Gas Exchange and Pulmonary Circulation Gas Pressure Gas









- Slides: 27

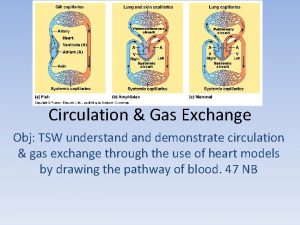
Gas Exchange and Pulmonary Circulation

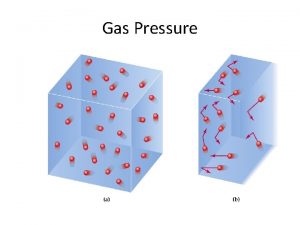
Gas Pressure • Gas pressure is caused by the molecules colliding with the surface. • In the lungs, the gas molecules are colliding with the surfaces of the respiratory passages and alveoli. • Higher concentrations of gas will produce more collisions and cause a higher pressure. • This idea of pressure applies to gases whether in air or water.

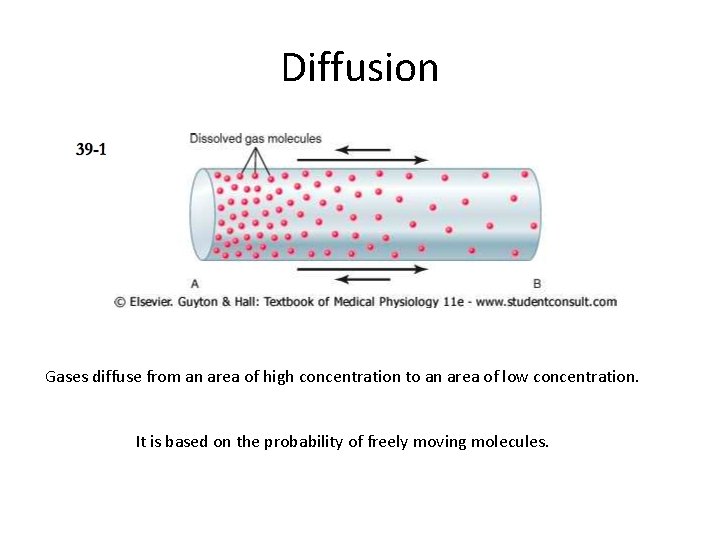
Diffusion Gases diffuse from an area of high concentration to an area of low concentration. It is based on the probability of freely moving molecules.

Direction of Diffusion • The net diffusion is determined by the difference between the partial pressures. • If the partial pressure of O 2 is greater in the alveolar air than in the blood, the net diffusion of O 2 will be into the blood.

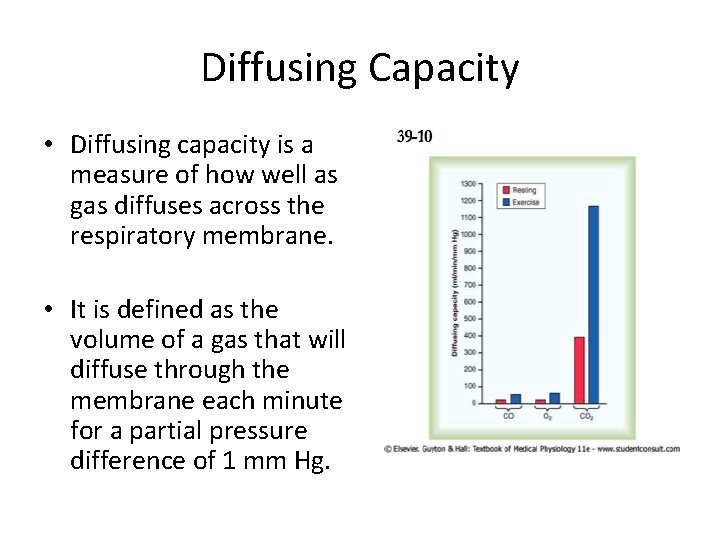
Diffusing Capacity • Diffusing capacity is a measure of how well as gas diffuses across the respiratory membrane. • It is defined as the volume of a gas that will diffuse through the membrane each minute for a partial pressure difference of 1 mm Hg.

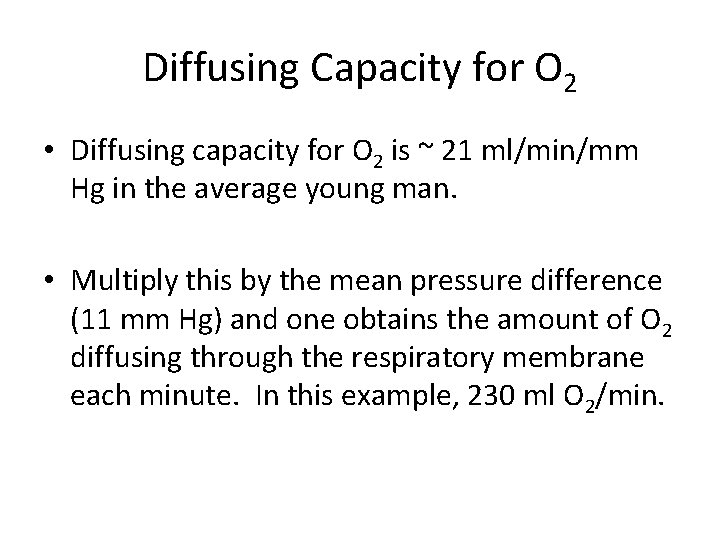
Diffusing Capacity for O 2 • Diffusing capacity for O 2 is ~ 21 ml/min/mm Hg in the average young man. • Multiply this by the mean pressure difference (11 mm Hg) and one obtains the amount of O 2 diffusing through the respiratory membrane each minute. In this example, 230 ml O 2/min.

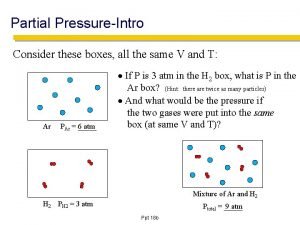
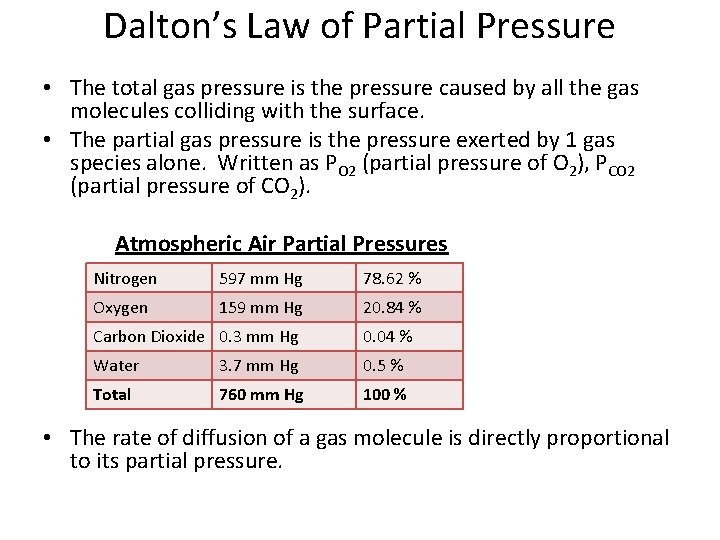
Dalton’s Law of Partial Pressure • The total gas pressure is the pressure caused by all the gas molecules colliding with the surface. • The partial gas pressure is the pressure exerted by 1 gas species alone. Written as PO 2 (partial pressure of O 2), PCO 2 (partial pressure of CO 2). Atmospheric Air Partial Pressures Nitrogen 597 mm Hg 78. 62 % Oxygen 159 mm Hg 20. 84 % Carbon Dioxide 0. 3 mm Hg 0. 04 % Water 3. 7 mm Hg 0. 5 % Total 760 mm Hg 100 % • The rate of diffusion of a gas molecule is directly proportional to its partial pressure.

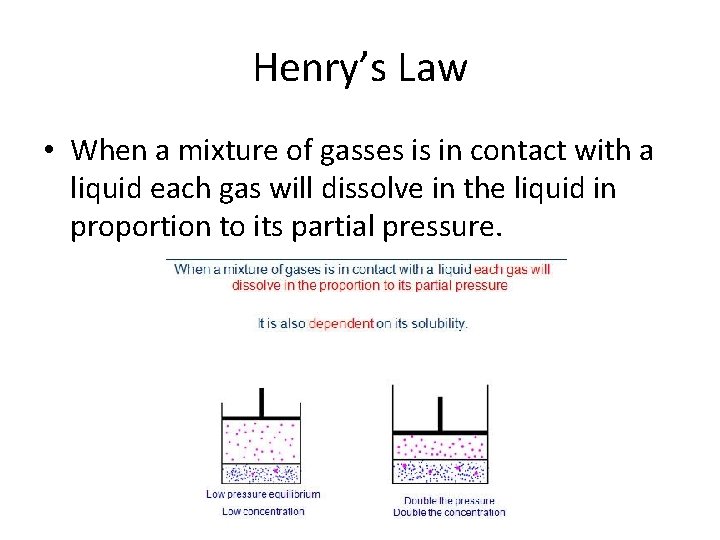
Henry’s Law • When a mixture of gasses is in contact with a liquid each gas will dissolve in the liquid in proportion to its partial pressure.

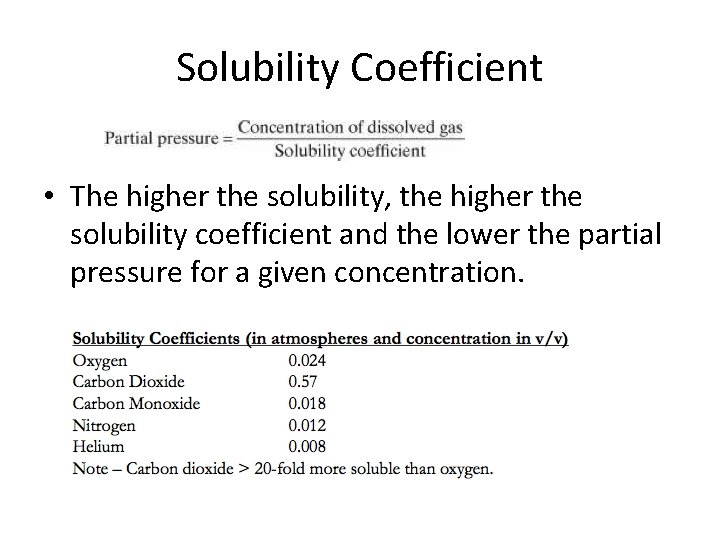
Solubility Coefficient • The higher the solubility, the higher the solubility coefficient and the lower the partial pressure for a given concentration.

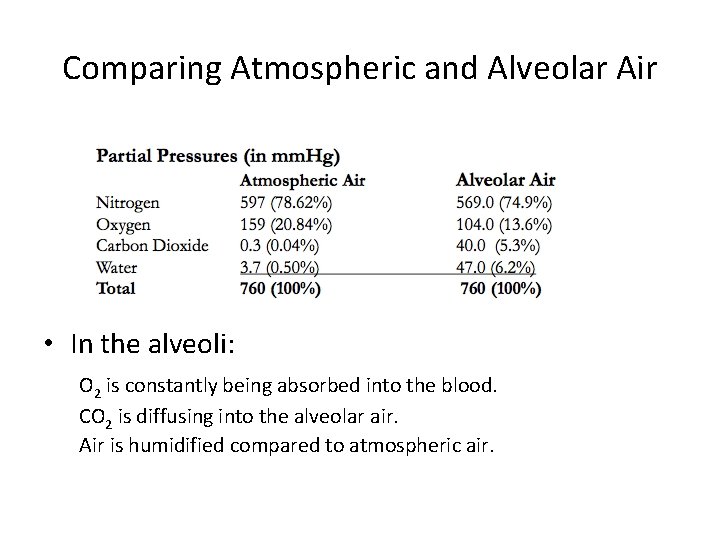
Comparing Atmospheric and Alveolar Air • In the alveoli: O 2 is constantly being absorbed into the blood. CO 2 is diffusing into the alveolar air. Air is humidified compared to atmospheric air.

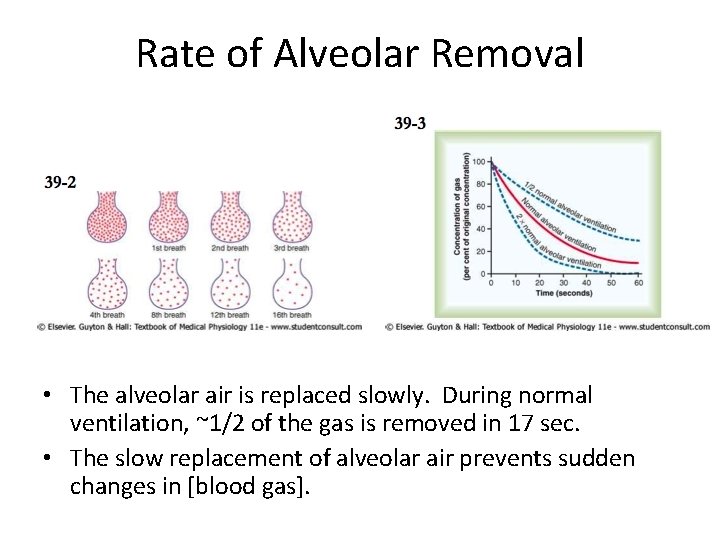
Rate of Alveolar Removal • The alveolar air is replaced slowly. During normal ventilation, ~1/2 of the gas is removed in 17 sec. • The slow replacement of alveolar air prevents sudden changes in [blood gas].

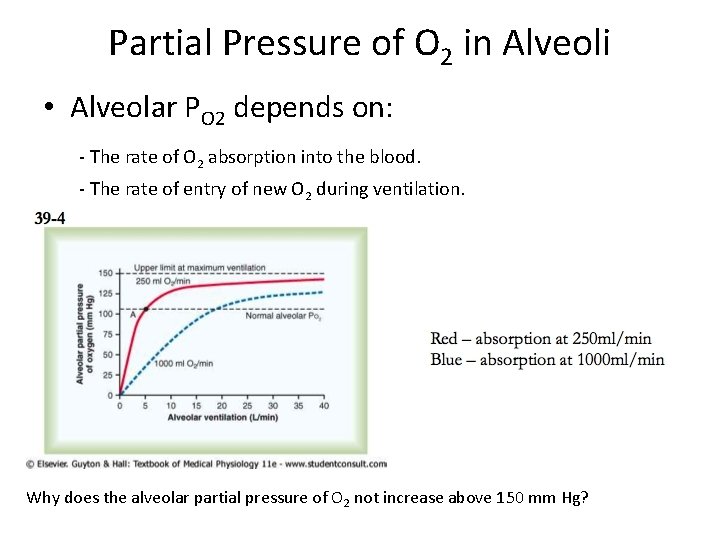
Partial Pressure of O 2 in Alveoli • Alveolar PO 2 depends on: - The rate of O 2 absorption into the blood. - The rate of entry of new O 2 during ventilation. Why does the alveolar partial pressure of O 2 not increase above 150 mm Hg?

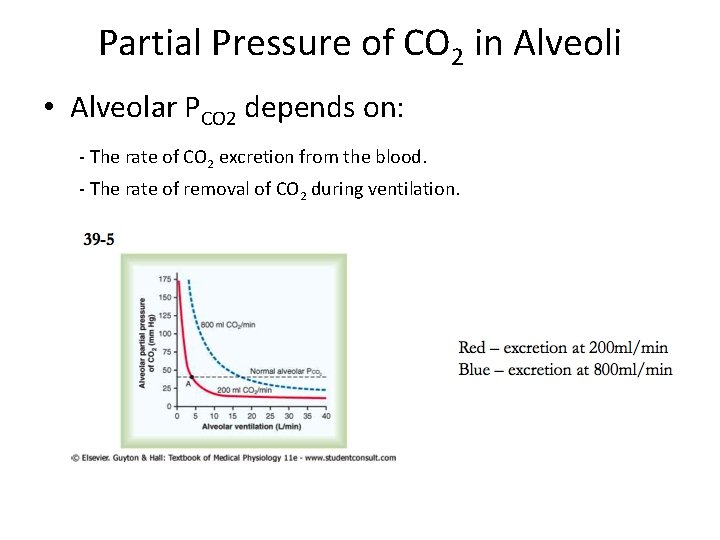
Partial Pressure of CO 2 in Alveoli • Alveolar PCO 2 depends on: - The rate of CO 2 excretion from the blood. - The rate of removal of CO 2 during ventilation.

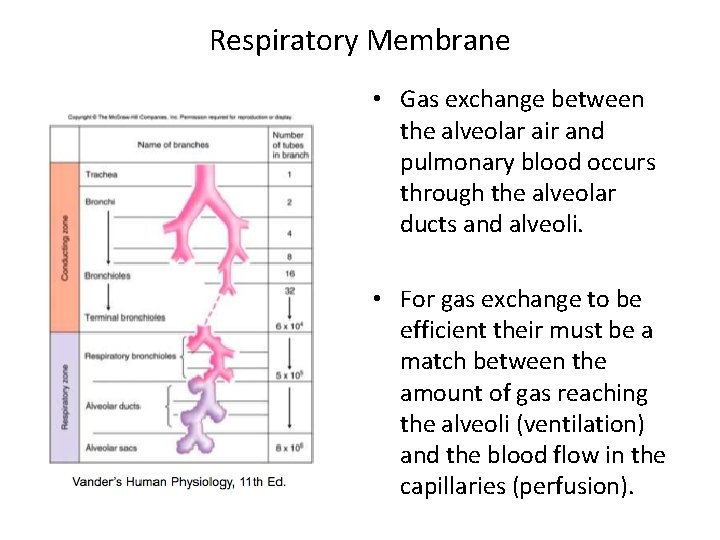
Respiratory Membrane • Gas exchange between the alveolar air and pulmonary blood occurs through the alveolar ducts and alveoli. • For gas exchange to be efficient their must be a match between the amount of gas reaching the alveoli (ventilation) and the blood flow in the capillaries (perfusion).

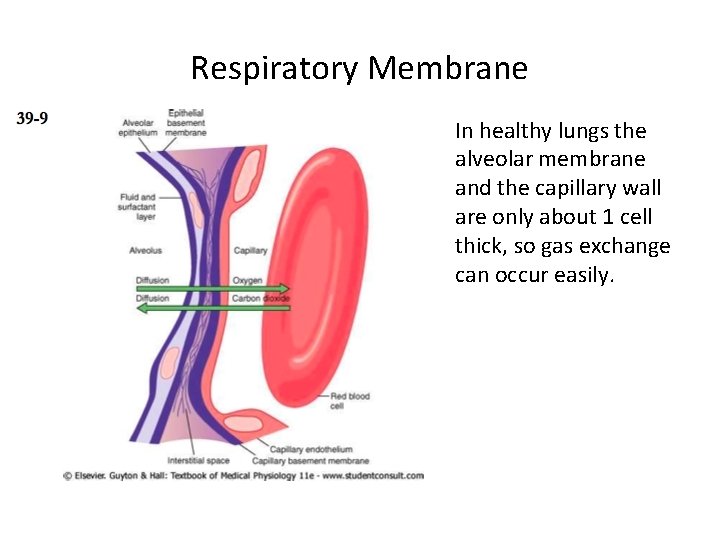
Respiratory Membrane In healthy lungs the alveolar membrane and the capillary wall are only about 1 cell thick, so gas exchange can occur easily.

Factors Affecting Diffusion through the Respiratory Membrane • Thickness of the membrane. • Surface area of the membrane. • Diffusion coefficient. • Difference in partial pressure.

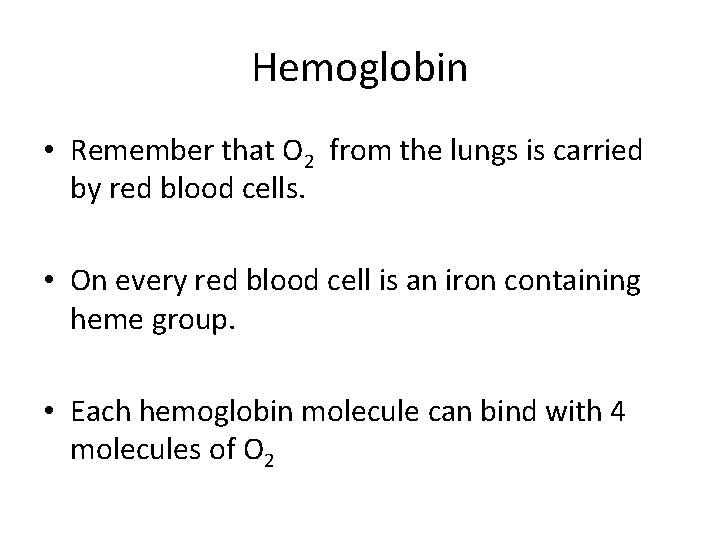
Hemoglobin • Remember that O 2 from the lungs is carried by red blood cells. • On every red blood cell is an iron containing heme group. • Each hemoglobin molecule can bind with 4 molecules of O 2

Hemoglobin • A hemoglobin with an oxygen is called an oxyhemoglobin. (Hb. O 2) • A hemoglobin that has released it’s oxygen is called a reduced or deoxyhemoglobin. (HHb)

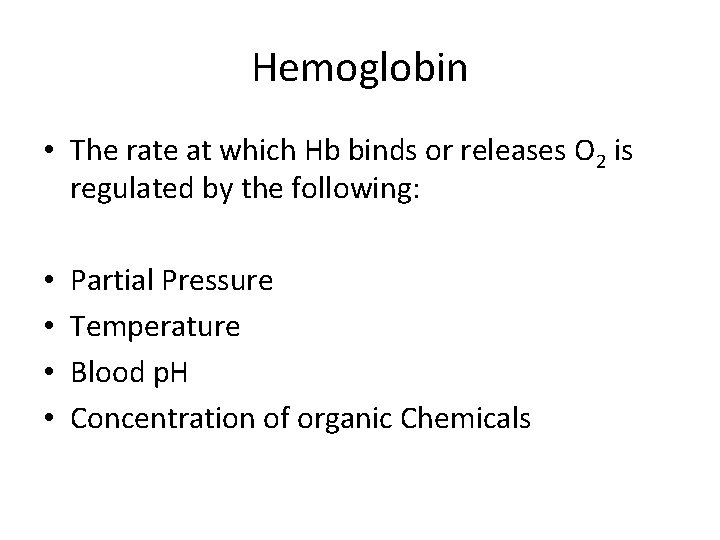
Hemoglobin • The rate at which Hb binds or releases O 2 is regulated by the following: • • Partial Pressure Temperature Blood p. H Concentration of organic Chemicals

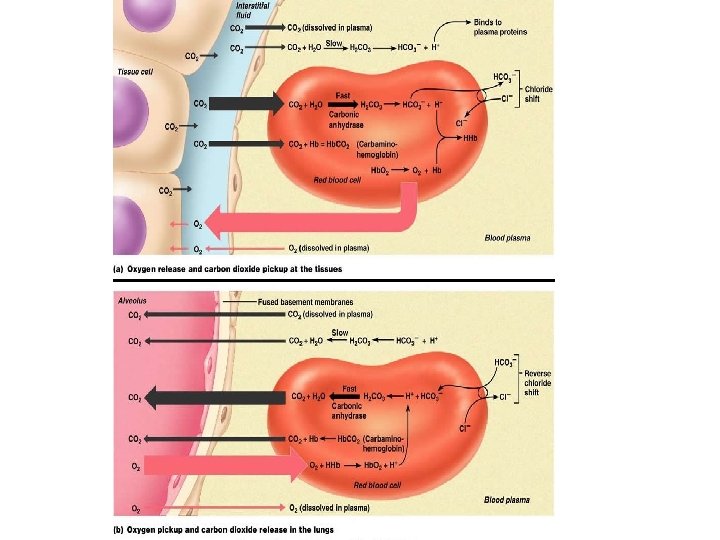
CO 2 Transport • A normal body cell produces 200 ml of carbon dioxide each minute. • Blood transports CO 2 from the tissues to the lungs in three forms – Dissolved in the plasma (7 -10%) – Bound to hemoglobin (roughly 20%) – As a bicarbonate ion in plasma (70%)

From CO 2 to Bicarbonate • CO 2 enters the plasma and then enters the RBC to be turned into Bicarbonate. • When CO 2 enters the blood cell it combine with water to make carbonic acid. • Carbonic acid is unstable and quickly disassociates into hydrogen ions and bicarbonate.


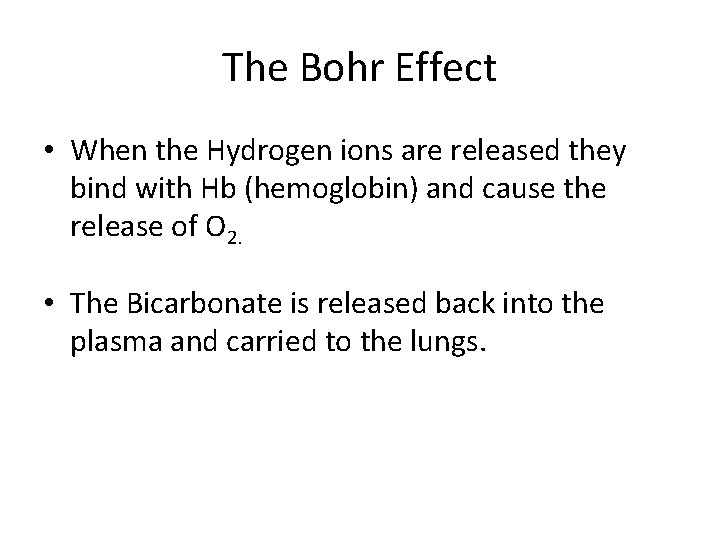
The Bohr Effect • When the Hydrogen ions are released they bind with Hb (hemoglobin) and cause the release of O 2. • The Bicarbonate is released back into the plasma and carried to the lungs.

From Bicarbonate to CO 2 • Once in the lungs the Bicarbonate returns to the RBC and the whole process is reversed producing CO 2, which you exhale.


Buffer System • The process of turning carbonic acid into bicarbonate and visa versa is how your body deals with p. H shifts. • If too many H+ are present bicarbonate in the plasma will bond with it forming carbonic acid. • If H+ are too low then carbonic acid will disassociate and release the hydrogen ions.

Acidosis/Alkalosis • Too much CO 2 in the blood will result in more carbonic acid and there fore a lower p. H level. • If prolonged this will cause acidosis and organ failure can occur. • Not enough CO 2 and the blood p. H will rise causing Alkalosis.
 Bronchial circulation and pulmonary circulation
Bronchial circulation and pulmonary circulation Chapter 42 circulation and gas exchange
Chapter 42 circulation and gas exchange What is the physiology of respiration
What is the physiology of respiration Single circulation and double circulation
Single circulation and double circulation Single circulation and double circulation
Single circulation and double circulation Gas exchange key events in gas exchange
Gas exchange key events in gas exchange Pulmonary circulation pathway
Pulmonary circulation pathway Major veins of the body
Major veins of the body Pa systolic pressure range
Pa systolic pressure range Mean pulmonary arterial pressure
Mean pulmonary arterial pressure Static pressure and dynamic pressure
Static pressure and dynamic pressure Osmolality vs osmolarity
Osmolality vs osmolarity High pressure and low pressure
High pressure and low pressure Tiefdruckgebiet
Tiefdruckgebiet Addison currency exchange
Addison currency exchange Pearl exchange activity
Pearl exchange activity Elmslie trillat
Elmslie trillat Pressure support vs pressure control
Pressure support vs pressure control Continuous bedside pressure mapping
Continuous bedside pressure mapping Intrapleural pressure
Intrapleural pressure Starling forces
Starling forces Hypergraph containers
Hypergraph containers Tripod position breathing
Tripod position breathing Regional metamorphism
Regional metamorphism Sore throat after surgery
Sore throat after surgery Capillary filtration coefficient
Capillary filtration coefficient Oncotic pressure vs hydrostatic pressure
Oncotic pressure vs hydrostatic pressure Hydrostatic oncotic pressure
Hydrostatic oncotic pressure