Ketones and Aldehydes Properties Nomenclature Preparation Reactions Synthesis








![Reduction Summary DIBAH [O] w/ PCC or Periodinane Reduction Summary DIBAH [O] w/ PCC or Periodinane](https://slidetodoc.com/presentation_image_h2/1863bb481ba47b448fdc1e0cd06ac734/image-14.jpg)











- Slides: 34

Ketones and Aldehydes Properties Nomenclature Preparation Reactions Synthesis

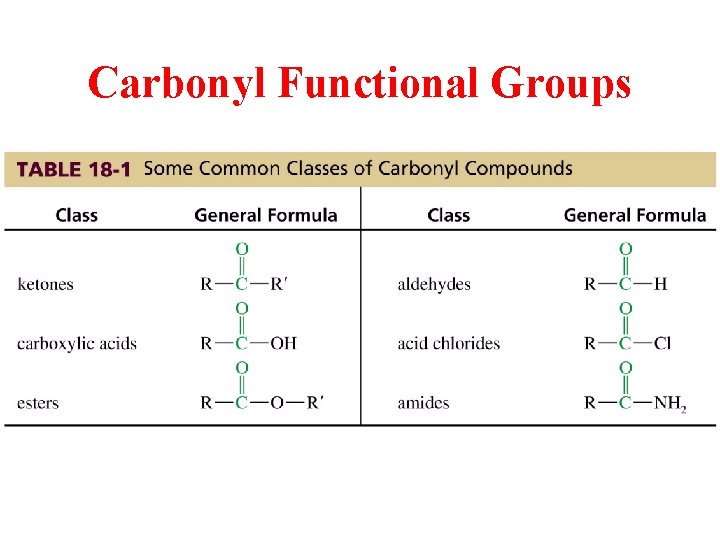
Carbonyl Functional Groups

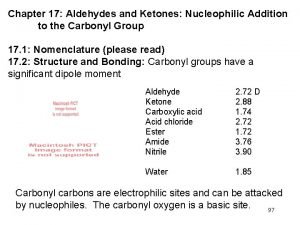
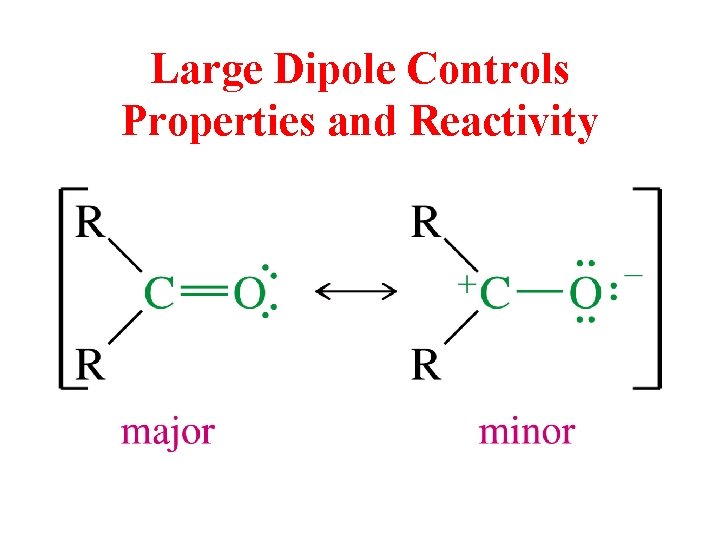
Large Dipole Controls Properties and Reactivity

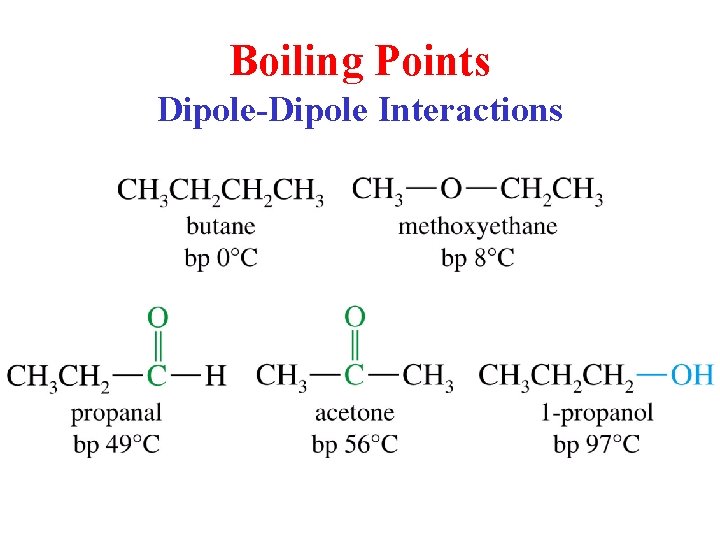
Boiling Points Dipole-Dipole Interactions

Adrogenic/Anabolic Steroids

Anabolic Steroids

IUPAC Nomenclature Ketones

IUPAC Nomenclature Aldehydes

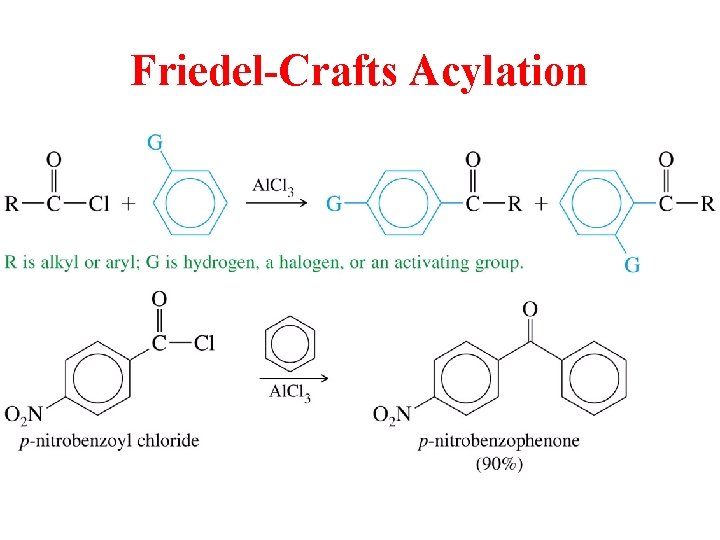
Preparation of Ketones and Aldehydes • Friedel-Crafts Acylation (ketones) • Hydration of Alkynes (ketones with oxymercuration, aldehydes with hydroboration) • Reduction of acids, acid chlorides and nitriles • Oxidation of alcohols

Friedel-Crafts Acylation

Oxymercuration Hydration Markovnikov

Hydroboration Hydration Anti-Markovnikov

DIBAH Diisobutyl Aluminum Hydride
![Reduction Summary DIBAH O w PCC or Periodinane Reduction Summary DIBAH [O] w/ PCC or Periodinane](https://slidetodoc.com/presentation_image_h2/1863bb481ba47b448fdc1e0cd06ac734/image-14.jpg)
Reduction Summary DIBAH [O] w/ PCC or Periodinane

How would you prepare the ff? 1. Pentanal from: a. pentan-1 -ol b. De-5 -ene c. Pentanoic acid 2. Hexan-2 -one from: a. hexan-2 -ol b. 2 -methyl-hex-1 -ene c. 1 -hexyne

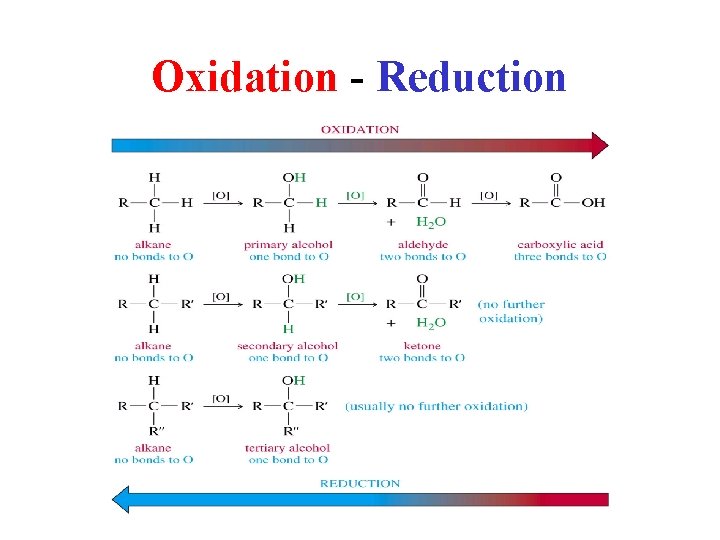
Oxidation - Reduction

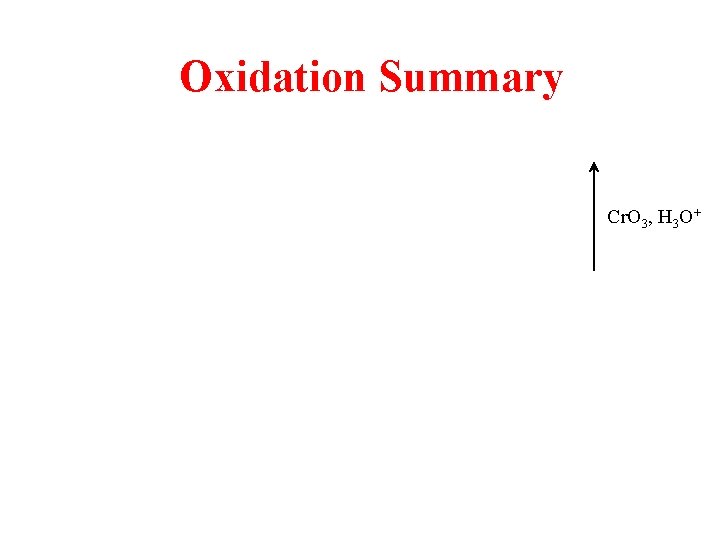
Oxidation Summary Cr. O 3, H 3 O+

Nucleophilic Addition Reactions: Strong Nucleophiles

Na. BH 4 Reduction

Some Examples

Grignard Reagents React With Ketones to form tertiary alcohols

Grignard Summary

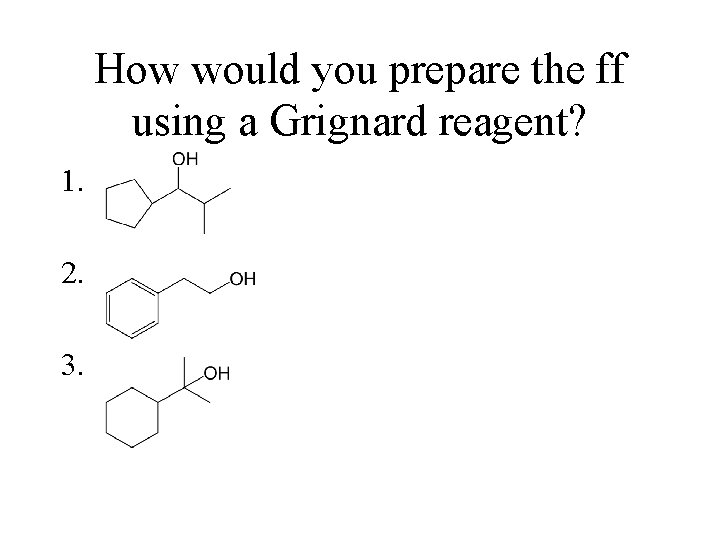
How would you prepare the ff using a Grignard reagent? 1. 2. 3.

Nucleophilic Addition Reactions: Weak Nucleophiles

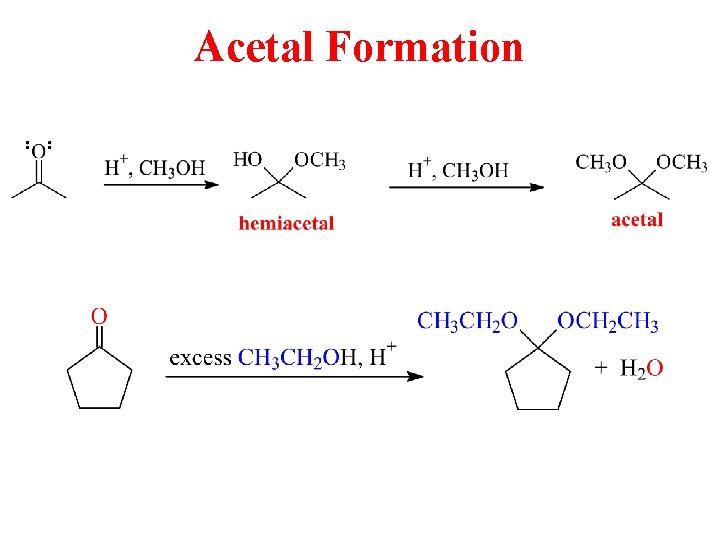
Acetal Formation

Acetal Mechanism

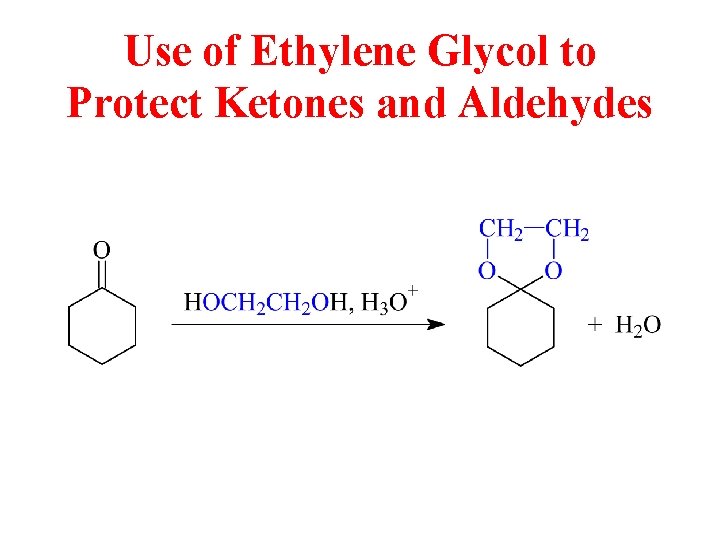
Use of Ethylene Glycol to Protect Ketones and Aldehydes

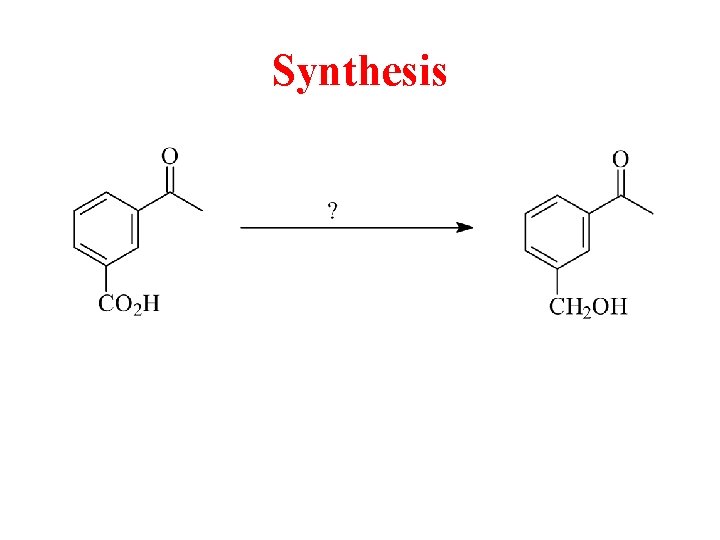
Synthesis

Synthesis

Aldehydes React Preferentially

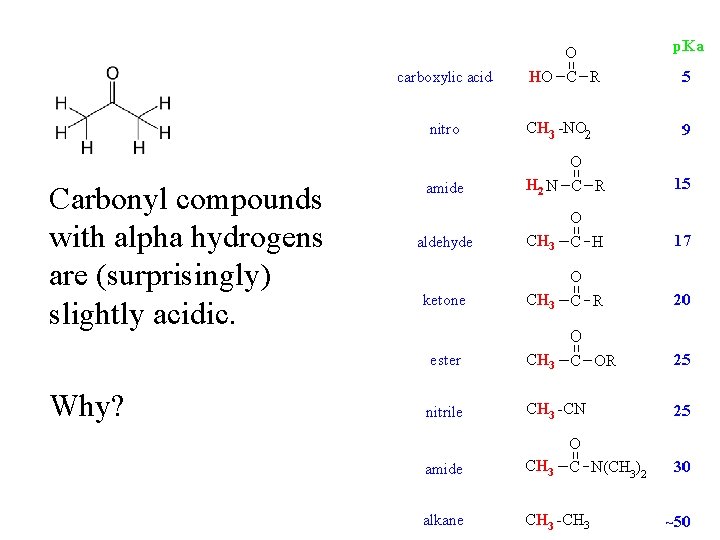
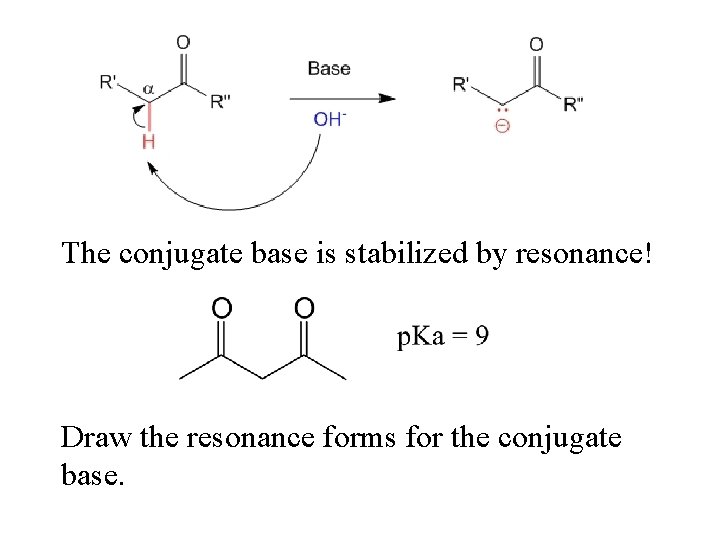
Carbonyl compounds with alpha hydrogens are (surprisingly) slightly acidic. Why?

The conjugate base is stabilized by resonance! Draw the resonance forms for the conjugate base.

Identify the most acidic proton: 1. 2. 3. 4. cyclohexane-1, 3 -dione Propanal Acetic acid 3, 3 -dimethylbutan-2 -one

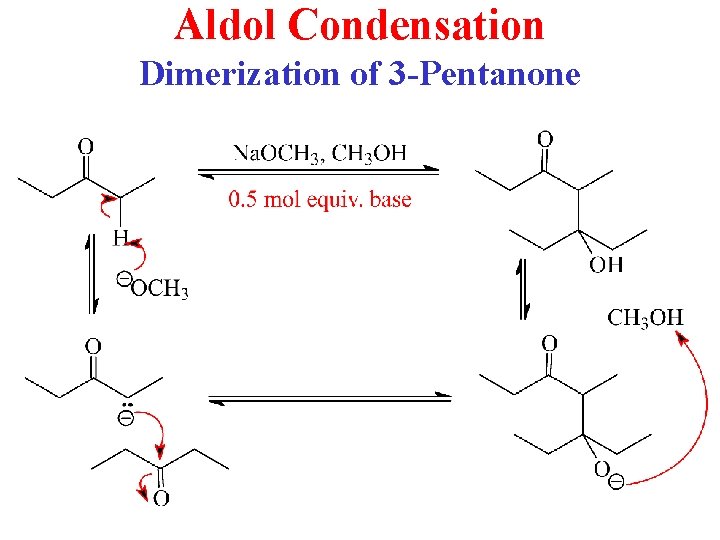
Aldol Condensation Dimerization of 3 -Pentanone
 Reactions of alcohols 2 chemsheets answers
Reactions of alcohols 2 chemsheets answers Reactions of aldehydes and ketones summary
Reactions of aldehydes and ketones summary Chemical properties of aldehydes
Chemical properties of aldehydes Aldehydes and ketones structure
Aldehydes and ketones structure Carbohydrates are polyhydroxy aldehydes and ketones
Carbohydrates are polyhydroxy aldehydes and ketones Aldehydes and ketones
Aldehydes and ketones Alkanals
Alkanals Test for alkanal
Test for alkanal Aldehydes and ketones
Aldehydes and ketones Ketones structure
Ketones structure Relative reactivity of aldehydes and ketones
Relative reactivity of aldehydes and ketones Carbonyl compounds
Carbonyl compounds Aldehydes and ketones nucleophilic addition
Aldehydes and ketones nucleophilic addition Cyanohydrin formation
Cyanohydrin formation Properties of ketones
Properties of ketones Ketone group
Ketone group Diol formation from alkene
Diol formation from alkene Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Balancing redox reactions in acidic solution
Balancing redox reactions in acidic solution Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Types of reactions
Types of reactions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers More predicting products of chemical reactions
More predicting products of chemical reactions What is galactosemia
What is galactosemia Aldehyde and ketones
Aldehyde and ketones Functional group of ketone
Functional group of ketone Aldehyde + ag2o
Aldehyde + ag2o Naming ether
Naming ether Carbonyl group
Carbonyl group Polyhydroxylated aldehydes
Polyhydroxylated aldehydes Eutectic solvent
Eutectic solvent Gmelin's test principle
Gmelin's test principle Aciclosis
Aciclosis Urinalysis sediment
Urinalysis sediment Oxidation of ketones
Oxidation of ketones