Lecture 3 Ionisation techniques Gas Phase Ionisation Techniques









- Slides: 33


Lecture 3 Ionisation techniques Gas Phase Ionisation Techniques : Chemical Ionisation

At the end of this lecture you should be able: • To explain how chemical ionisation works • To instruct a MS operator about the type of chemical ionisation reagent gas required for your experiment

Ionisation Techniques: Overview Gas-Phase Methods • Electron Impact (EI) • Chemical Ionization (CI) Desorption Methods • Secondary Ion MS (SIMS) and Liquid SIMS • Fast Atom Bombardment (FAB) • Laser Desorption/Ionization (LDI) • Matrix-Assisted Laser Desorption/Ionization (MALDI) Spray Methods • Atmospheric Pressure Chemical Ionization (APCI) • Electrospray (ESI)

EI: electron ionisation: recap • 1 st step: sample must be in gas phase • 2 nd step: bombarded by electron beam • Generates high-energy analyte ions, which can fragment • Analyte ions are always odd-electron • Advantages: Simple to use, provides librarysearchable fingerprint data • Disadvantages: – Applicable only to volatile (i. e. small) and thermally stable compounds – Extensive fragmentation, can be difficult to detect molecular ion

Chemical ionisation • • • Introduced by Munson and Field 1966 Ion source similar to that for EI Suitable for small, volatile molecules Higher pressures: ca. 1 Torr for ionisation, 10 -4 Torr for injection into mass analyser Generates less energetic, more stable ions CI yields even-electron ions: more stable Mainly molecular ion Simple spectra – But: fragmentation not straightforward Good for mixtures and quantitation Routinely used in gas chromatography (GC-MS)

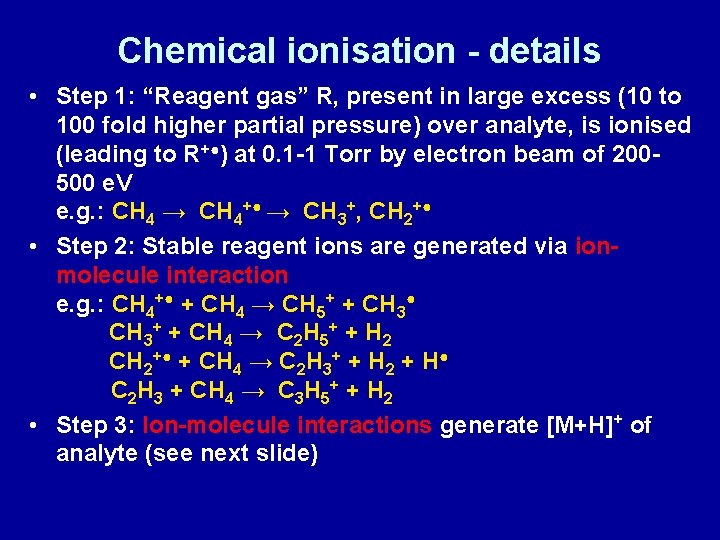
Chemical ionisation - details • Step 1: “Reagent gas” R, present in large excess (10 to 100 fold higher partial pressure) over analyte, is ionised (leading to R+●) at 0. 1 -1 Torr by electron beam of 200500 e. V e. g. : CH 4 → CH 4+● → CH 3+, CH 2+● • Step 2: Stable reagent ions are generated via ionmolecule interaction e. g. : CH 4+● + CH 4 → CH 5+ + CH 3● CH 3+ + CH 4 → C 2 H 5+ + H 2 CH 2+● + CH 4 → C 2 H 3+ + H 2 + H● C 2 H 3 + CH 4 → C 3 H 5+ + H 2 • Step 3: Ion-molecule interactions generate [M+H]+ of analyte (see next slide)

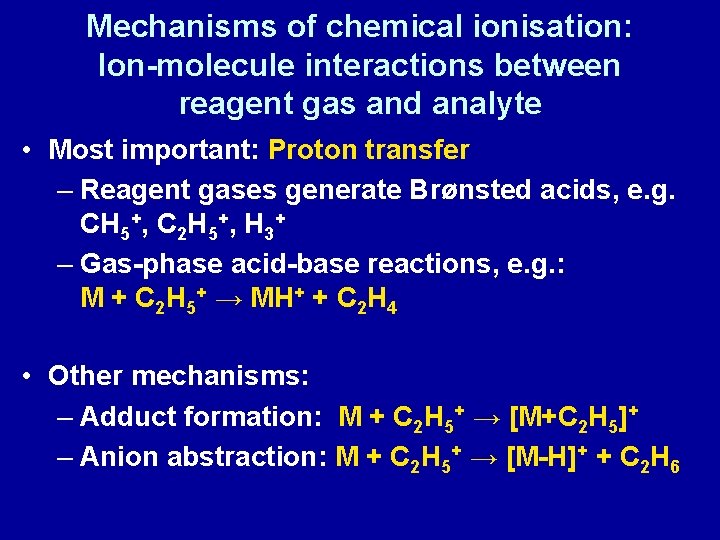
Mechanisms of chemical ionisation: Ion-molecule interactions between reagent gas and analyte • Most important: Proton transfer – Reagent gases generate Brønsted acids, e. g. CH 5+, C 2 H 5+, H 3+ – Gas-phase acid-base reactions, e. g. : M + C 2 H 5+ → MH+ + C 2 H 4 • Other mechanisms: – Adduct formation: M + C 2 H 5+ → [M+C 2 H 5]+ – Anion abstraction: M + C 2 H 5+ → [M-H]+ + C 2 H 6

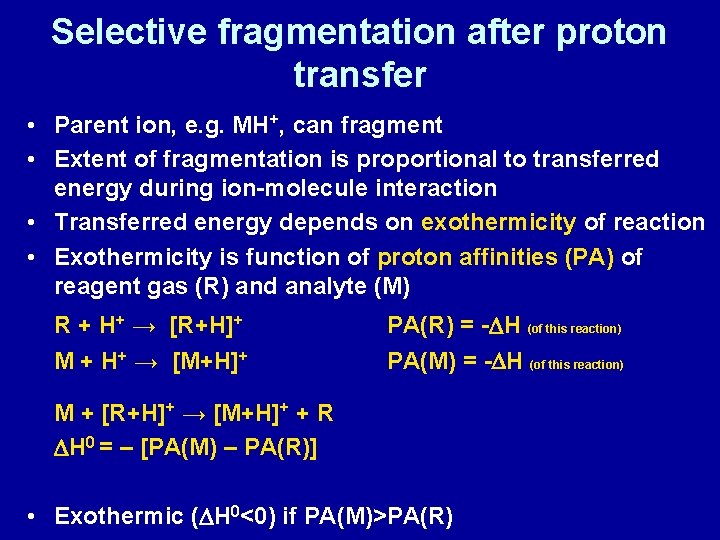
Selective fragmentation after proton transfer • Parent ion, e. g. MH+, can fragment • Extent of fragmentation is proportional to transferred energy during ion-molecule interaction • Transferred energy depends on exothermicity of reaction • Exothermicity is function of proton affinities (PA) of reagent gas (R) and analyte (M) R + H+ → [R+H]+ M + H+ → [M+H]+ PA(R) = -DH (of this reaction) PA(M) = -DH (of this reaction) M + [R+H]+ → [M+H]+ + R DH 0 = – [PA(M) – PA(R)] • Exothermic (DH 0<0) if PA(M)>PA(R)

Proton affinities of common reagent gases (k. J/mole) • • • Methane, CH 4 Ammonia, NH 3 Iso-butane, (CH 3)3 CH Ethane Water Methanol Hydrogen Acetone Methylamine 423 854 819 601 697 761 423 882

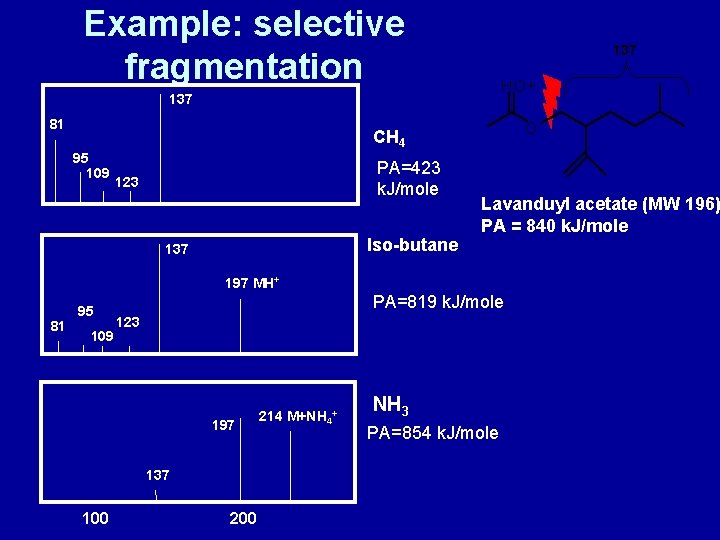
Example: selective fragmentation 137 HO+ 137 81 O CH 4 95 109 PA=423 k. J/mole 123 Iso-butane 137 Lavanduyl acetate (MW 196) PA = 840 k. J/mole 197 MH+ 81 95 109 PA=819 k. J/mole 123 197 137 100 214 M+NH 4+ NH 3 PA=854 k. J/mole

Other modes of CI • Charge-Exchange Chemical Ionisation: with toluene, benzene, NO, CS 2, COS, Xe, CO 2, CO, N 2, Ar, He as reagent gas: – M + X+● → M+● + X (creates radical cations) – Can use mixtures to generate both kinds of ions (conventional CI and CE-CI) • Negative CI: electron capture

Self-assessment questions • Q 1 Describe chemical ionisation mass spectrometry. How does it work, what is the nature of the reagent gas, what function(s) does the gas serve, and what type of mass spectra are generated from the analyte species ? • Q 2 Compare and contrast EI and CI • Q 3 Explain why EI and CI are not applicable to large non-volatile samples. • Q 4 Explain how the choice of reagent gas (eg NH 3 or CH 4) affects the appearance of the mass spectra in chemical ionisation with respect to ionisation by proton transfer.

Lecture 4 Condensed phase ionisation techniques (1): Desorption methods

At the end of this lecture you should be able to: • describe the differences and similarities of SIMS, LSIMS and FAB • explain how laser desorption works • describe MALDI and preparation of samples

Condensed phase ionisation techniques (1): solid state samples • • • Field desorption (FD) Plasma desorption (PD) Secondary-ion Mass Spectrometry (SIMS) Fast Atom Bombardment (FAB) Laser Desorption/Ionisation (LDI) MALDI

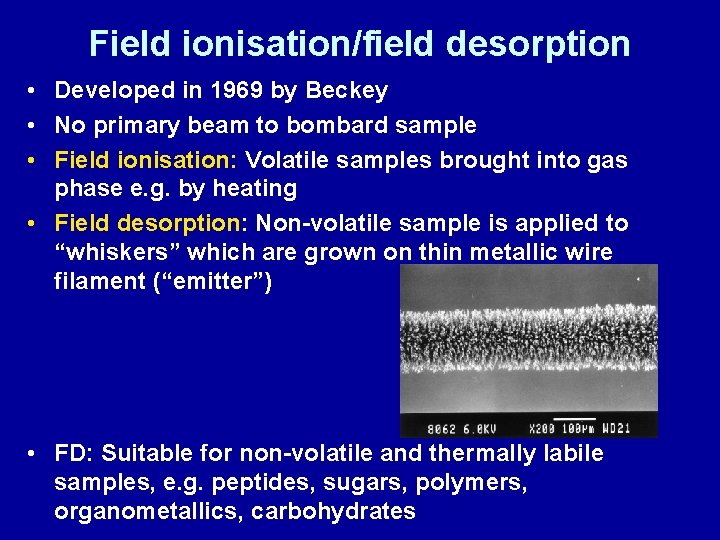
Field ionisation/field desorption • Developed in 1969 by Beckey • No primary beam to bombard sample • Field ionisation: Volatile samples brought into gas phase e. g. by heating • Field desorption: Non-volatile sample is applied to “whiskers” which are grown on thin metallic wire filament (“emitter”) • FD: Suitable for non-volatile and thermally labile samples, e. g. peptides, sugars, polymers, organometallics, carbohydrates

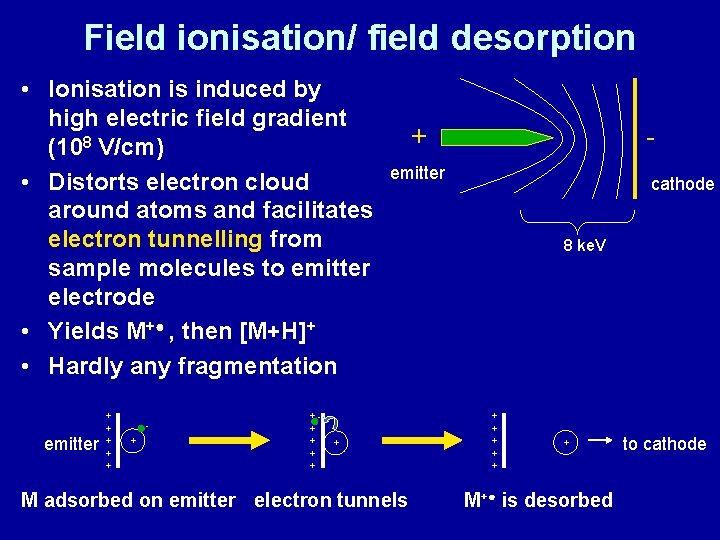
Field ionisation/ field desorption • Ionisation is induced by high electric field gradient (108 V/cm) • Distorts electron cloud around atoms and facilitates electron tunnelling from sample molecules to emitter electrode • Yields M+● , then [M+H]+ • Hardly any fragmentation emitter + + + ++ + - + emitter + M adsorbed on emitter electron tunnels cathode 8 ke. V + + + M+● is desorbed to cathode

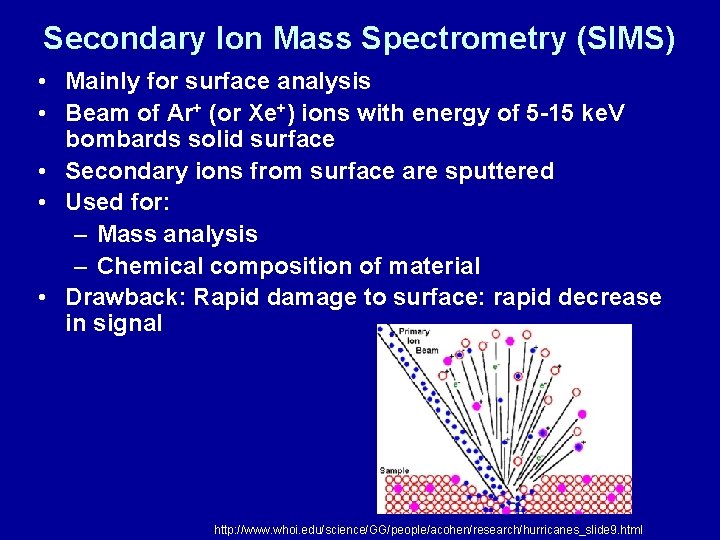
Secondary Ion Mass Spectrometry (SIMS) • Mainly for surface analysis • Beam of Ar+ (or Xe+) ions with energy of 5 -15 ke. V bombards solid surface • Secondary ions from surface are sputtered • Used for: – Mass analysis – Chemical composition of material • Drawback: Rapid damage to surface: rapid decrease in signal http: //www. whoi. edu/science/GG/people/acohen/research/hurricanes_slide 9. html

Variations of SIMS: Fast atom bombardment (FAB) and Liquid SIMS • • FAB: Developed in 1980 by Barber et al. Improved version of SIMS Sample is dissolved in inert liquid matrix Common Matrix: Glycerol (amongst others). Protects sample from destruction and helps ionisation and desorption FAB: Bombardment with high-energy ATOMS (e. g. Xe) LSIMS: Similar, but bombardment with IONS (e. g. Cs+ at 25 -40 ke. V) instead of ATOMS Mass limits: 7 k. Da standard, 24 k. Da possible Often used in conjunction with magnetic sector mass analysers

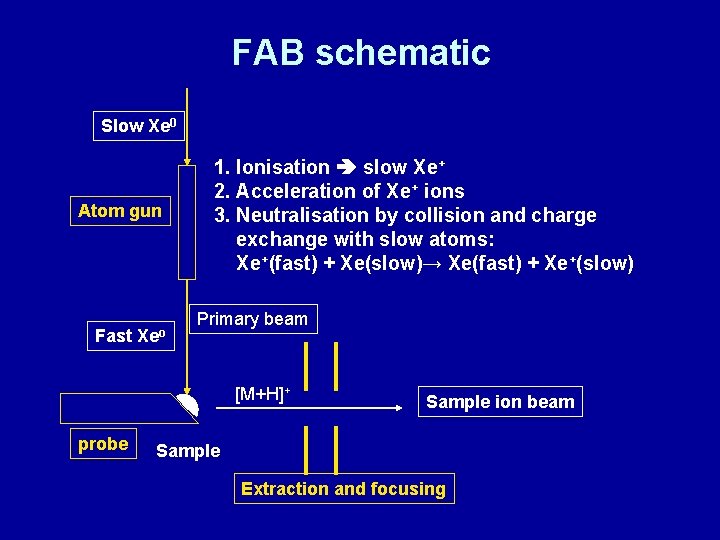
FAB schematic Slow Xe 0 Atom gun Fast Xeo 1. Ionisation slow Xe+ 2. Acceleration of Xe+ ions 3. Neutralisation by collision and charge exchange with slow atoms: Xe+(fast) + Xe(slow)→ Xe(fast) + Xe+(slow) Primary beam [M+H]+ probe Sample ion beam Sample Extraction and focusing

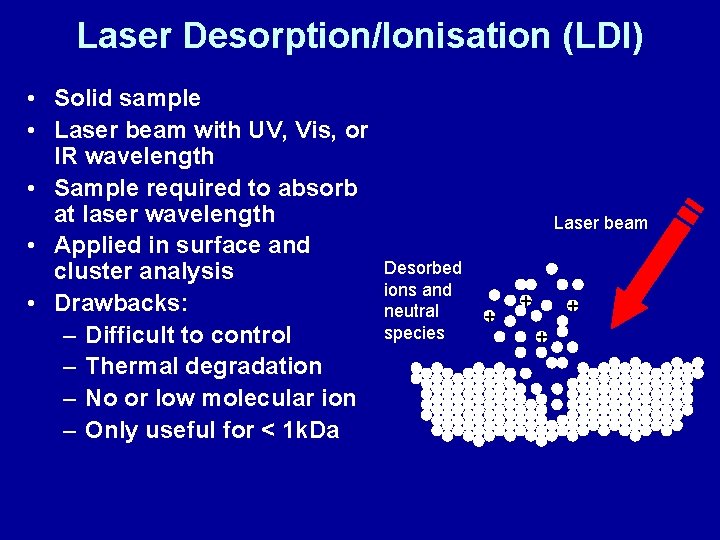
Laser Desorption/Ionisation (LDI) • Solid sample • Laser beam with UV, Vis, or IR wavelength • Sample required to absorb at laser wavelength • Applied in surface and cluster analysis • Drawbacks: – Difficult to control – Thermal degradation – No or low molecular ion – Only useful for < 1 k. Da Laser beam Desorbed ions and neutral species + +

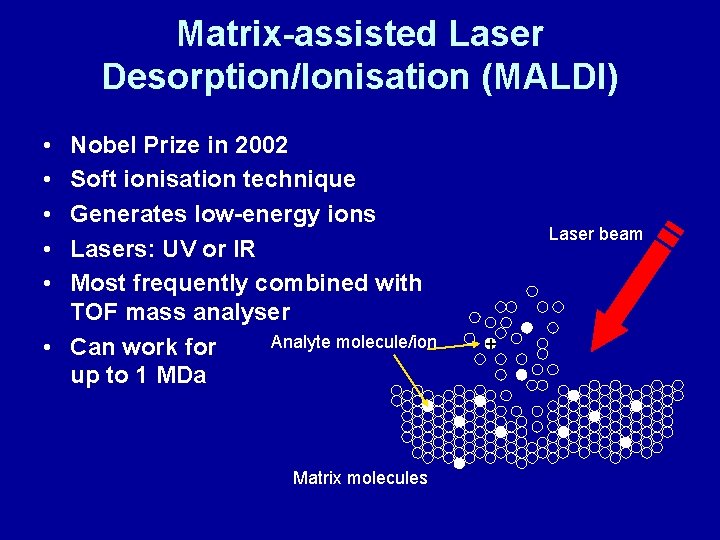
Matrix-assisted Laser Desorption/Ionisation (MALDI) • • • Nobel Prize in 2002 Soft ionisation technique Generates low-energy ions Lasers: UV or IR Most frequently combined with TOF mass analyser Analyte molecule/ion • Can work for up to 1 MDa Matrix molecules Laser beam +

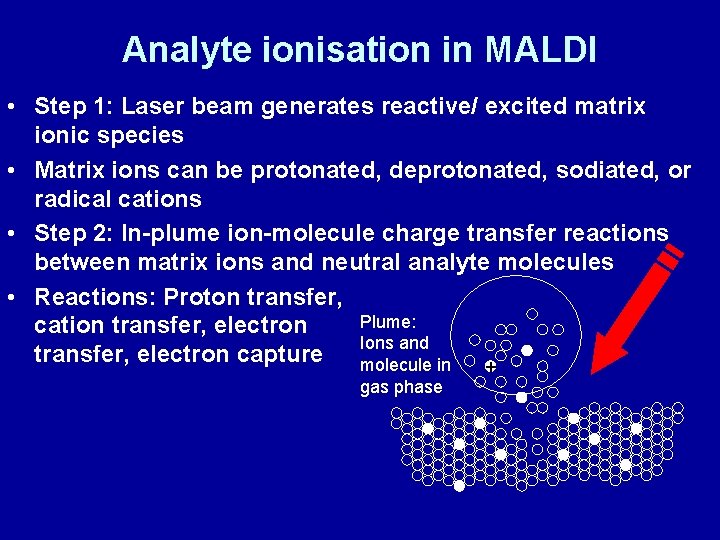
Analyte ionisation in MALDI • Step 1: Laser beam generates reactive/ excited matrix ionic species • Matrix ions can be protonated, deprotonated, sodiated, or radical cations • Step 2: In-plume ion-molecule charge transfer reactions between matrix ions and neutral analyte molecules • Reactions: Proton transfer, Plume: cation transfer, electron Ions and transfer, electron capture molecule in + gas phase

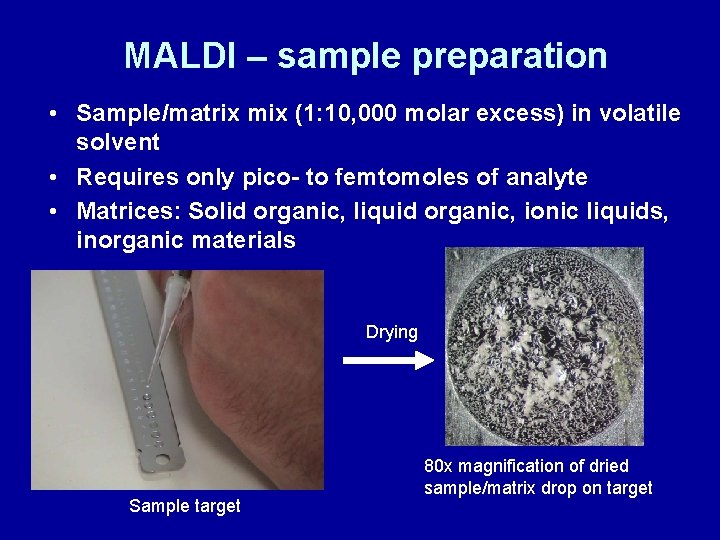
MALDI – sample preparation • Sample/matrix mix (1: 10, 000 molar excess) in volatile solvent • Requires only pico- to femtomoles of analyte • Matrices: Solid organic, liquid organic, ionic liquids, inorganic materials Drying Sample target 80 x magnification of dried sample/matrix drop on target

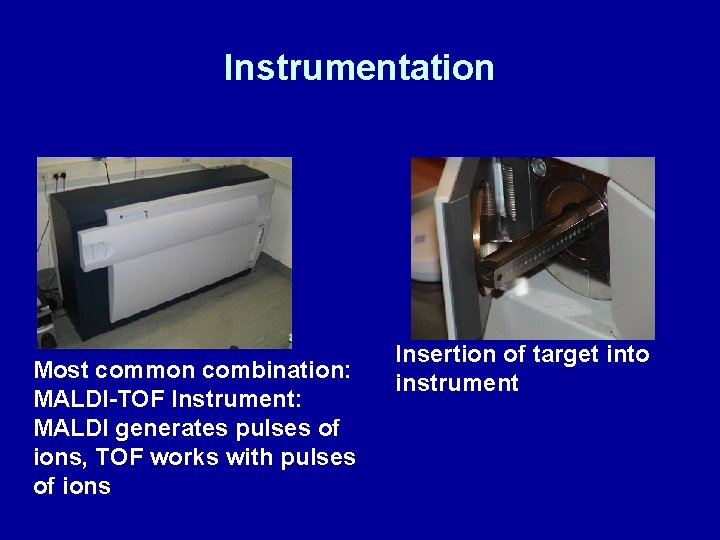
Instrumentation Most common combination: MALDI-TOF Instrument: MALDI generates pulses of ions, TOF works with pulses of ions Insertion of target into instrument

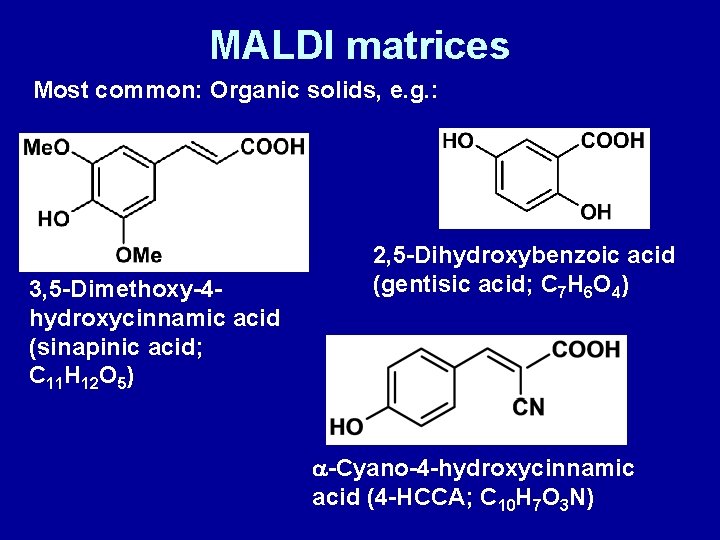
MALDI matrices Most common: Organic solids, e. g. : 3, 5 -Dimethoxy-4 hydroxycinnamic acid (sinapinic acid; C 11 H 12 O 5) 2, 5 -Dihydroxybenzoic acid (gentisic acid; C 7 H 6 O 4) a-Cyano-4 -hydroxycinnamic acid (4 -HCCA; C 10 H 7 O 3 N)

MALDI matrix • absorbs photon energy and transfers it to analyte • minimises aggregation between analyte molecules • Matrix must – Absorb strongly at Laser wavelength – Have low sublimation temperature – Have good mixing and solvent compatibility with analyte – Have ability to participate in photochemical reaction

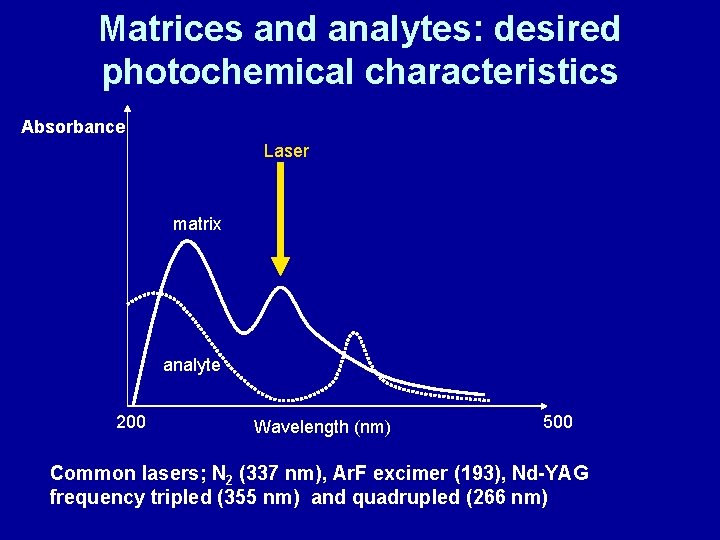
Matrices and analytes: desired photochemical characteristics Absorbance Laser matrix analyte 200 Wavelength (nm) 500 Common lasers; N 2 (337 nm), Ar. F excimer (193), Nd-YAG frequency tripled (355 nm) and quadrupled (266 nm)

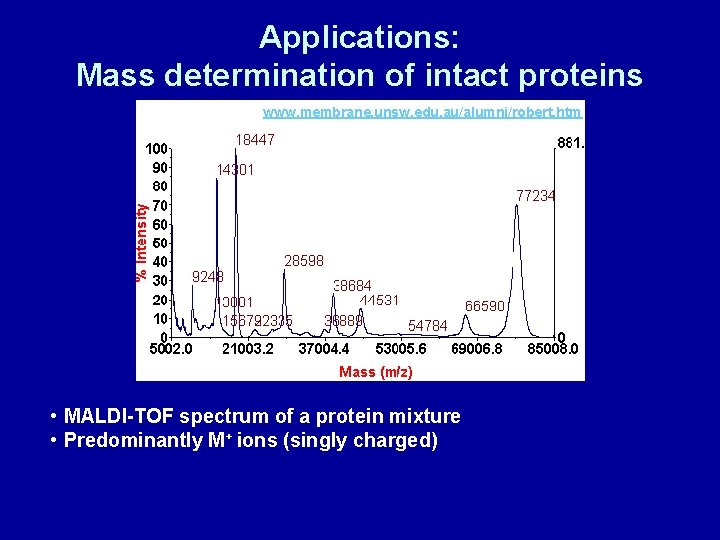
Applications: Mass determination of intact proteins www. membrane. unsw. edu. au/alumni/robert. htm • MALDI-TOF spectrum of a protein mixture • Predominantly M+ ions (singly charged)

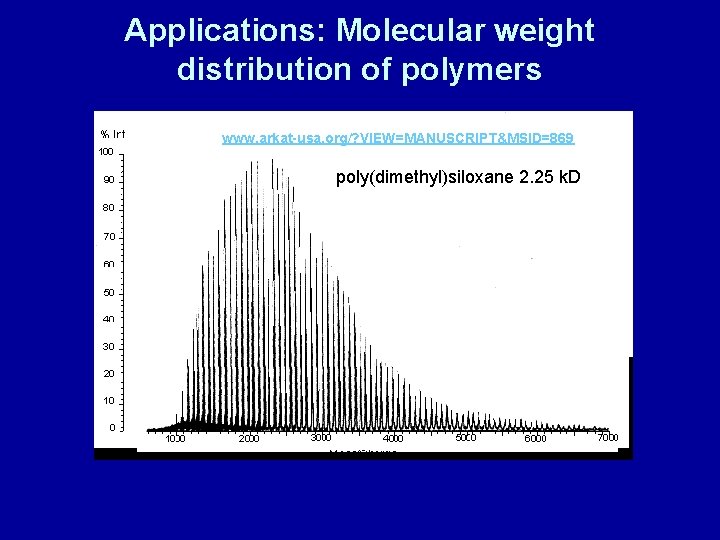
Applications: Molecular weight distribution of polymers www. arkat-usa. org/? VIEW=MANUSCRIPT&MSID=869 poly(dimethyl)siloxane 2. 25 k. D

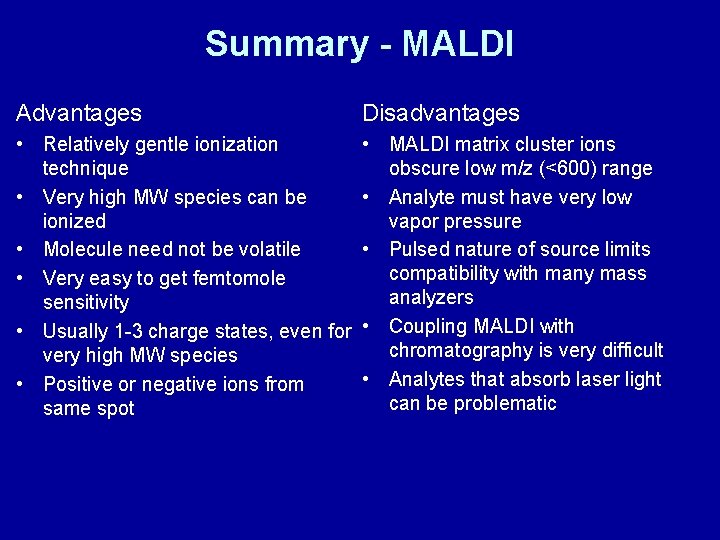
Summary - MALDI Advantages Disadvantages • Relatively gentle ionization technique • Very high MW species can be ionized • Molecule need not be volatile • Very easy to get femtomole sensitivity • Usually 1 -3 charge states, even for very high MW species • Positive or negative ions from same spot • MALDI matrix cluster ions obscure low m/z (<600) range • Analyte must have very low vapor pressure • Pulsed nature of source limits compatibility with many mass analyzers • Coupling MALDI with chromatography is very difficult • Analytes that absorb laser light can be problematic

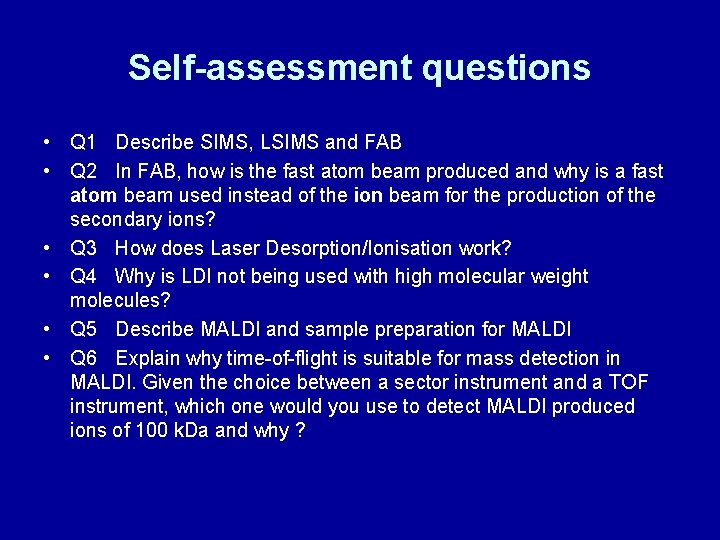
Self-assessment questions • Q 1 Describe SIMS, LSIMS and FAB • Q 2 In FAB, how is the fast atom beam produced and why is a fast atom beam used instead of the ion beam for the production of the secondary ions? • Q 3 How does Laser Desorption/Ionisation work? • Q 4 Why is LDI not being used with high molecular weight molecules? • Q 5 Describe MALDI and sample preparation for MALDI • Q 6 Explain why time-of-flight is suitable for mass detection in MALDI. Given the choice between a sector instrument and a TOF instrument, which one would you use to detect MALDI produced ions of 100 k. Da and why ?