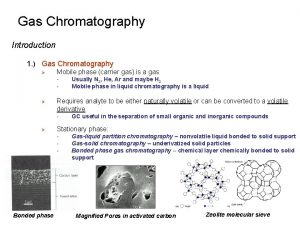
Gas Chromatography GC Gas Chromatograph GC COMPONENTS Gas















![Chemical Ionization �Heated sample. �+ [M+H]+ often visible, less fragmentation than EI or [M-H]+ Chemical Ionization �Heated sample. �+ [M+H]+ often visible, less fragmentation than EI or [M-H]+](https://slidetodoc.com/presentation_image_h2/470fc1dd8db06b748f256e48ebdf4be4/image-58.jpg)


- Slides: 73

Gas Chromatography GC

Gas Chromatograph � GC COMPONENTS

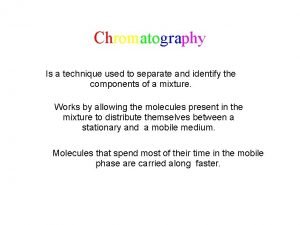
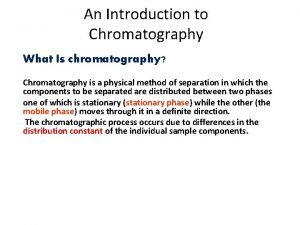
�Gas chromatography (GC), is a common type of chromatography used in analytic chemistry for separating and analyzing compounds that can be vaporized without decomposition.

Principle: �A method of analysis by which the analyte is vaporized and introduced into a stream of carrier gas. � It is conducted through a chromatographic column and separated into its constituents. � These fractions pass through the column at characteristic rates, and are detected as they emerge in a time sequence. � The detecting cell responses are recorded on a chart, from which the components can be identified both qualitatively and quantitatively.

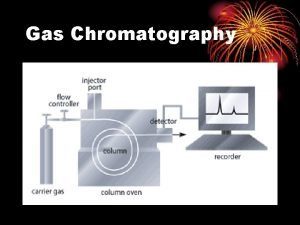
� In GC, the moving phase (mobile phase) is a carrier gas, usually an inert gas such as helium or nitrogen. � The stationary phase is a microscopic layer of liquid or polymer on an inert solid support, inside a piece of glass or metal tubing called a column. � The instrument used to perform GC is called a gas chromatograph.

� The gaseous compounds being analyzed interact with the walls of the column which is coated with different stationary phases. � This causes each compound to elute at a different time, known as the retention time of the compound. � The comparison of retention times is what gives GC its analytical usefulness.

� Gas chromatography is in principle similar to column chromatography , but has several notable differences: ◦ The process of separating the compounds in a mixture is carried out between a liquid stationary phase and a gas moving phase, whereas in column chromatography the stationary phase is a solid and the moving phase is a liquid. ◦ The column through which the gas phase passes is located in an oven where the temperature of the gas can be controlled, whereas column chromatography has no such temperature control. ◦ The concentration of a compound in the gas phase is solely a function of the vapor pressure of the gas.

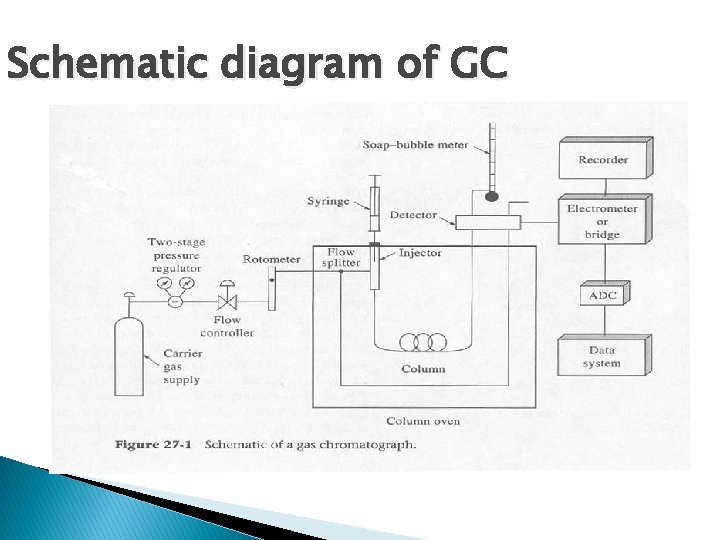
GC Instrument

Schematic diagram of GC

Carrier gas: �The carrier gas must be chemically inert. Commonly used gases include nitrogen, helium, argon, and carbon dioxide. �The choice of carrier gas is often dependant upon the type of detector which is used. �The carrier gas system also associated with pressure regulators and flow meters. �In addition, it contains a molecular sieve to remove water and other impurities.


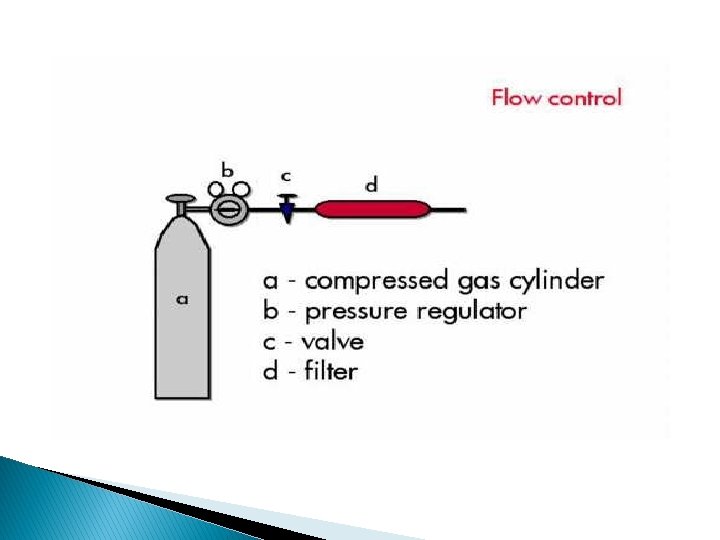
Flow control: � Flow rates are controlled by a 2 stage pressure regulator: ◦ At the gas cylinder. ◦ Mounted in the chromatograph. � Inlet pressure 10 -50 psi → F = 25 -100 ml/min with packed column F = 1 -20 ml/min with capillary column The flow rate will be constant if the inlet pressure remains constant.


Sample injection port: � The injector is a piece of hardware attached to the column head. � It provides the means to introduce a sample into a continuous flow of carrier gas. � For optimum column efficiency, the sample should not be too large, and should be introduced onto the column as vapor. � Slow injection of large samples causes band broadening and loss of resolution.

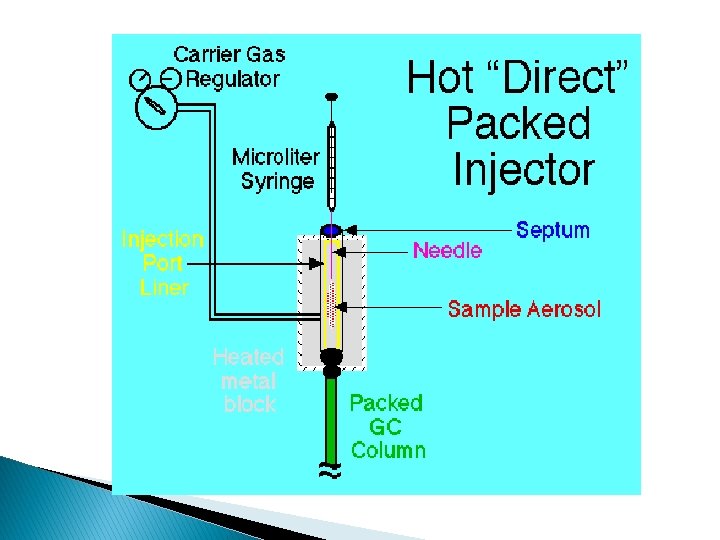
Common injector types are: � Microflash vaporizer direct injector: ◦ It involves the use of a microsyringe to inject the sample through a rubber septum into a flash vaporizer port located at the head of the column. ◦ The temperature of the sample port is usually about 50°C higher than the boiling point of the least volatile component of the sample. ◦ Used for packed columns, where the sample size vary from a few tenth of microliter to 20 ul.


Capillary columns require much smaller -3 samples ( 10 ul), so a sample splitter system is used to deliver only a small fraction of the injected sample to the column head, with the rest going to waste.

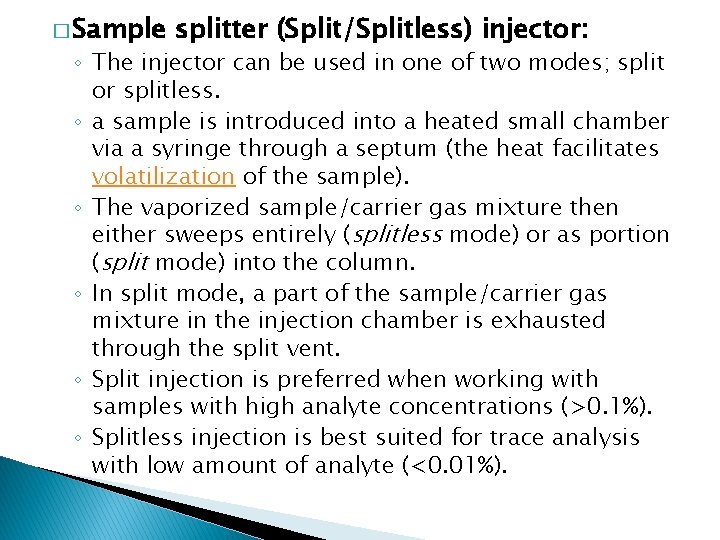
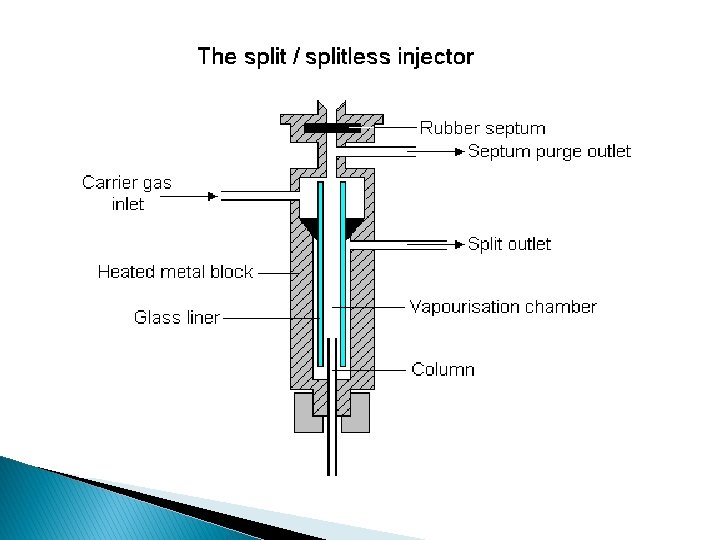
� Sample splitter (Split/Splitless) injector: ◦ The injector can be used in one of two modes; split or splitless. ◦ a sample is introduced into a heated small chamber via a syringe through a septum (the heat facilitates volatilization of the sample). ◦ The vaporized sample/carrier gas mixture then either sweeps entirely (splitless mode) or as portion (split mode) into the column. ◦ In split mode, a part of the sample/carrier gas mixture in the injection chamber is exhausted through the split vent. ◦ Split injection is preferred when working with samples with high analyte concentrations (>0. 1%). ◦ Splitless injection is best suited for trace analysis with low amount of analyte (<0. 01%).


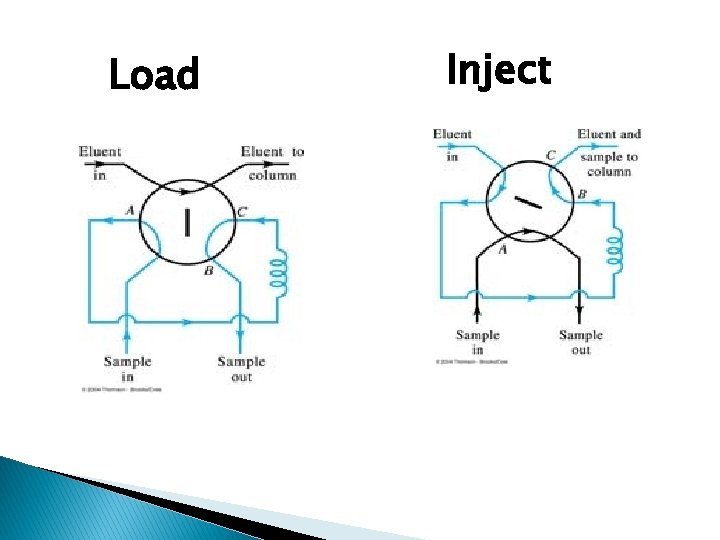
For quantitative work, more reproducible sample size are required and this can be obtained by a rotary sample valve.

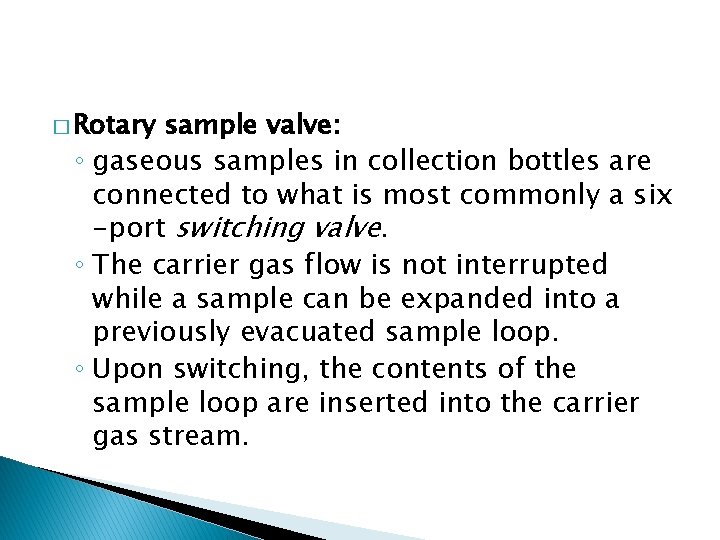
� Rotary sample valve: ◦ gaseous samples in collection bottles are connected to what is most commonly a six -port switching valve. ◦ The carrier gas flow is not interrupted while a sample can be expanded into a previously evacuated sample loop. ◦ Upon switching, the contents of the sample loop are inserted into the carrier gas stream.

Load Inject

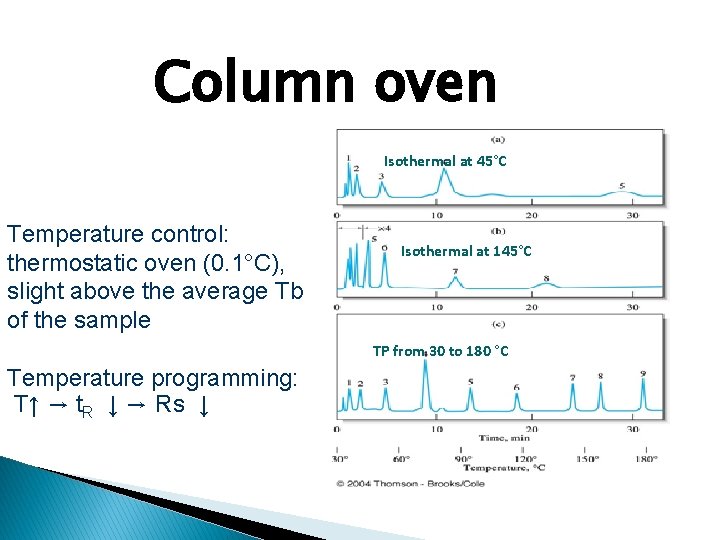
Column oven Isothermal at 45°C Temperature control: thermostatic oven (0. 1°C), slight above the average Tb of the sample Isothermal at 145°C TP from 30 to 180 °C Temperature programming: T↑ → t. R ↓ → Rs ↓

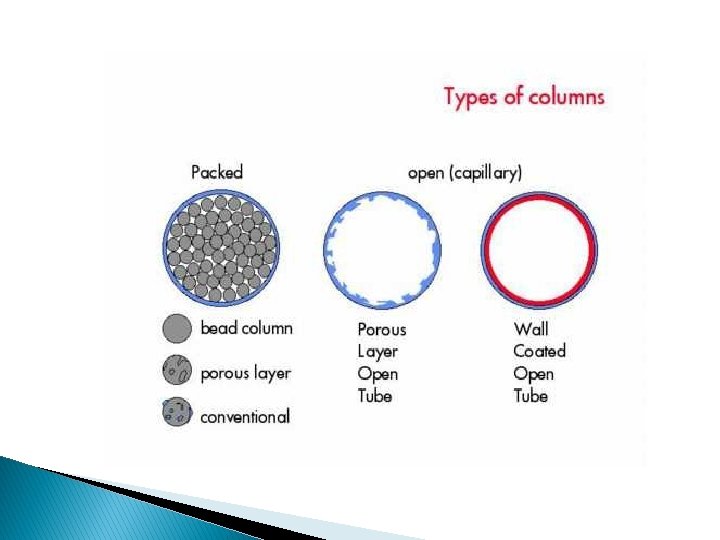
Column: � There are two general types of column: ◦ Packed column ◦ Capillary column (open tubular). � All the GC studies in the early 1950 s were carried out on packed column. � In the late 1950 s capillary column were constructed that much superior in speed and column efficiency (≈ 300000 plates).


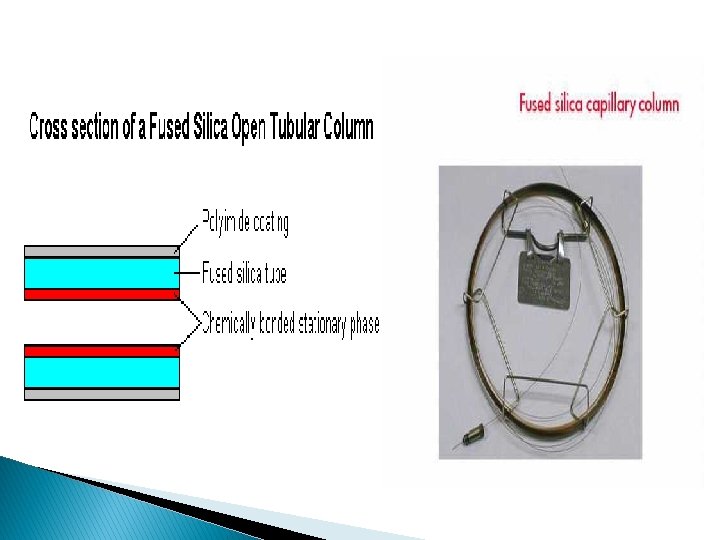
Capillary column: � Capillary columns have an internal diameter of a few tenths of a millimeter. � They were constructed of stain-less steel, aluminum, copper, plastic, or glass. � They can be one of three types; wall-coated open tubular (WCOT) or support-coated open tubular (SCOT) or porous layer open tubular column(PLOT). � Wall-coated columns consist of a capillary tube whose walls are coated with liquid stationary phase. � In support-coated columns, the inner wall of the capillary is lined with a thin layer of support material, onto which the stationary phase has been adsorbed. � SCOT columns are generally less efficient than WCOT columns, but both types are more efficient than packed columns.


� In 1979, a new type of WCOT column appeared, the Fused Silica Open Tubular (FSOT) column. � It was drawn from specially purified silica that contains metal oxides. � These have much thinner walls than the glass capillary columns, and are given strength by an outside protective polyimide coating. � These columns are flexible and can be bent into coils. � They have the advantages of physical strength, flexibility and low reactivity.


Packed column: � Packed columns contain a finely divided, inert, solid support material coated with a thin layer of liquid stationary phase. � Most packed columns are 1 - 6 m in length and have an internal diameter of 2 4 mm. � They are made from glass, metals, or Teflon.

Solid support materials: � Hold the liquid stationary phase. � Consists of small, uniform, spherical particles with good mechanical strength. � It should be inert at elevated temperature. � The most widely used is prepared from the naturally occurring diatomaceous earth (skeleton of thousands of single-celled plants -diatoms- lives in lakes and seas). � The efficiency of a GC column increases with decreasing particle size of the solid support.

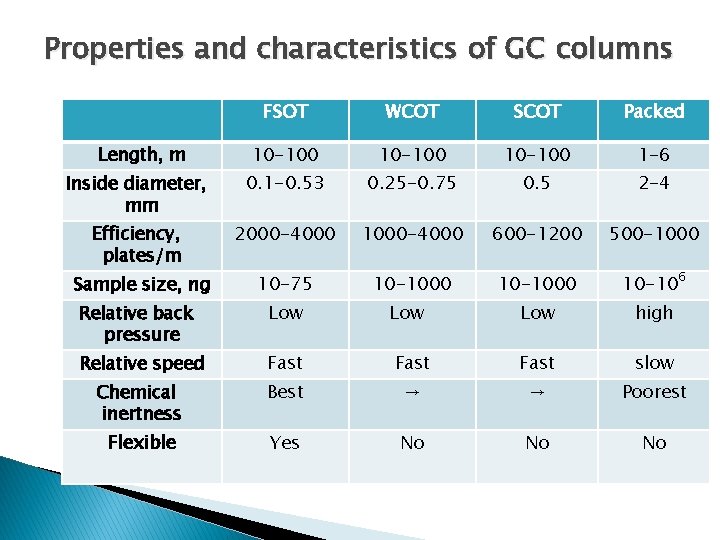
Properties and characteristics of GC columns FSOT WCOT SCOT Packed 10 -100 1 -6 Inside diameter, mm 0. 1 -0. 53 0. 25 -0. 75 0. 5 2 -4 Efficiency, plates/m 2000 -4000 1000 -4000 600 -1200 500 -1000 Sample size, ng 10 -75 10 -1000 10 -106 Length, m Relative back pressure Low Low high Relative speed Fast slow Chemical inertness Best → → Poorest Flexible Yes No No No

Common liquid stationary phases for GC Applications

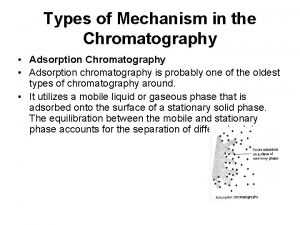
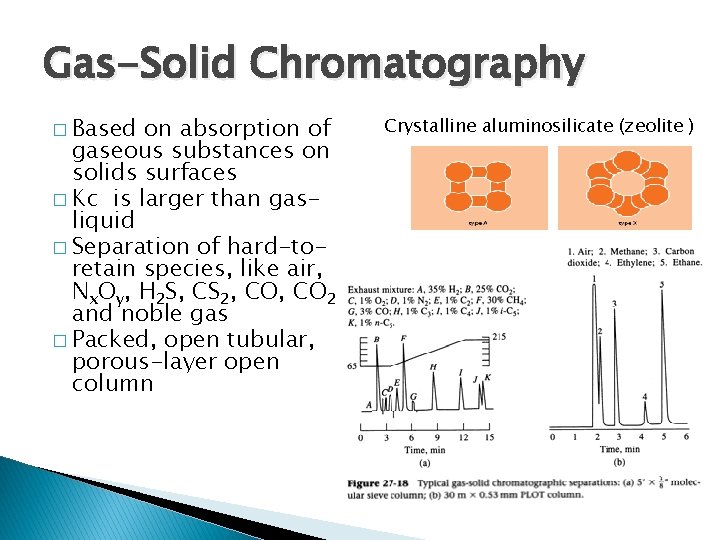
Gas-Solid Chromatography � Based on absorption of gaseous substances on solids surfaces � Kc is larger than gasliquid � Separation of hard-toretain species, like air, Nx. Oy, H 2 S, CS 2, CO 2 and noble gas � Packed, open tubular, porous-layer open column Crystalline aluminosilicate (zeolite )

Characteristics of ideal detector �Sensitivity �Good stability & reproducibility �Linear response �Temperature range �Short response time independent of flow rate �High reliability & ease of use �Should be non-destructive

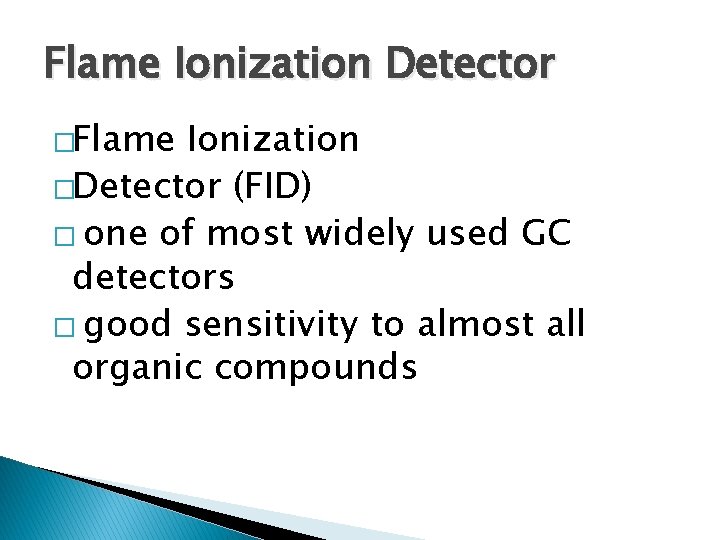
Flame Ionization Detector �Flame Ionization �Detector (FID) � one of most widely used GC detectors � good sensitivity to almost all organic compounds


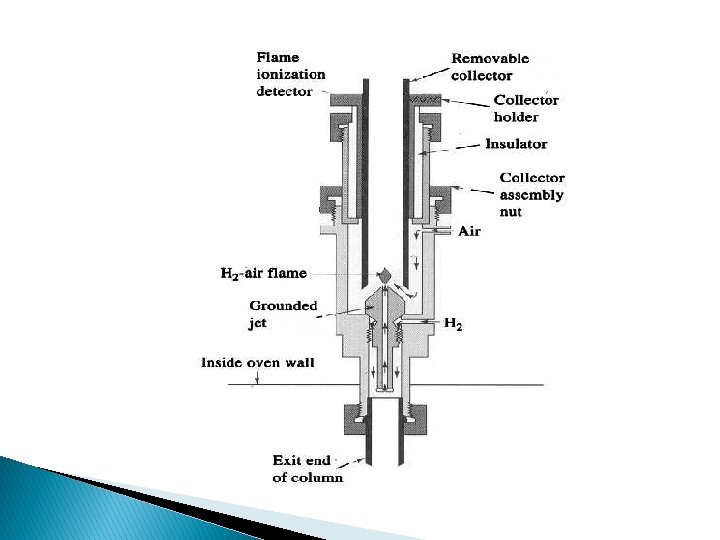
Thermal Conductivity Detector (TCD) �One of earliest GC detectors �Not popular today �Low sensitivity �Several designs �Use heated wire or semiconductor �Resistance of wire changes with analyte vs carrier

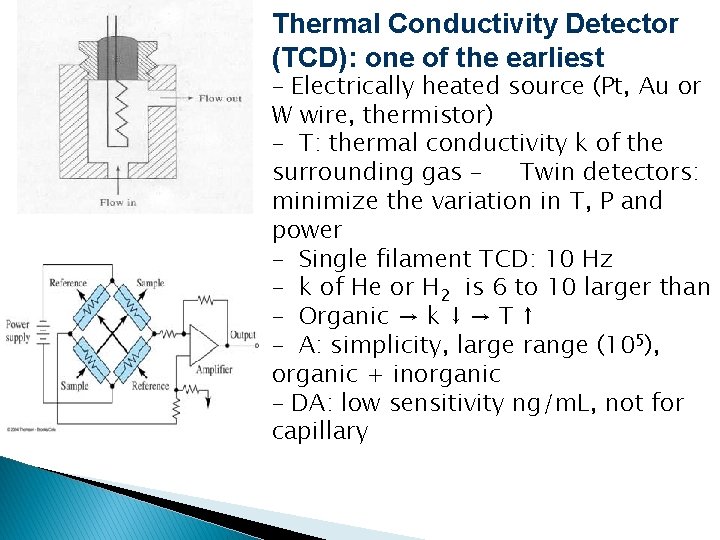
Thermal Conductivity Detector (TCD): one of the earliest – Electrically heated source (Pt, Au or W wire, thermistor) – T: thermal conductivity k of the surrounding gas – Twin detectors: minimize the variation in T, P and power – Single filament TCD: 10 Hz – k of He or H 2 is 6 to 10 larger than – Organic → k ↓ → T ↑ – A: simplicity, large range (105), organic + inorganic – DA: low sensitivity ng/m. L, not for capillary

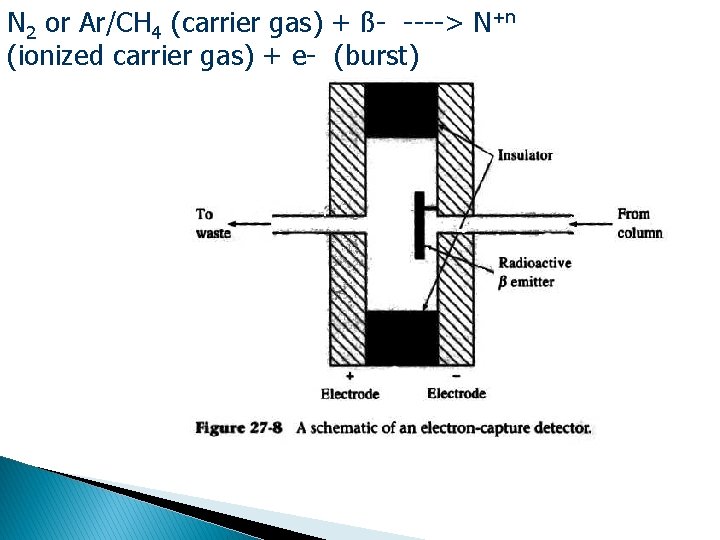
Electron Capture Detector (ECD) � Sample passes over b emitter (radioactive) like 63 Ni foil or 3 H adsorbed on Pt or Ti foil � β particles (i. e. electrons) hit carrier gas (usually N 2) causing a burst of e- to be released & measured by electrode → standing current or constant signal � When analyte molecule that absorbs e-passes through, current is reduced → signal � Response is non-linear unless pulsed

N 2 or Ar/CH 4 (carrier gas) + ß- ----> N+n (ionized carrier gas) + e- (burst)

ECD Advantages �Responds well to molecules with electronegative atoms like halogens (F, Cl, Br, I), peroxides, quinones, & nitro groups �Insensitive to amines, alcohols, hydrocarbons �Chlorinated pesticides are big application � Highly sensitive � Easy to use � Pretty reliable, although foil can get coated � Selective

ECD Disadvantages �Narrow linear range � Radioactive � Regular wipe test � Bake out contaminants � Some limits to applicability because highly selective

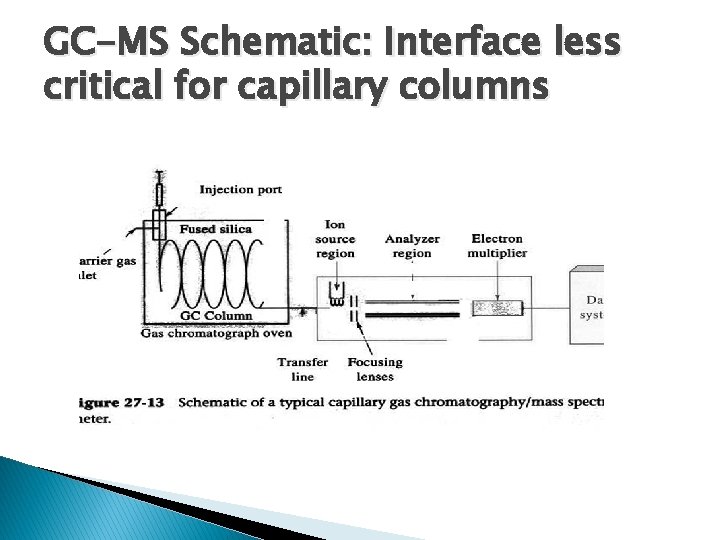
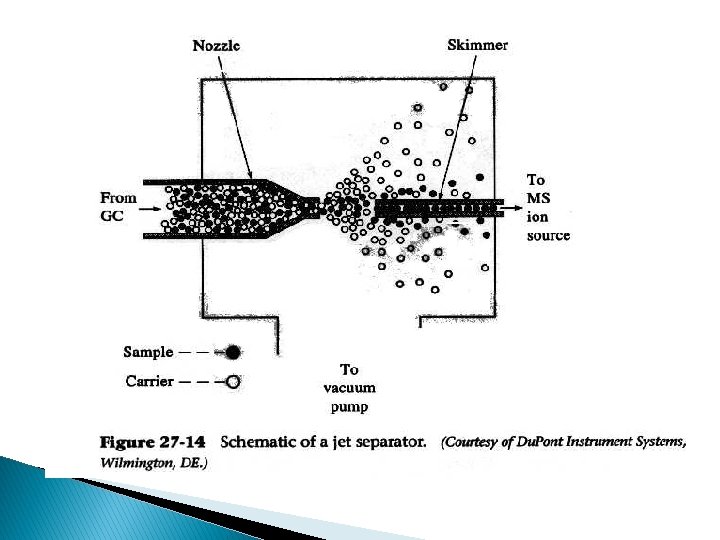
GC – Mass Spectrometry (GC-MS) � Interfacing GC & MS normally difficult � GC at pressure above atmospheric while MS under high vacuum � Need � Jet special interfaces for packed columns separator � Membrane separator – a membrane sandwich between spiral channels, column efluent on one side under pressure, MS on other side under vacuum – relies on differential permeability of carrier gas vs analyte molecules

GC-MS Schematic: Interface less critical for capillary columns


GC-MS INTERFACE

What kind of info can mass spec give you? �Molecular weight �Elemental composition (low MW with high resolution instrument) �Structural CID) info (EI-hard ionization or

How does it work? �Gas-phase ions are separated according to mass/charge ratio and sequentially detected

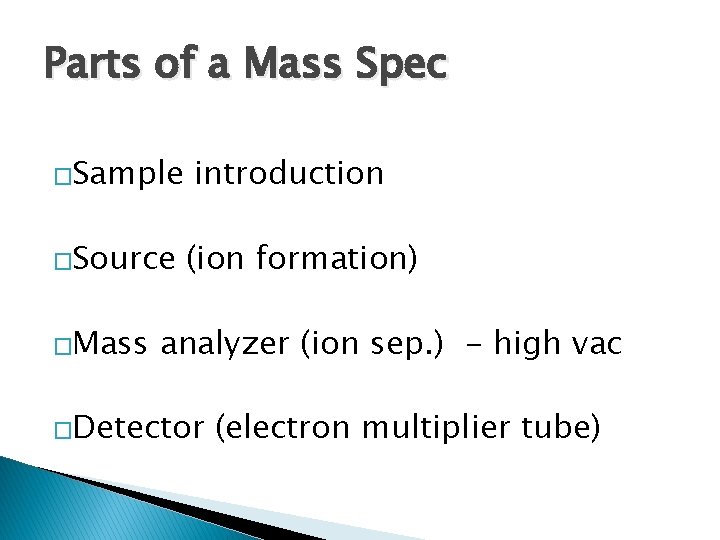
Parts of a Mass Spec �Sample �Source �Mass introduction (ion formation) analyzer (ion sep. ) - high vac �Detector (electron multiplier tube)

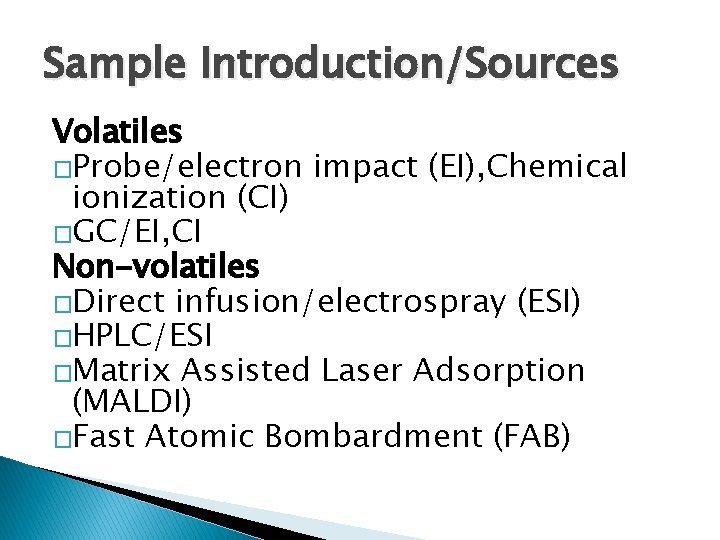
Sample Introduction/Sources Volatiles �Probe/electron impact (EI), Chemical ionization (CI) �GC/EI, CI Non-volatiles �Direct infusion/electrospray (ESI) �HPLC/ESI �Matrix Assisted Laser Adsorption (MALDI) �Fast Atomic Bombardment (FAB)

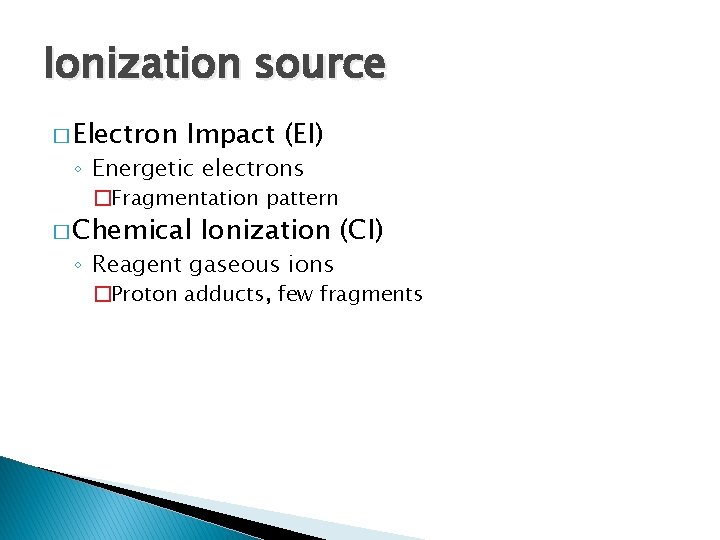
Ionization source � Electron Impact (EI) ◦ Energetic electrons �Fragmentation pattern � Chemical Ionization (CI) ◦ Reagent gaseous ions �Proton adducts, few fragments

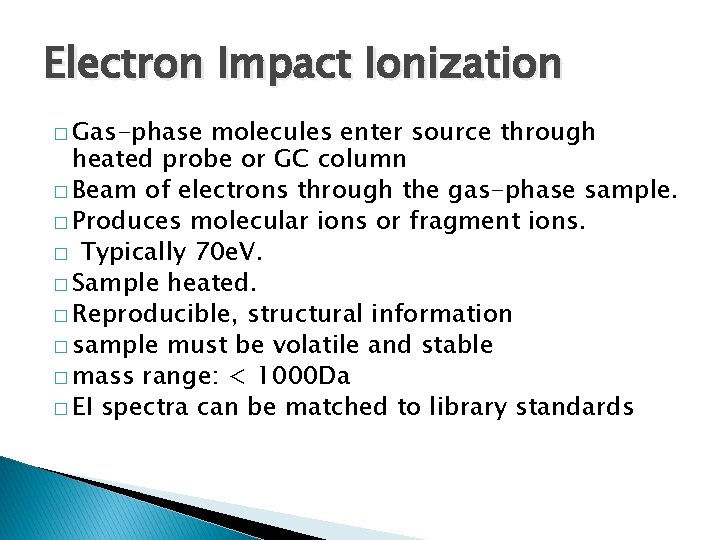
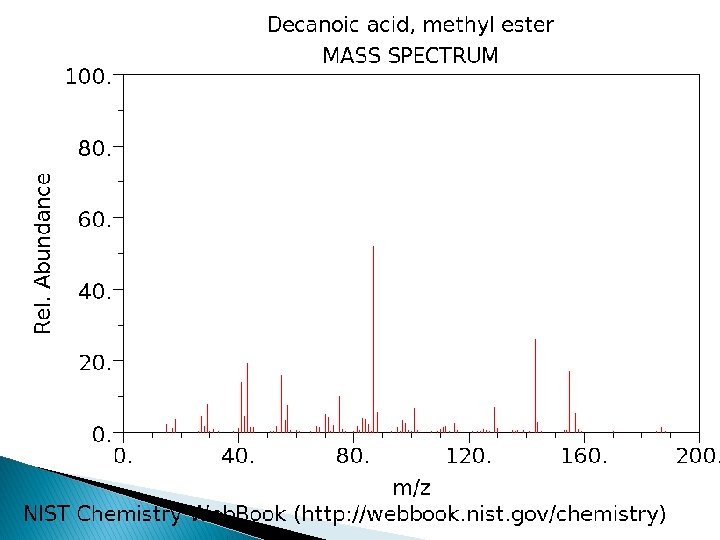
Electron Impact Ionization � Gas-phase molecules enter source through heated probe or GC column � Beam of electrons through the gas-phase sample. � Produces molecular ions or fragment ions. � Typically 70 e. V. � Sample heated. � Reproducible, structural information � sample must be volatile and stable � mass range: < 1000 Da � EI spectra can be matched to library standards

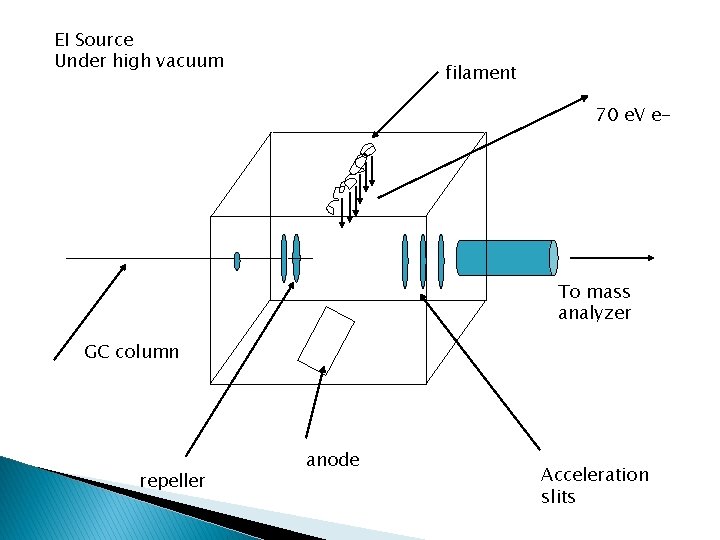
EI Source Under high vacuum filament 70 e. V e- To mass analyzer GC column repeller anode Acceleration slits

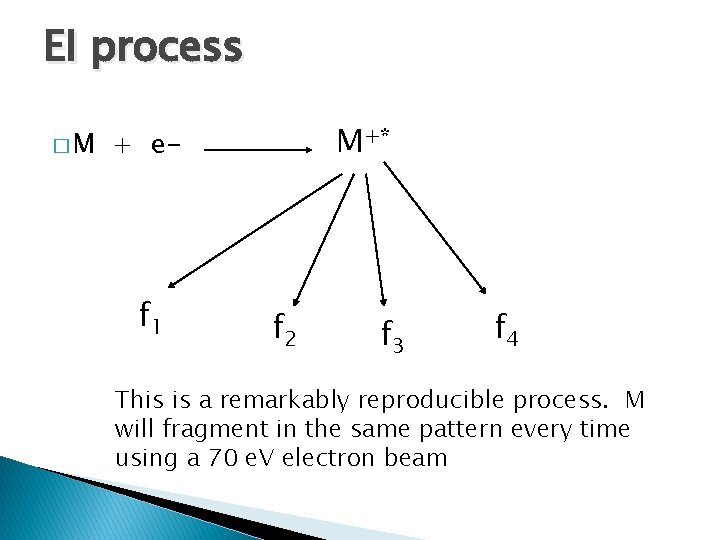
EI process �M M+* + e- f 1 f 2 f 3 f 4 This is a remarkably reproducible process. M will fragment in the same pattern every time using a 70 e. V electron beam


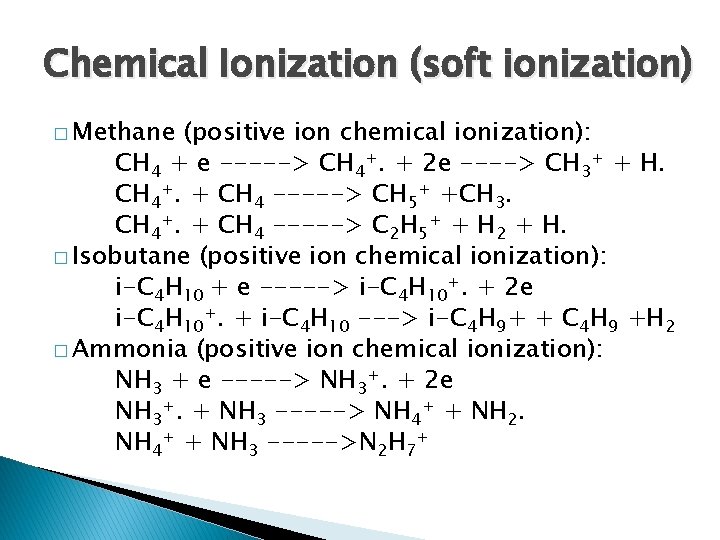
Chemical Ionization (soft ionization) � Methane (positive ion chemical ionization): CH 4 + e -----> CH 4+. + 2 e ----> CH 3+ + H. CH 4+. + CH 4 -----> CH 5+ +CH 3. CH 4+. + CH 4 -----> C 2 H 5+ + H 2 + H. � Isobutane (positive ion chemical ionization): i-C 4 H 10 + e -----> i-C 4 H 10+. + 2 e i-C 4 H 10+. + i-C 4 H 10 ---> i-C 4 H 9+ + C 4 H 9 +H 2 � Ammonia (positive ion chemical ionization): NH 3 + e -----> NH 3+. + 2 e NH 3+. + NH 3 -----> NH 4+ + NH 2. NH 4+ + NH 3 ----->N 2 H 7+
![Chemical Ionization Heated sample MH often visible less fragmentation than EI or MH Chemical Ionization �Heated sample. �+ [M+H]+ often visible, less fragmentation than EI or [M-H]+](https://slidetodoc.com/presentation_image_h2/470fc1dd8db06b748f256e48ebdf4be4/image-58.jpg)
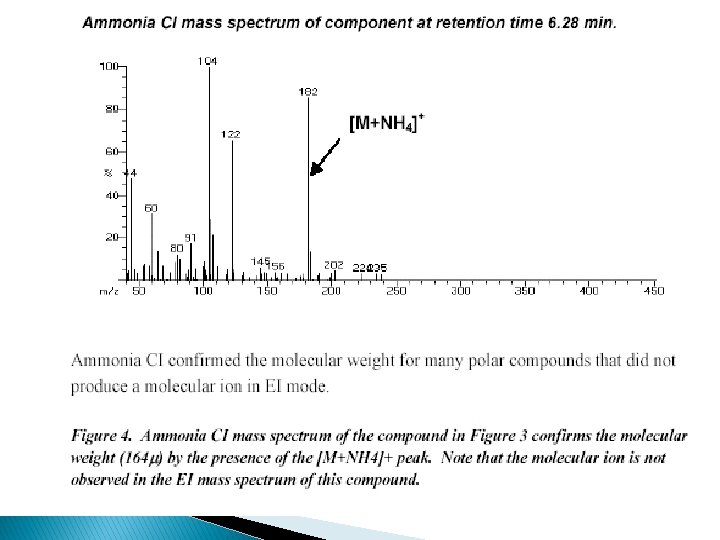
Chemical Ionization �Heated sample. �+ [M+H]+ often visible, less fragmentation than EI or [M-H]+ �sample must be volatile and stable, �less structural info than EI �mass range: < 1000 Da


Mass Analyzers � Mass Spectrometers separate ions according to their mass-to charge (m/z) ratios � Low resolution ◦ Quadrupole ◦ Ion trap � High resolution � Ultra high resolution ◦ TOF time of flight ◦ Sector instruments (magnet) ◦ ICR ion cyclotron resonance

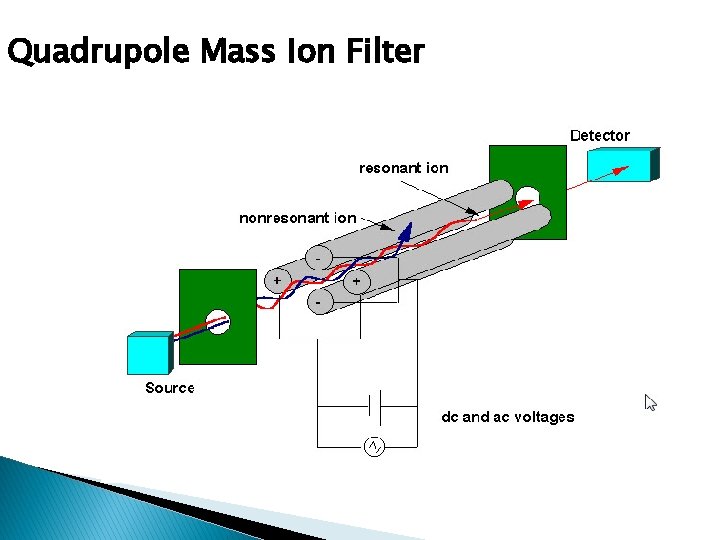
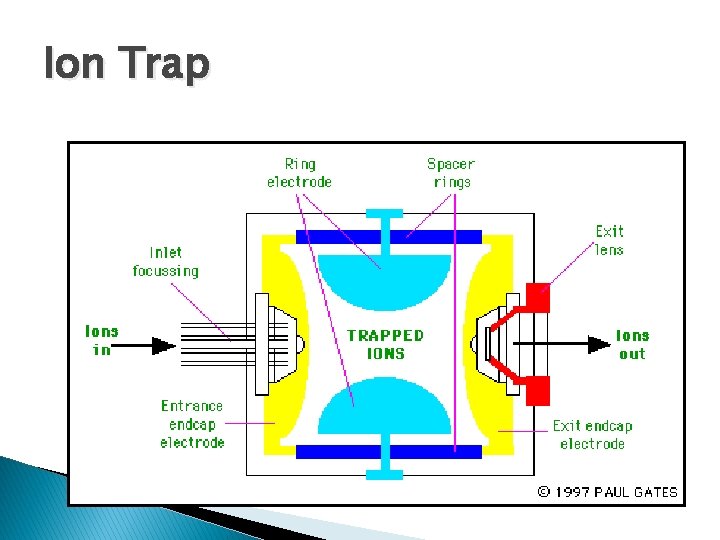
Analyzers � Separates the ions according to m/z values � Most common in GCMS is ◦ Quadrupole: Ion motion in dc and radiofrequency fields. Only certain m/z values are passed. ◦ Ion trap: Storage of ion in space defined by ring and end cap electrodes. Electric field sequentially ejects ions of increasing m/z values

Several types of Mass Specs available � Rarely magnetic sector or time of flight � Usually quadrapole or ion trap for GC-MS � Less expensive � Less maintenance � Easy to use � Normally use electron multiplier as detector � All MS systems need ion source, either electron impact or chemical ionization

Quadrupole Mass Ion Filter

Ion Trap

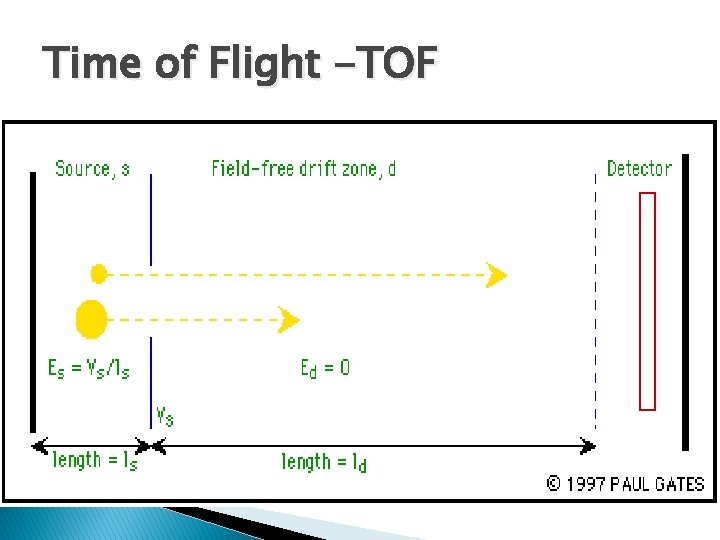
Time of Flight -TOF

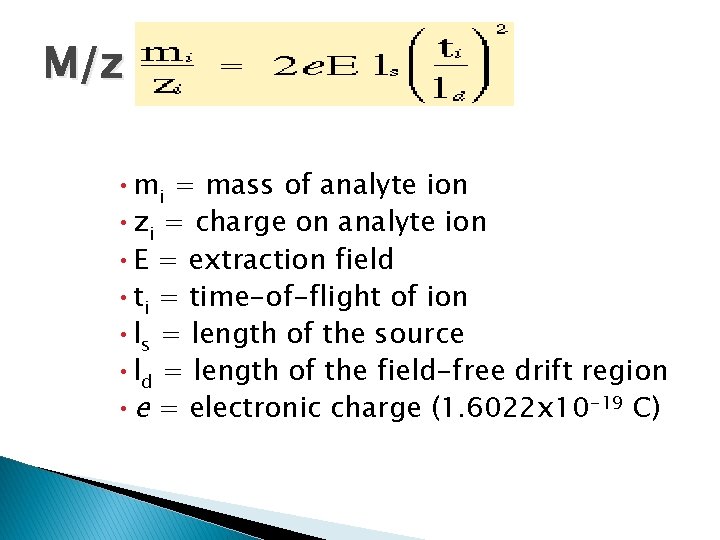
M/z • mi = mass of analyte ion • zi = charge on analyte ion • E = extraction field • ti = time-of-flight of ion • ls = length of the source • ld = length of the field-free drift region • e = electronic charge (1. 6022 x 10 -19 C)

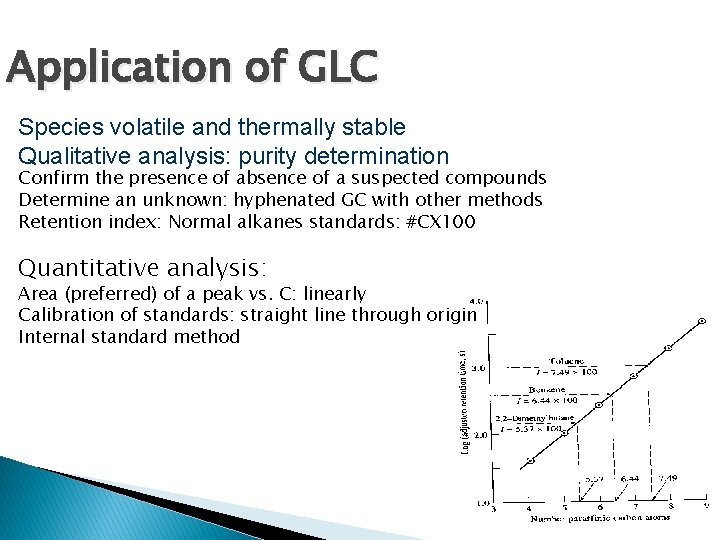
Application of GLC Species volatile and thermally stable Qualitative analysis: purity determination Confirm the presence of absence of a suspected compounds Determine an unknown: hyphenated GC with other methods Retention index: Normal alkanes standards: #CX 100 Quantitative analysis: Area (preferred) of a peak vs. C: linearly Calibration of standards: straight line through origin Internal standard method

Common liquid stationary phases for GC Applications

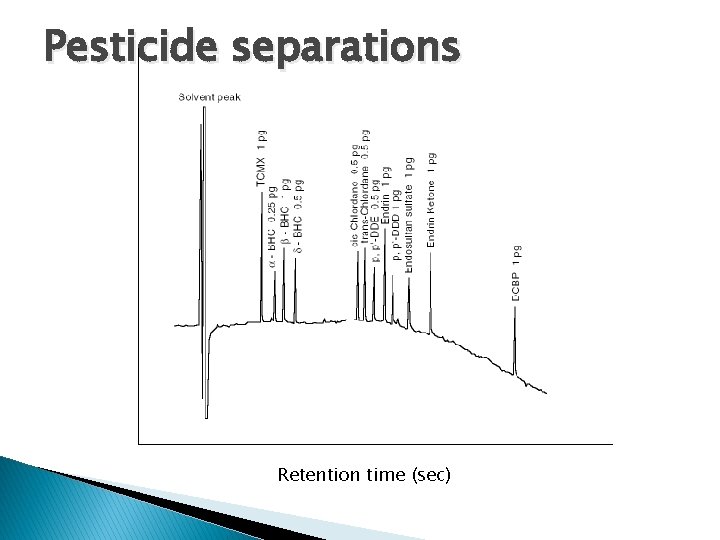
Pesticides � Analysis of pesticide residues in soil, water, and food is crucial for maintaining safe levels in the environment. The PDD in the ECD mode is highly selective for monitoring electron capturing compounds such as chlorinated pesticides and other halogens. This chromatogram illustrates the sensitivity of the non-radioactive PDECD for such compounds. Sample: Pesticide calibration mix Detector mode: Electron capture Detector temp: 330°C Column: 25 m x 0. 32 mm x 25 µm, HP-5 Column temp: 150°C to 300°C at 10°C/min Sample volume: 1 µL, 10: 1 split Discharge gas: Helium, 30 m. L/min Dopant gas: 5% methane in helium, 2. 4 m. L/min Attenuation: 1

Pesticide separations Retention time (sec)

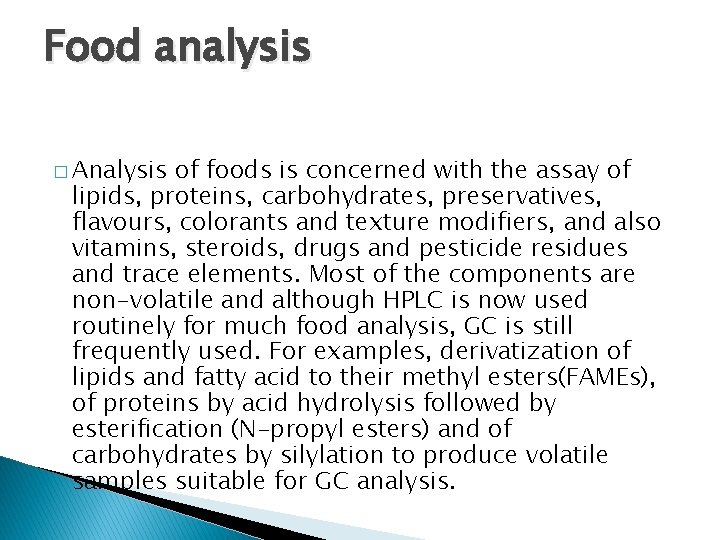
Food analysis � Analysis of foods is concerned with the assay of lipids, proteins, carbohydrates, preservatives, flavours, colorants and texture modifiers, and also vitamins, steroids, drugs and pesticide residues and trace elements. Most of the components are non-volatile and although HPLC is now used routinely for much food analysis, GC is still frequently used. For examples, derivatization of lipids and fatty acid to their methyl esters(FAMEs), of proteins by acid hydrolysis followed by esterification (N-propyl esters) and of carbohydrates by silylation to produce volatile samples suitable for GC analysis.

GC Food � � GC quality control analysis of food products can confirm the presence and quantities of the analytes For example, fruits, fruit derived foodstuffs, vegetables and soft drinks, tea and coffee, were analyzed for their polybasic and hydroxy acid contents (citric, maleic acids) as TMS derivatives. All food and beverage products on sale today must be carefully assayed for contamination with pesticides, herbicides and many other materials that are considered a health risk. The analysis of food involves separating and identifying very complex mixtures, the components of which are present at very low concentrations. GC is the ideal technique for use in food and beverage assays and tests. Furthermore, the origin of many herbs and spices can often be identified from the peak pattern of the chromatograms.

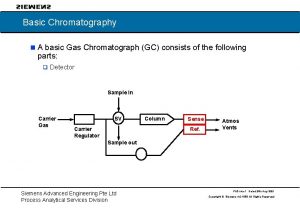
 Siemens chromatography
Siemens chromatography Rosemount gas chromatograph
Rosemount gas chromatograph Gas chromatograph
Gas chromatograph Basic gas chromatography
Basic gas chromatography Van deemter equation for open tubular column
Van deemter equation for open tubular column Gas chromatography
Gas chromatography Contoh soal menghitung nilai rf
Contoh soal menghitung nilai rf Gas liquid chromatography
Gas liquid chromatography Gas chromatography history
Gas chromatography history Hplc
Hplc Gas chromatography
Gas chromatography Percent composition gas chromatography
Percent composition gas chromatography Youtube
Youtube Fid detector principle
Fid detector principle Hplc slideshare
Hplc slideshare Mobile phase
Mobile phase Introduction of gas chromatography
Introduction of gas chromatography Gc basic principle
Gc basic principle Gas chromatography theory
Gas chromatography theory Dual fuel solutions
Dual fuel solutions How does chromatography work polarity
How does chromatography work polarity Protein chromatography
Protein chromatography High performance liquid chromatography introduction
High performance liquid chromatography introduction Advantages of column chromatography
Advantages of column chromatography Rumus waktu retensi
Rumus waktu retensi Elution order
Elution order Who discovered chromatography
Who discovered chromatography Paper chromatography separation of cations and dyes
Paper chromatography separation of cations and dyes Nickel affinity chromatography
Nickel affinity chromatography Stationary phase chromatography
Stationary phase chromatography Paper chromatography
Paper chromatography Adsorption chromatography types
Adsorption chromatography types Chromatography is a technique used to separate
Chromatography is a technique used to separate Retention factor chromatography
Retention factor chromatography Introduction to chromatography
Introduction to chromatography Hydrophobic interaction chromatography principle
Hydrophobic interaction chromatography principle Kav chromatography
Kav chromatography Introduction of chromatography
Introduction of chromatography Who invented thin layer chromatography
Who invented thin layer chromatography Partition chromatography
Partition chromatography Affinity chromatography animation
Affinity chromatography animation How does paper chromatography work
How does paper chromatography work Advantages of gel filtration chromatography
Advantages of gel filtration chromatography Affinity chromatography principle
Affinity chromatography principle Tlc plate
Tlc plate Plate theory of chromatography
Plate theory of chromatography Tswett
Tswett High performance liquid chromatography hplc machine
High performance liquid chromatography hplc machine Principle of simple distillation
Principle of simple distillation Principle hplc
Principle hplc Definition of chromatography
Definition of chromatography Anion exchange chromatography
Anion exchange chromatography Size exclusion chromatography animation
Size exclusion chromatography animation Ion chromatography
Ion chromatography Normal phase vs reverse phase chromatography
Normal phase vs reverse phase chromatography Normal phase vs reverse phase chromatography
Normal phase vs reverse phase chromatography Application of chromatography
Application of chromatography Gel filtration calibration kit
Gel filtration calibration kit Principle of paper chromatography
Principle of paper chromatography Ammonium chloride reversible reaction
Ammonium chloride reversible reaction Uses of paper chromatography
Uses of paper chromatography Ion exchange chromatography
Ion exchange chromatography Theoretical plates in hplc
Theoretical plates in hplc Gel permeation chromatography instrument
Gel permeation chromatography instrument Ferrocene
Ferrocene Chromatography lecture
Chromatography lecture Difference between affinity and ion exchange chromatography
Difference between affinity and ion exchange chromatography Ni-nta
Ni-nta Rf value in paper chromatography
Rf value in paper chromatography Greyhound chromatography
Greyhound chromatography Smb chromatography
Smb chromatography Chromatography uses
Chromatography uses How to calculate resolution chromatography
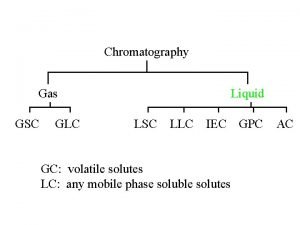
How to calculate resolution chromatography Gsc glc
Gsc glc