 5 Colligative Properties of Solutions Will not cover: 13. 6 The Structure and Properties of Colloids

Definitions 1. Solution » 2. homogeneous mixture with only one phase present Mixture » 2 or more substances physically mixed together » Composition is variable » Properties of components are retained

Formation of Solutions 1. Driving Force: • Tendency toward Randomness (T 1) • Nature favors processes that result in an increase in entropy (more randomness or less order) – Explains why solutions of gases always form. 2. Why don’t solutions always form with solids and liquids? » Must consider IMF’s

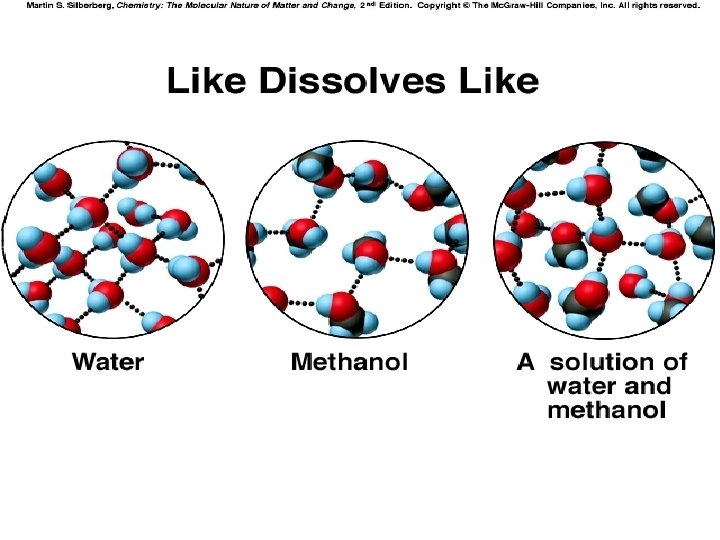
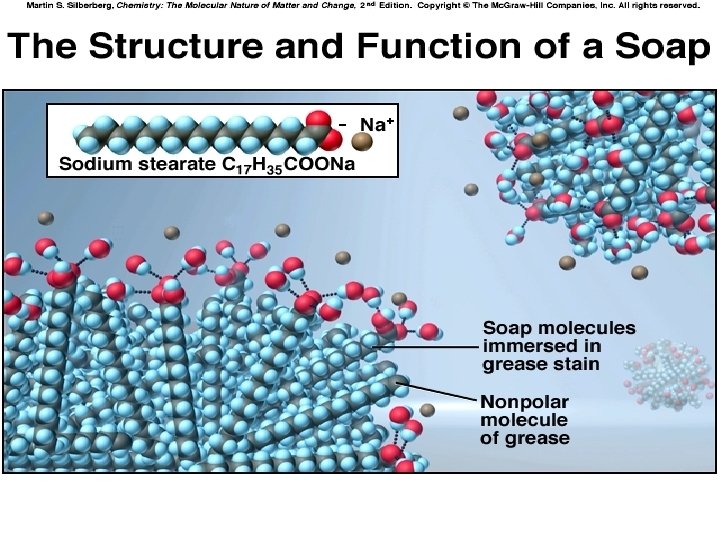
Formation of Solutions • Solute and solvent particles must be attracted to one another: “Like Dissolves Like” • For a solution form…. . Solute-solvent IMF’s > Solvent-Solvent & solute-solute IMFS



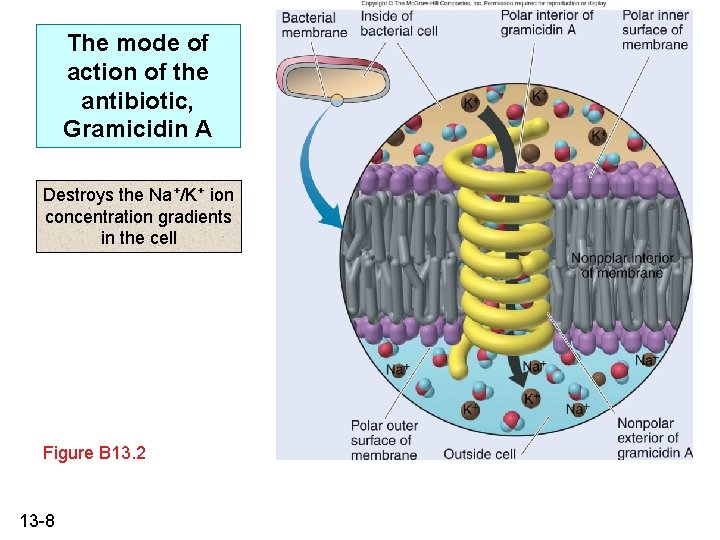
The mode of action of the antibiotic, Gramicidin A Destroys the Na+/K+ ion concentration gradients in the cell Figure B 13. 2 13 -8

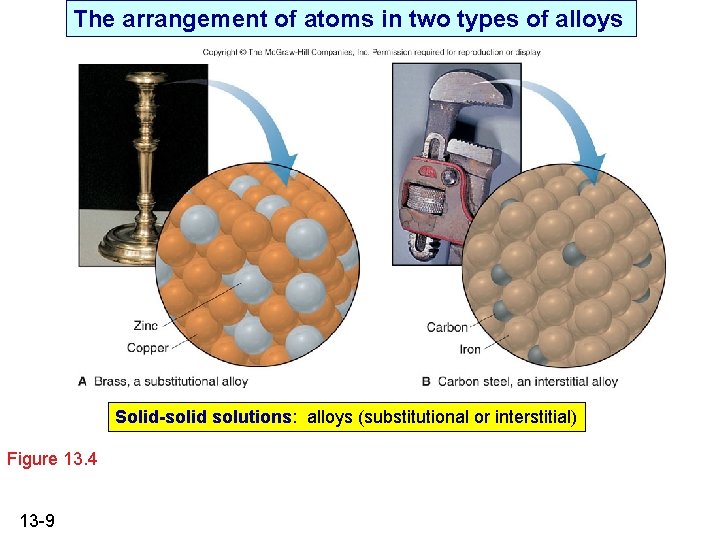
The arrangement of atoms in two types of alloys Solid-solid solutions: alloys (substitutional or interstitial) Figure 13. 4 13 -9

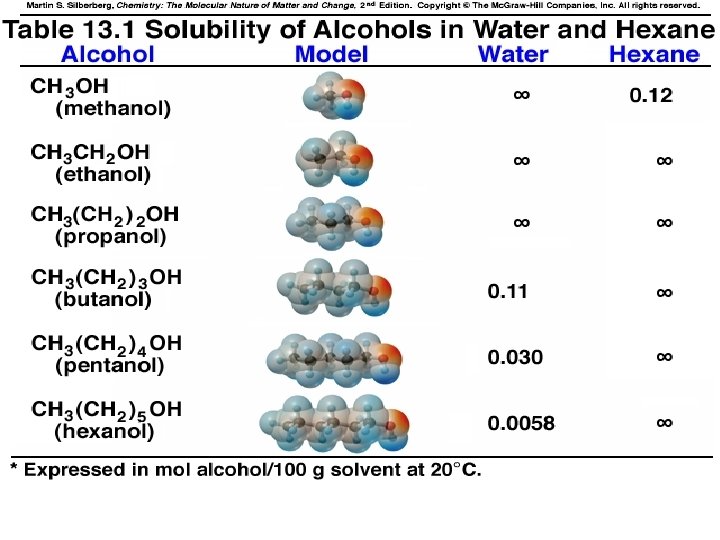
Solutions involving Liquids • • Molecules of each liquid must be pushed apart for a solution to form Why doesn’t water form a solution with n. Hexane, C 6 H 14? » Water molecules too strongly attracted to each other to be pushed aside to make room for hexane molecules


Application Questions: Solutions involving Liquids 1. Why does a solution form between water and ethanol? • 2. H-Bonding between water and ethanol molecules is responsible for solution formation – Allows water molecules to be pushed aside to make room for ethanol molecules Why do all nonpolar liquids mix to form solutions? » e. g. Oil and n-Hexane

SAMPLE PROBLEM 13. 1 Predicting Relative Solubilities of Substances Predict which solvent will dissolve more of the given solute: (a) Ethylene glycol (HOCH 2 OH) in hexane (CH 3 CH 2 CH 2 CH 3) or in water. (b) Diethyl ether (CH 3 CH 2 OCH 2 CH 3) in water or in ethanol (CH 3 CH 2 OH) (c) Sodium chloride in methanol (CH 3 OH) or in propanol (CH 3 CH 2 OH) PLAN: Consider the intermolecular forces which can exist between solute molecules and consider whether the solvent can provide such interactions and thereby substitute.

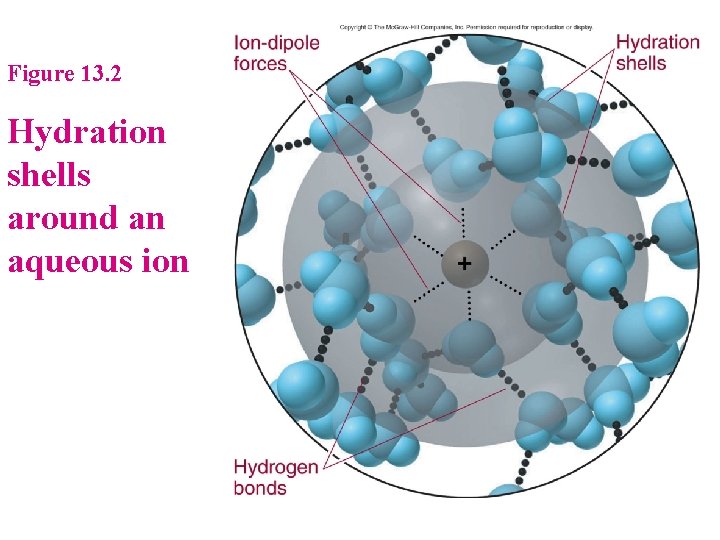
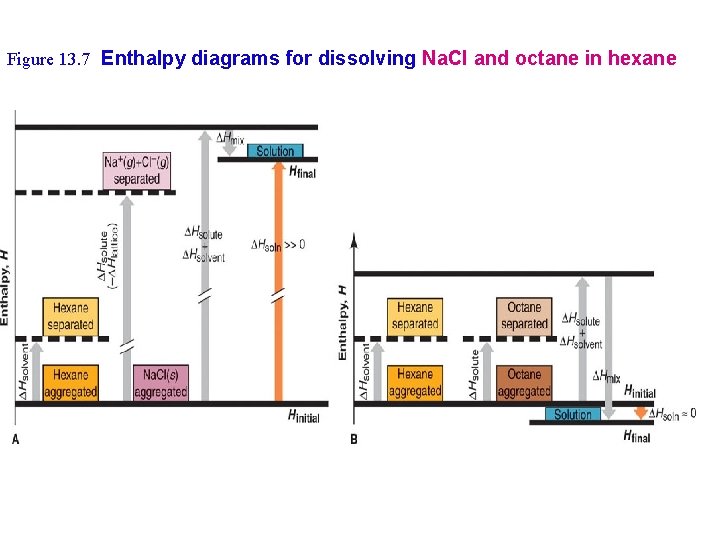
Solids Dissolved in Liquids 1. 2. Solid particles must separate for a solution to form (T 2) » Solute must be attracted to solvent – Ion-Dipole attractions – Solvation vs Hydration What happens if the attractive forces within solute and solvent differ greatly? » e. g. Hexane and Na. Cl

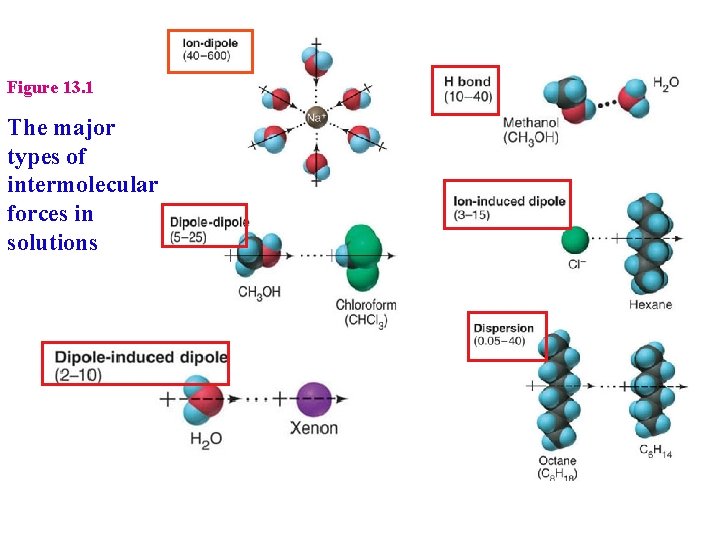
Figure 13. 1 The major types of intermolecular forces in solutions

Figure 13. 2 Hydration shells around an aqueous ion

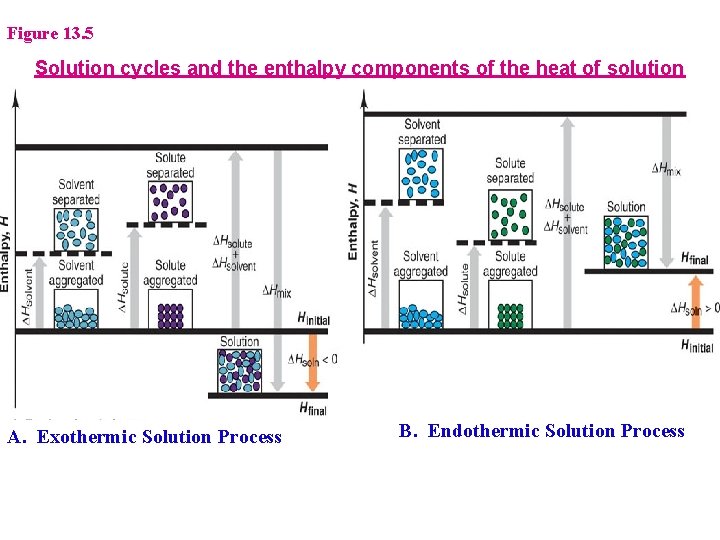
What determines if the DHsoln for a solid with a liquid is exo- or endothermic? (T 3 -5) 1. DHsoln = Lattice Energy + Solvation Energy 2. Lattice Energy » E needed to separate particles: Always Endothermic 3. Solvation Energy » E released upon solvation » Always Exothermic

Figure 13. 5 Solution cycles and the enthalpy components of the heat of solution A. Exothermic Solution Process B. Endothermic Solution Process

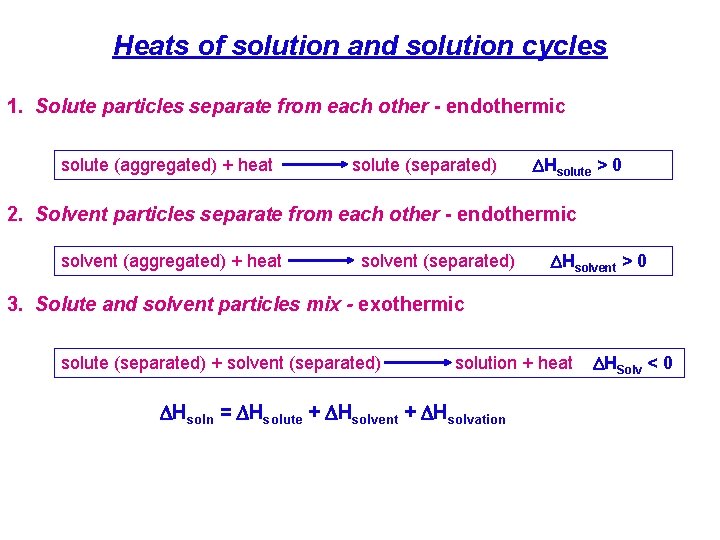
Heats of solution and solution cycles 1. Solute particles separate from each other - endothermic solute (aggregated) + heat solute (separated) DHsolute > 0 2. Solvent particles separate from each other - endothermic solvent (aggregated) + heat solvent (separated) DHsolvent > 0 3. Solute and solvent particles mix - exothermic solute (separated) + solvent (separated) solution + heat DHsoln = DHsolute + DHsolvent + DHsolvation DHSolv < 0

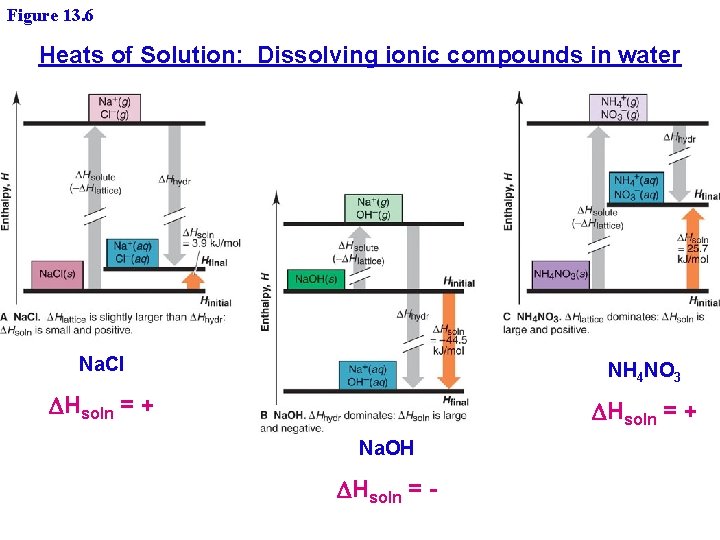
Figure 13. 6 Heats of Solution: Dissolving ionic compounds in water Na. Cl NH 4 NO 3 DHsoln = + Na. OH DHsoln = -

Figure 13. 7 Enthalpy diagrams for dissolving Na. Cl and octane in hexane

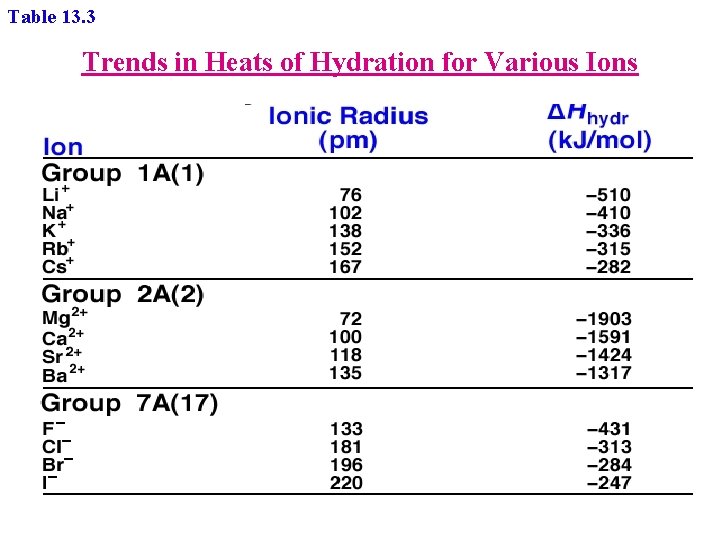
Table 13. 3 Trends in Heats of Hydration for Various Ions

Heats of Solution: Application Questions 1. 2. 3. 4. Why is the DHsoln always exothermic (negative) for solutions between gases and liquids? Why is the DHsoln = 0 for solutions between gases? Why is the dissolving of Calcium Chloride, Ca. Cl 2, in water exothermic? » Ca. Cl 2 (along with Na. Cl) are used to salt roads Why is the dissolving of ammonium nitrate, NH 4 NO 3, in water endothermic? » NH 4 NO 3 is used in chemical cold packs

The Effect of Temperature on Solubility Objectives: » Describe the effect of temperature on solubility of gases, liquids, and solids in liquids

Solubility 1. Equation describing a saturated solution at equilibrium Solute + Solvent Saturated Solution 2. Most Common Units: » Mass solute/100 g solvent at a given temperature

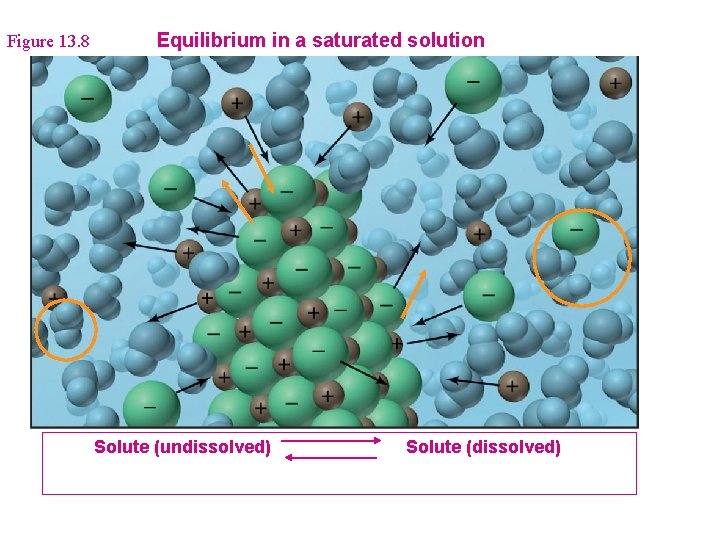
Figure 13. 8 Equilibrium in a saturated solution Solute (undissolved) Solute (dissolved)

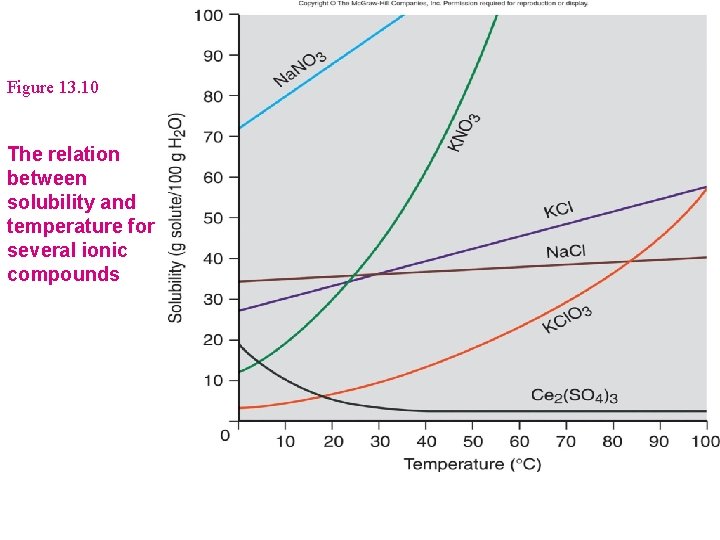
Figure 13. 10 The relation between solubility and temperature for several ionic compounds

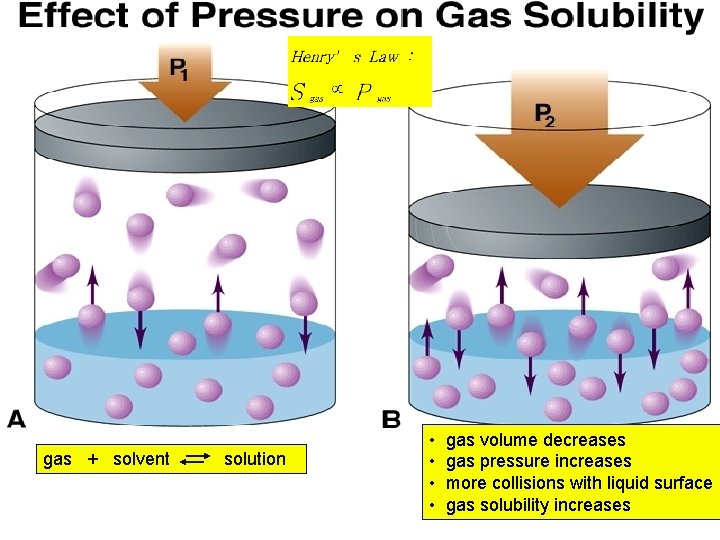
Effect of Temp. on the Solubility of a Gas in a Liquid 1. 2. 3. Solubility of a gas always decreases as Temp. increases. Why? ? Le Chatelier’s Principle is used to predict how an increase in temp. affects the solubility of a gas in a liquid. Recall DHsoln is exothermic for all gases in a liquid: Gas + Liquid Gas dissolved + E

Solubility of a Gas in a Liquid: Applications 1. 2. Thermal Pollution Decreases O 2 Solubility » Streams and Rivers – Salmon/Trout habitat restoration » Deep lakes – Warm water at surface, cold water deep Why are the richest fisheries in the coldest waters of the world?

Solubility of a Gas in a Liquid: Application Questions 1. 2. 3. Why do bubbles form on the side of a glass of water? What do these bubbles consist of? Why do sodas get flat as they sit? Why are some ice cubes clear, others cloudy? » How are clear ice cubes made?

gas + solvent solution • • gas volume decreases gas pressure increases more collisions with liquid surface gas solubility increases

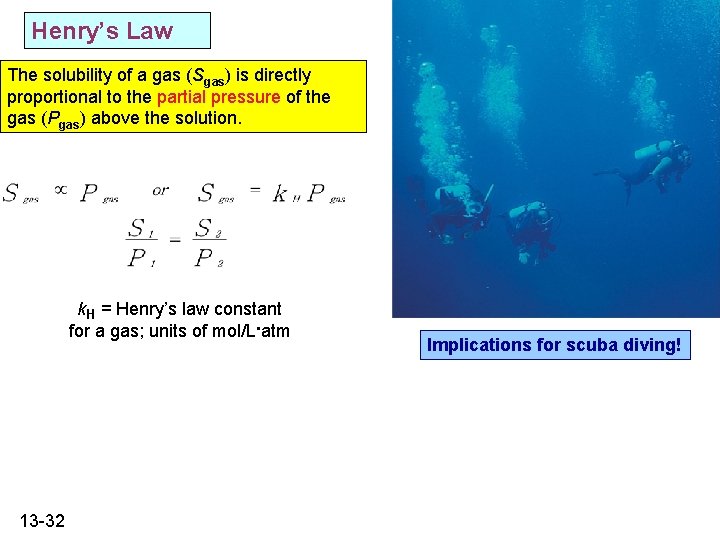
Henry’s Law The solubility of a gas (Sgas) is directly proportional to the partial pressure of the gas (Pgas) above the solution. k. H = Henry’s law constant for a gas; units of mol/L. atm 13 -32 Implications for scuba diving!

Application of Henry’s Law 1. 2. Why does a soda start to bubble immediately after opening the bottle? The solubility of methane , the chief component in natural o gas, in water at 20. 0 C and 1. 0 atm pressure is 0. 025 g/L. o What will the solubility be at 1. 5 atm pressure and 20. 0 C ? » Answer: 0. 038 g/L

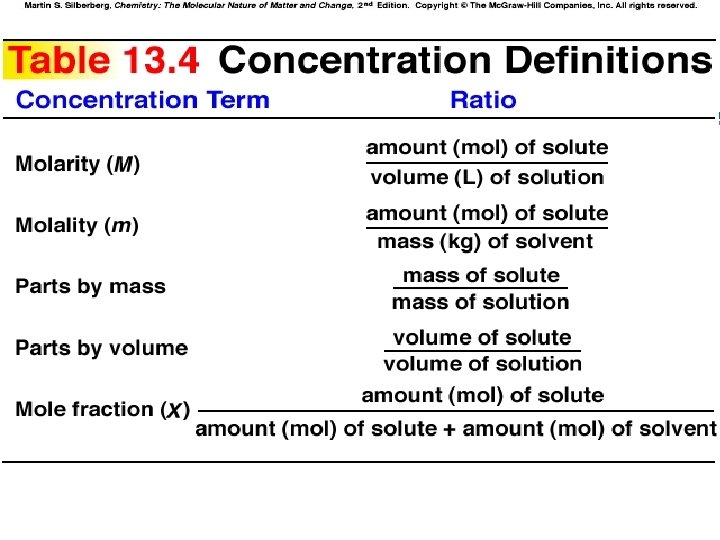
Section 13. 4 Concentrations of Solutions 1. 2. Be able to convert from one concentration unit to another: • Molarity: Review Section 3. 5 • Molality • Mass Fraction and Mass Percent • Mole fraction and Mole Percent Practice!!


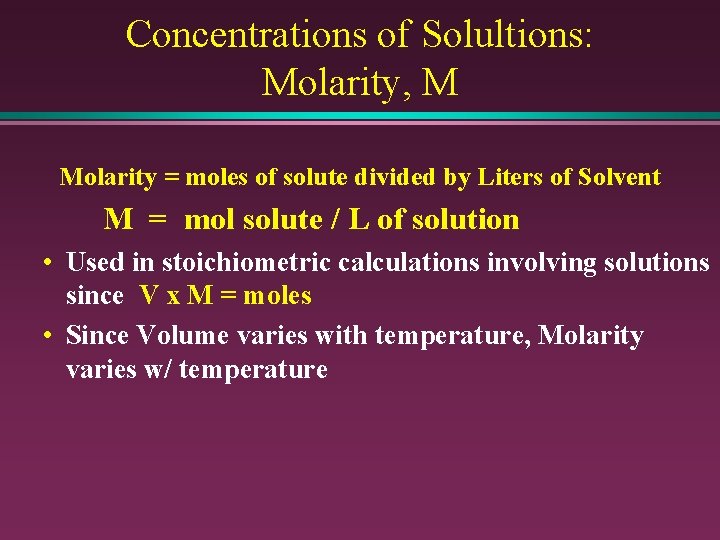
Concentrations of Solultions: Molarity, M Molarity = moles of solute divided by Liters of Solvent M = mol solute / L of solution • Used in stoichiometric calculations involving solutions since V x M = moles • Since Volume varies with temperature, Molarity varies w/ temperature

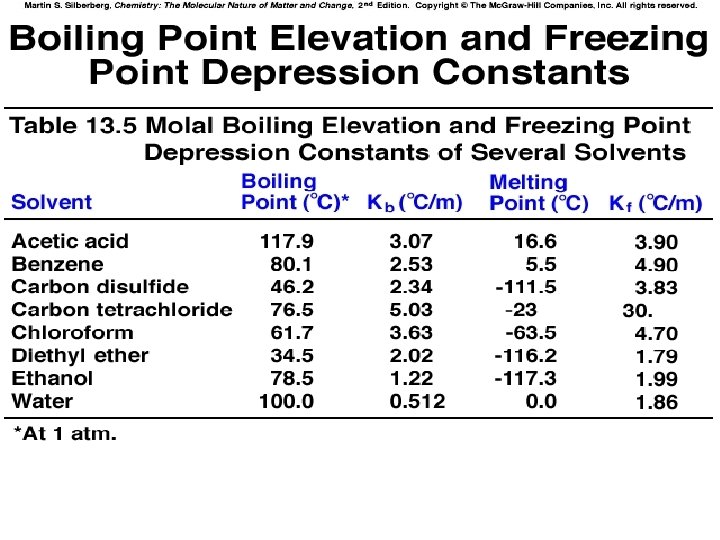
Concentrations of Solutions: Molality, m 1. Molality =Moles of solute divided by kg of Solvent m = mol solute / kg solvent 2. Does not change with Temp. – Used in BP elevation and FP depression calculations

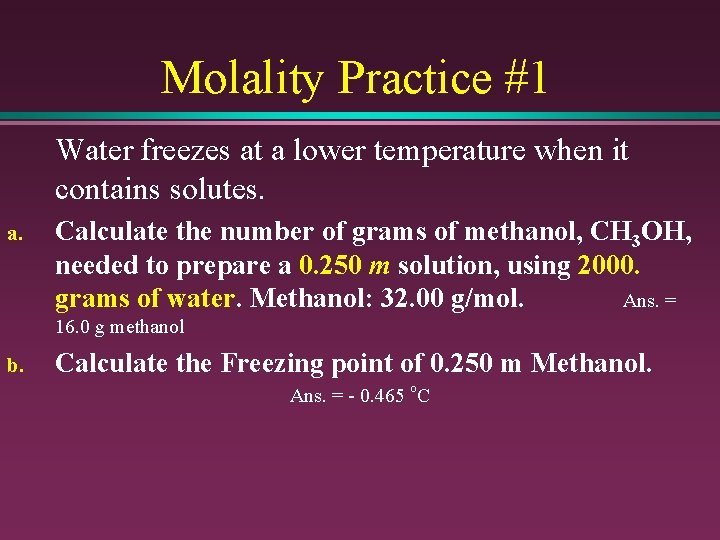
Molality Practice #1 Water freezes at a lower temperature when it contains solutes. a. Calculate the number of grams of methanol, CH 3 OH, needed to prepare a 0. 250 m solution, using 2000. grams of water. Methanol: 32. 00 g/mol. Ans. = 16. 0 g methanol b. Calculate the Freezing point of 0. 250 m Methanol. Ans. = - 0. 465 o. C

FP Lowering DTf = Kf m DTf = amount FP is lowered Kf = Freezing point depession constant Kf is solvent Dependent o 1. 86 C/m for water o 5. 07 C/m for benzene o 20. 0 C/m for cyclohexane

Molality Practice #2 a. If you prepare a solution by dissolving 4. 00 g of Na. OH in 250. g of water, what is the molality of the solution? Na. OH: 40. 00 g/mol Ans. = 0. 400 m b. At what temperature would the solution boil? o Ans. = 100. 2 C

BP Elevation DTb = Kb m • Kb = Boiling point elevation constant • Kb is solvent Dependent o 0. 51 C/m for water o 2. 53 C/m for benzene o 2. 69 C/m for cyclohexane

Parts by Mass (or Percent by Mass) 1. Mass of component divided by total mass of solution Mass % = (msolute / m solution) x 100 2. Also known as weight percent: w/w or % w/w

Mass Percent Practice #1 How many grams of Na. OH are needed to prepare 250. 0 g of 1. 00% Na. OH in water? Ans. = 2. 50 g Na. OH How many grams of water are needed? Ans. = 247. 5 g How many m. L of water at 20. 0 o. C are needed? Ans. = 250. 455 m. L a. b. c. • d. dwater at 20. 0 o. C = 0. 9882 g/m. L What is the molality of the solution? Ans. = 0. 2525 m Na. OH d. What is the approximate FP of the solution? Ans. = -0. 939 o. C

Mass Percent Practice #2 a. Concentrated hydrochloric acid can be purchased from chemical supply houses as a solution that is 37% HCl by mass. HCl = 36. 46 g/mol What mass of conc. HCl is needed to make 1. 0 liter of 0. 1 M HCl? Ans. = 9. 854 or 10 g conc HCl b. How would you make the 0. 1 M HCl solution?

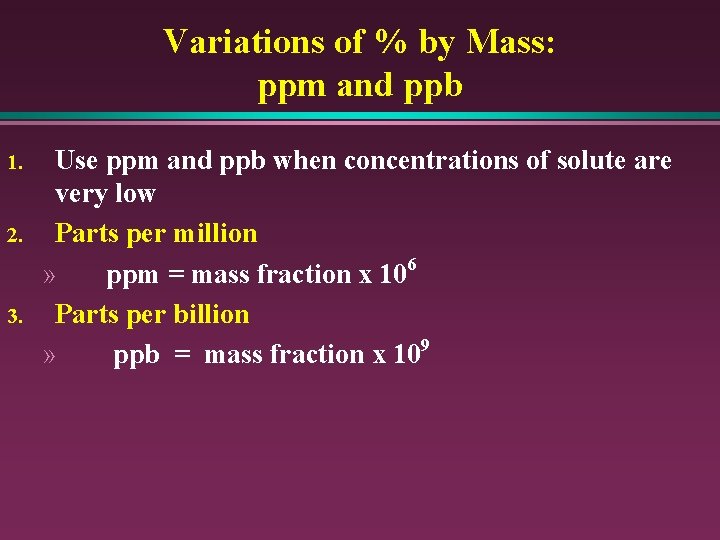
Variations of % by Mass: ppm and ppb 1. 2. 3. Use ppm and ppb when concentrations of solute are very low Parts per million » ppm = mass fraction x 106 Parts per billion » ppb = mass fraction x 109

Concentration Unit Conversion Problems 1. Strategies. . a. Determine the Units of Concentration involved – What are the units you are starting with? – What are the units you are converting to? b. Figure our what conversion factors are needed to go get you to the desired units of concentration

Concentration Unit Conversion Practice #1 a. Calculate the molality 2. 00 % Na. Cl = 58. 4425 g/mol Ans. = 0. 349 m Na. Cl b. How would you prepare • 1. 00 liter of 2. 00 % Na. Cl (w/v)? • 500. m. L of 2. 00 % Na. Cl (w/v)? • 250. m. L of 2. 00 % Na. Cl (w/v)?

Concentration Unit Conversion Practice #2 l Conc. hydrobromic acid can be purchased as 40. 0% HBr. The density of the solution is 1. 38 g/m. L. What is the molar concentration of 40. 0% HBr? HBr = 80. 912 g/mol Ans. = 6. 82 M HBr

Colligative Properties of a solution that depend on the number of solute particles, not on their identity: » Vapor Pressure Lowering » Freezing Point Lowering » Boiling Point Elevation » Osmotic Pressure (will not cover)

Vapor Pressures of Solutions Which is higher, the vapor pressure of salt water or that of pure water?

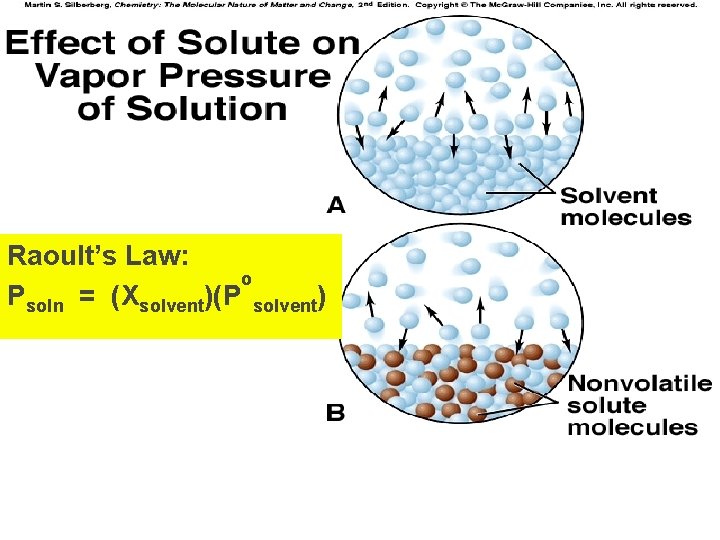
Vapor Pressures of Solutions 1. A solution has a lower vapor pressure than that of the pure solvent (if the solute is nonvolatile). Why? 2. Solute particles impede evaporation, but do not affect condensation

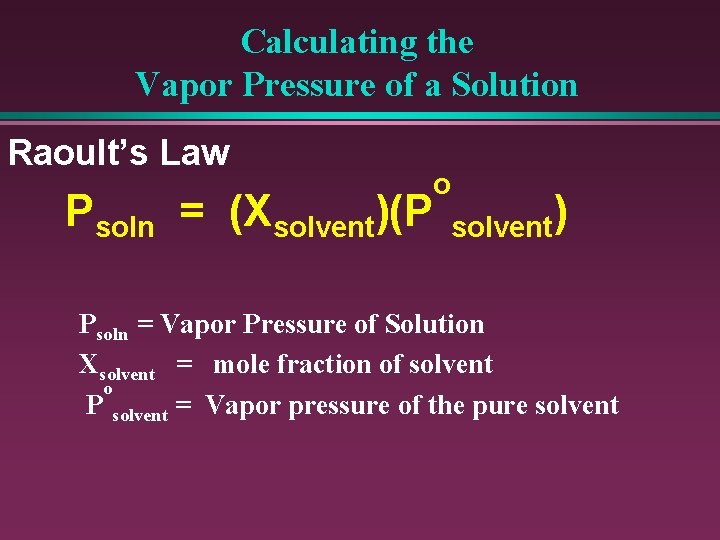
Raoult’s Law: o Psoln = (Xsolvent)(P solvent)

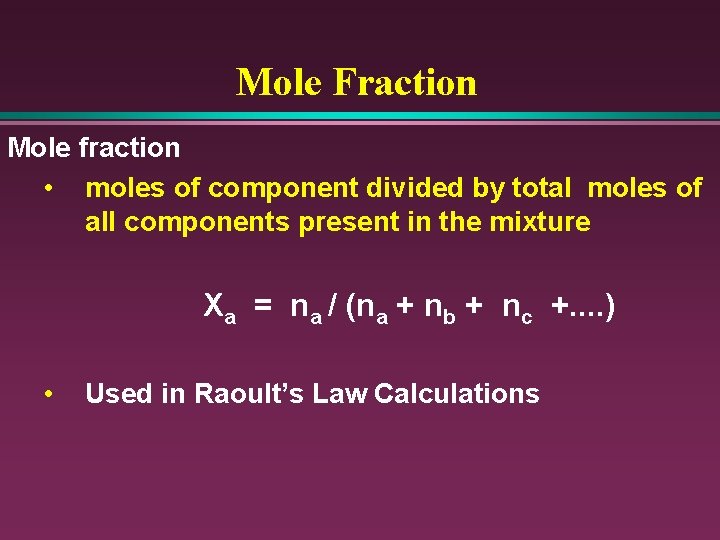
Mole Fraction Mole fraction • moles of component divided by total moles of all components present in the mixture Xa = na / (na + nb + nc +. . ) • Used in Raoult’s Law Calculations

Calculating the Vapor Pressure of a Solution Raoult’s Law Psoln = (Xsolvent)(P o solvent) Psoln = Vapor Pressure of Solution Xsolvent = mole fraction of solvent o P solvent = Vapor pressure of the pure solvent

Raoult’s Law: Practice makes perfect? 1. Dibutyl phthalate, C 16 H 22 O 4 (mw = 278 g/mol), is an oil sometimes worked into plastic articles to give them softness. It has a negligible vapor pressure (P = 1 torr @ 148 o. C). What is the vapor pressure at 20. 0 o. C of a solution of 20. 0 g dibutyl phthalate in 50. 0 g of octane, C 8 H 18 (mw = 114 g/mol)? » The vapor pressure of pure octane at 20. 0 o. C is 10. 5 torr. Ans. = 9. 02 torr



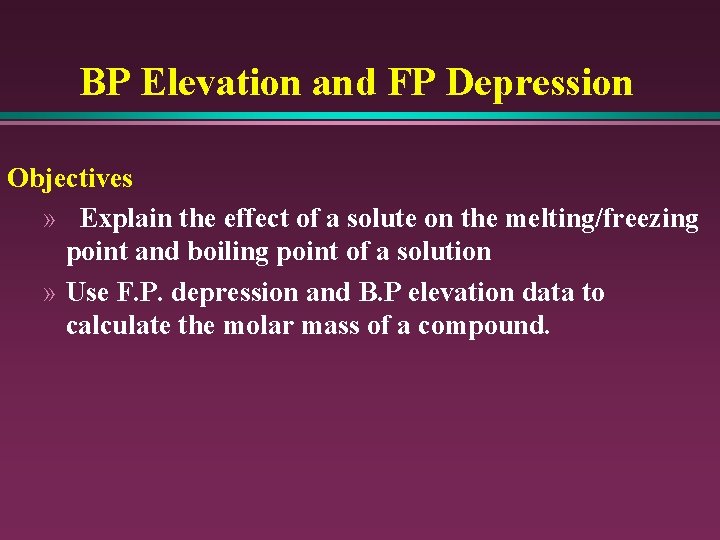
BP Elevation and FP Depression Objectives » Explain the effect of a solute on the melting/freezing point and boiling point of a solution » Use F. P. depression and B. P elevation data to calculate the molar mass of a compound.

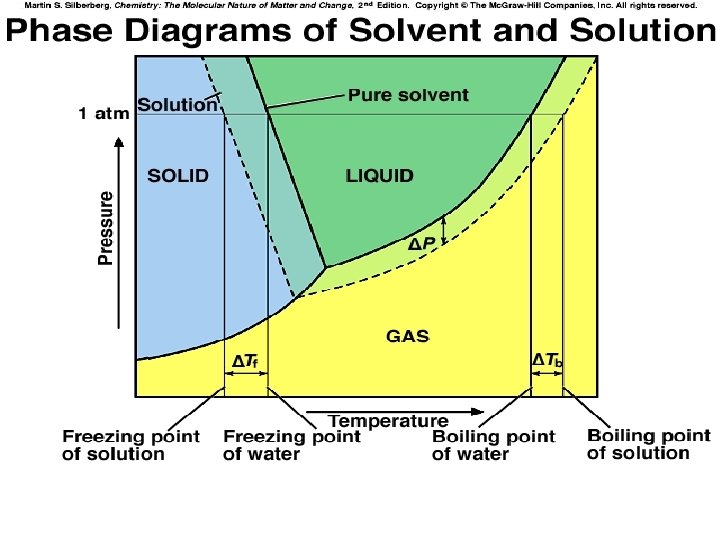
BP Elevation and FP Depression 1. Nonvolatile Solutes Lower the Vapor Pressure of a Solvent (T 11) » Causes…. BP Elevation, DTb FP Depression, DTf » Colligative Properties: Depends on conc. of solute, not identity of solute

BP Elevation 1. DTb = Kb m » Kb = Boiling point elevation constant » Kb is solvent Dependent o 0. 51 C/m for water o 2. 53 C/m for benzene o 2. 69 C/m for cyclohexane

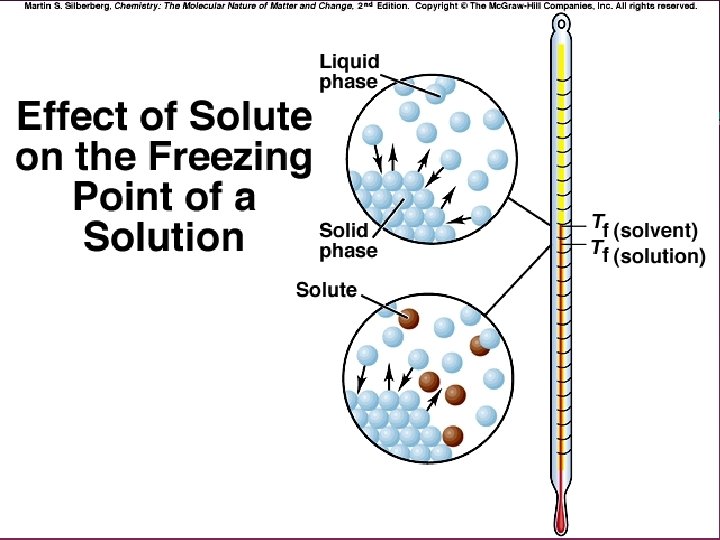
FP Depression DTf = Kf m » Kf = Freezing point depession constant » Kf is solvent Dependent o 1. 86 C/m for water o 5. 07 C/m for benzene o 20. 0 C/m for cyclohexane


Practice makes perfect. . 1. A solution made by dissolving 3. 46 g of an unknown o compound in 85. 0 g of benzene (Kf = 5. 07 C/m, FP = o o 5. 45 C) froze at 4. 13 C. What is the molar mass of the compound? Answer: 157 g/mol 2. At what temperature will a 10. 0 % aqueous sucrose, C 12 H 22 O 11, solution boil? Answer: 100. 60 o. C

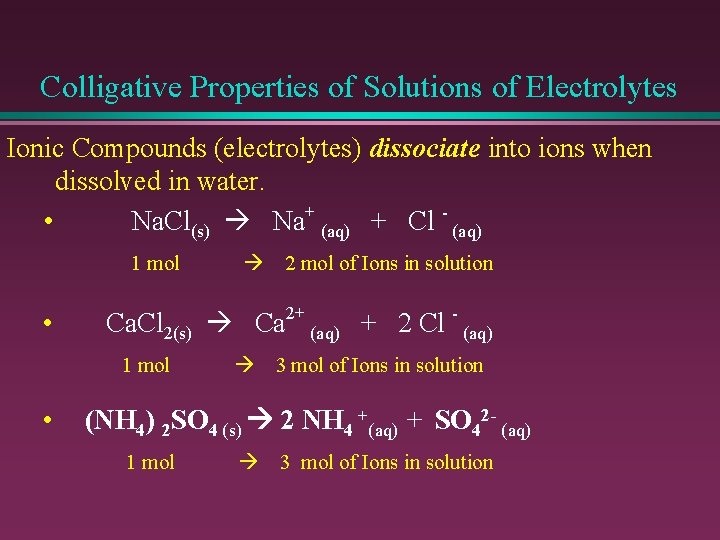
Colligative Properties of Solutions of Electrolytes Ionic Compounds (electrolytes) dissociate into ions when dissolved in water. • Na. Cl(s) Na+ (aq) + Cl - (aq) 1 mol • 2 mol of Ions in solution Ca. Cl 2(s) Ca 2+ (aq) + 2 Cl - (aq) 1 mol • 3 mol of Ions in solution (NH 4) 2 SO 4 (s) 2 NH 4 +(aq) + SO 42 - (aq) 1 mol 3 mol of Ions in solution


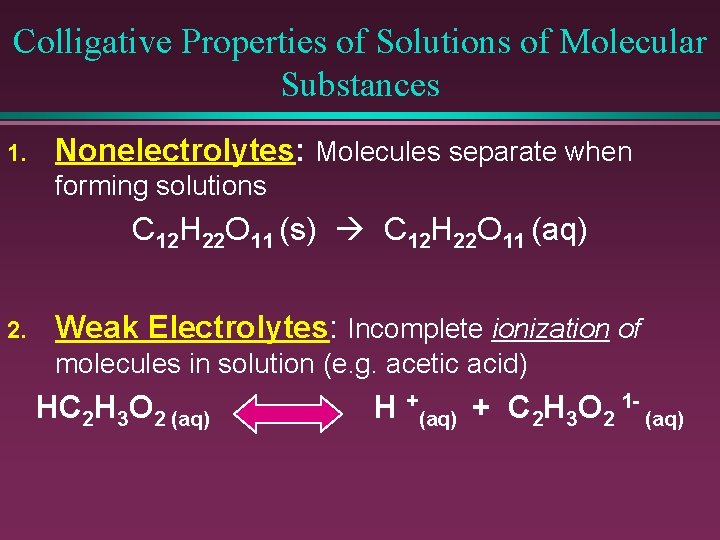
Colligative Properties of Solutions of Molecular Substances 1. Nonelectrolytes: Molecules separate when forming solutions C 12 H 22 O 11 (s) C 12 H 22 O 11 (aq) 2. Weak Electrolytes: Incomplete ionization of molecules in solution (e. g. acetic acid) HC 2 H 3 O 2 (aq) H +(aq) + C 2 H 3 O 2 1 - (aq)

Predicting Freezing Points Estimate the FP of the following aqueous solutions o DTf = Kf m Kf for water = 1. 86 C/m) 1. 1. 00 m sucrose, C 12 H 22 O 11 2. 2. 00 m sucrose, C 12 H 22 O 11 3. 1. 00 m Na. Cl 4. 1. 00 m Mg. SO 4 5. 1. 00 m HCl 6. 1. 00 m Acetic Acid, HC 2 H 3 O 2

Why are the expected FP’s higher than the observed values for some solutions? Expected 1. 2. 3. 4. 5. 6. 1. 00 m sucrose: 2. 00 m sucrose: 1. 00 m Na. Cl: 1. 00 m Mg. SO 4: 1. 00 m HCl: 1. 00 m HC 2 H 3 O 2: o Observed o -1. 86 C vs. -1. 86 C o o -3. 72 C vs. -3. 72 C o o -3. 72 C vs. -3. 53 C o o -3. 72 C vs. -2. 42 C o o -3. 72 C vs. -3. 53 C o o -1. 86 C vs. -1. 90 C

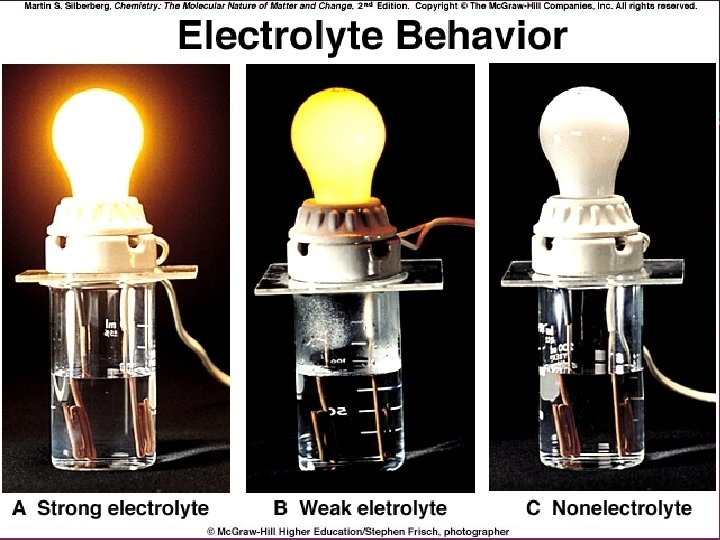
Effects of Interionic Attractions 1. 2. Dissociation into ions is not 100% Ion pairs exist in solution. . Thus. . » Number of moles of ions in a 1. 0 m Na. Cl solution is not double of the molality » Causes DTb and DTf to be smaller than expected


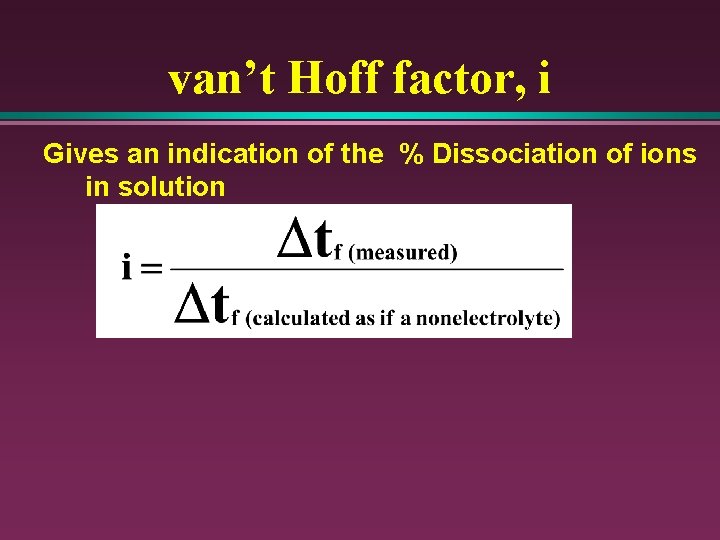
van’t Hoff factor, i Gives an indication of the % Dissociation of ions in solution

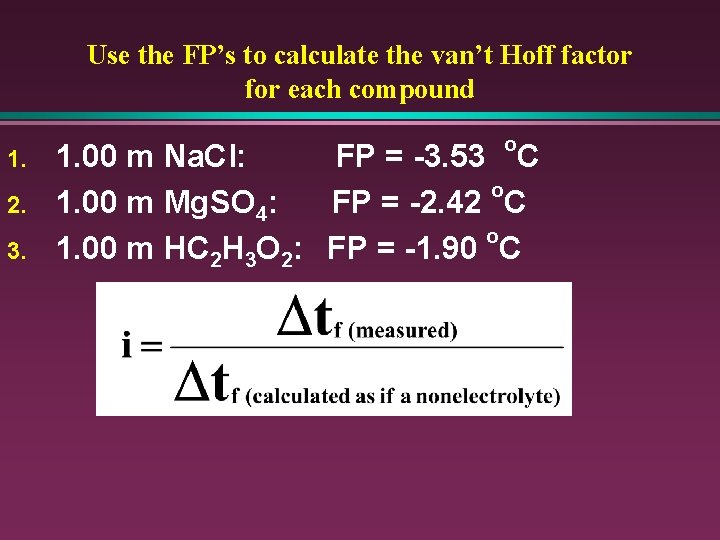
Use the FP’s to calculate the van’t Hoff factor for each compound 1. 2. 3. o 1. 00 m Na. Cl: FP = -3. 53 C o 1. 00 m Mg. SO 4: FP = -2. 42 C o 1. 00 m HC 2 H 3 O 2: FP = -1. 90 C

van’t Hoff factors for ideal solutions If 100 % dissociation, then for a. Na. Cl : i = 2 b. KCl : i = 2 c. Mg. SO 4 : i = 2 d. K 2 SO 4 : i = 3 e. Na 3 PO 4: i = 4

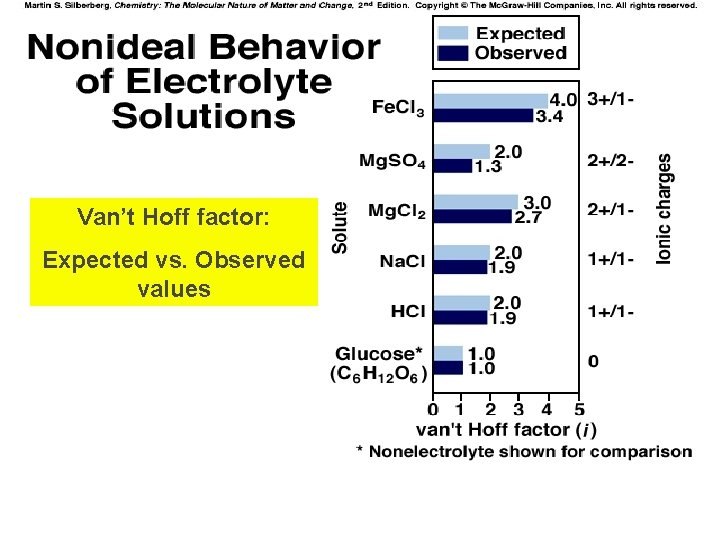
Van’t Hoff factor: Expected vs. Observed values

The End Good luck on your final exams!!!