How many protons neutrons and electrons do the








- Slides: 37


How many protons, neutrons, and electrons do the following have Helium Rhodium Yttrium

Bohr Models are NOT Bohring! How to Draw Bohr Diagrams

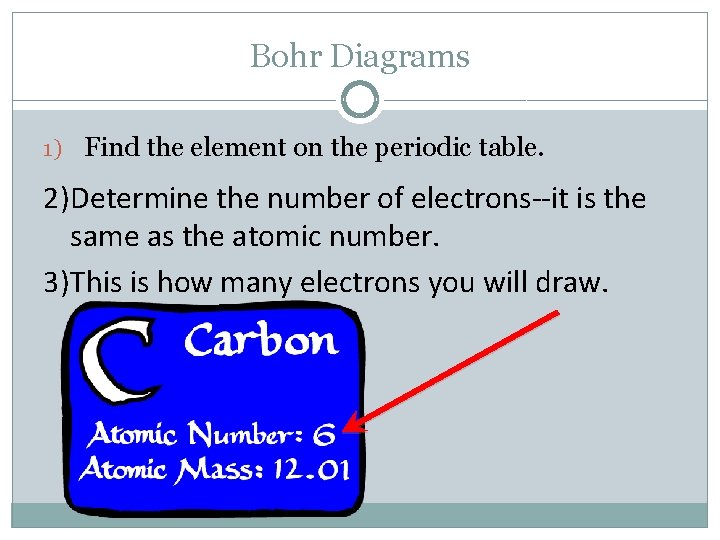
Bohr Diagrams 1) Find the element on the periodic table. 2)Determine the number of electrons--it is the same as the atomic number. 3)This is how many electrons you will draw.

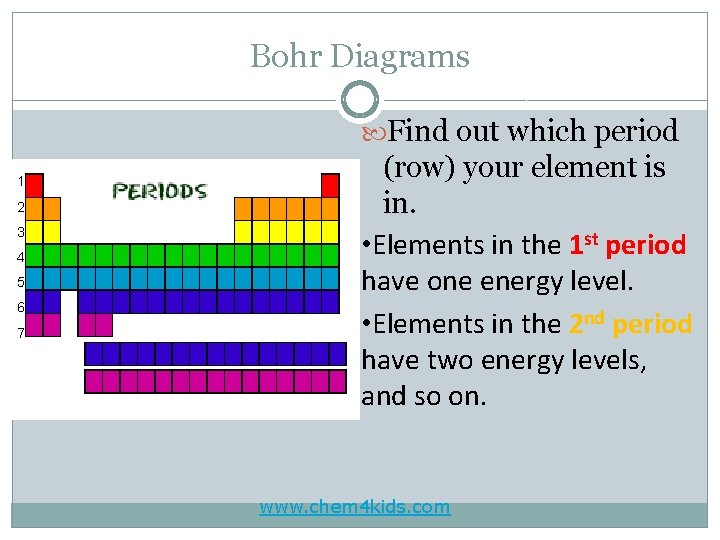
Bohr Diagrams Find out which period 1 2 3 4 5 6 7 (row) your element is in. • Elements in the 1 st period have one energy level. • Elements in the 2 nd period have two energy levels, and so on. www. chem 4 kids. com

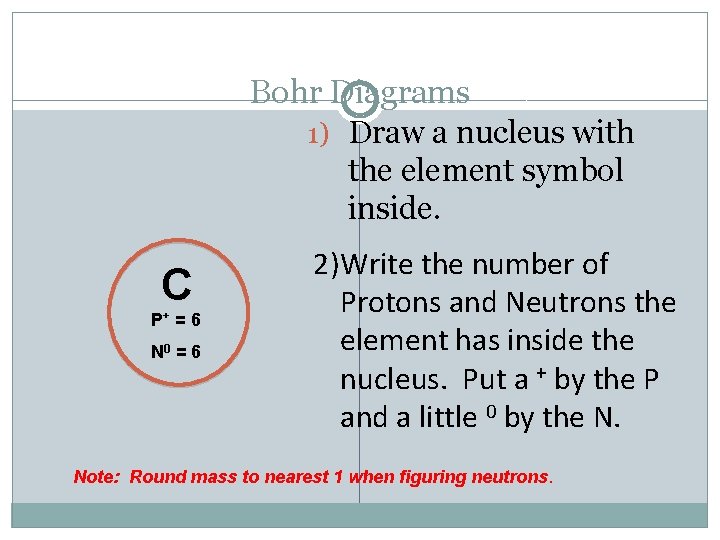
Bohr Diagrams 1) Draw a nucleus with the element symbol inside. C P+ = 6 N 0 = 6 2)Write the number of Protons and Neutrons the element has inside the nucleus. Put a + by the P and a little 0 by the N. Note: Round mass to nearest 1 when figuring neutrons.

The electron shells surrounding the nucleus each hold a particular number of electrons. Shells are named with letters in physics: 1 = K shell 2 = L shell 3 = M shell 4 = N shell 5 -7 = O, P, Q = up to 2 electrons = up to 8 (for the third periods) OR 18 electrons (for the other periods) = up to 18 electrons = up to 32 electrons ***The outer shell of an atom (no matter what letter) can only hold 8 electrons!

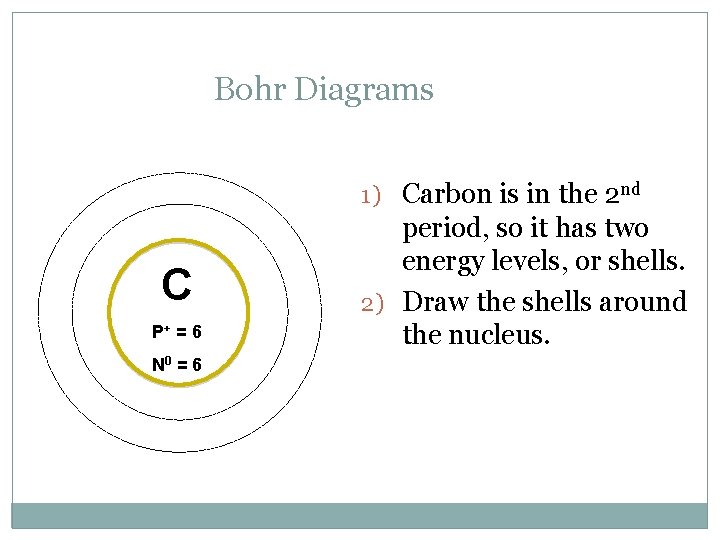
Bohr Diagrams 1) Carbon is in the 2 nd C P+ = 6 N 0 = 6 period, so it has two energy levels, or shells. 2) Draw the shells around the nucleus.

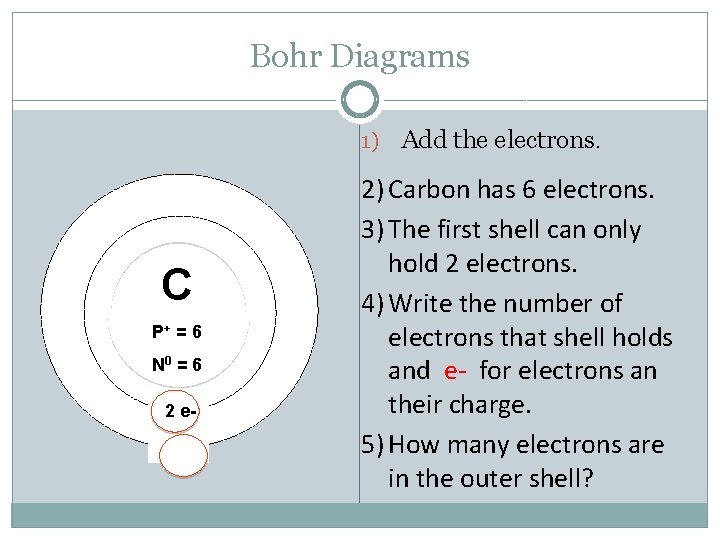
Bohr Diagrams 1) C P+ = 6 N 0 = 6 2 e- Add the electrons. 2) Carbon has 6 electrons. 3) The first shell can only hold 2 electrons. 4) Write the number of electrons that shell holds and e- for electrons an their charge. 5) How many electrons are in the outer shell?

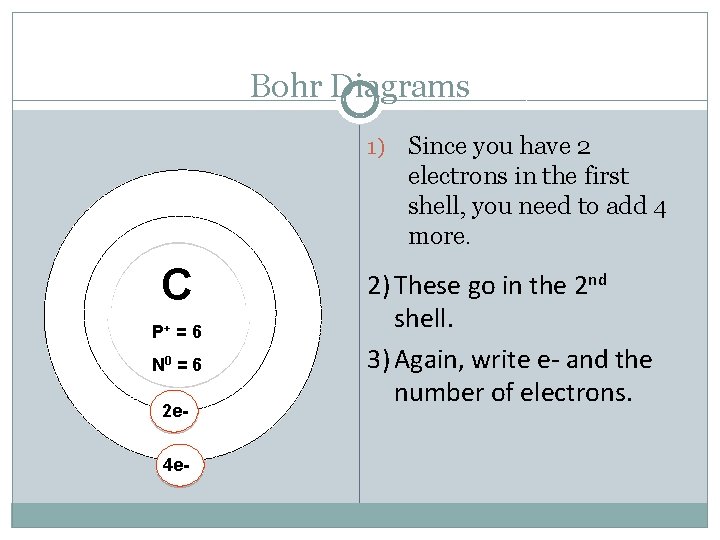
Bohr Diagrams 1) C P+ = 6 N 0 = 6 2 e 4 e- Since you have 2 electrons in the first shell, you need to add 4 more. 2) These go in the 2 nd shell. 3) Again, write e- and the number of electrons.

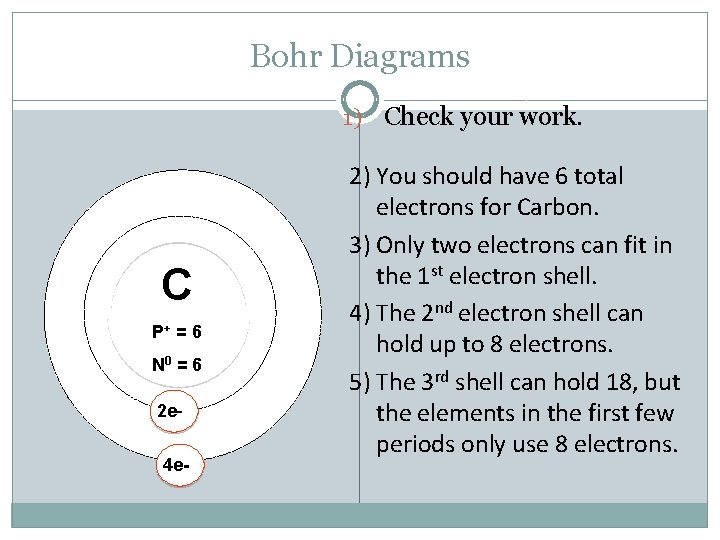
Bohr Diagrams 1) Check your work. C P+ = 6 N 0 = 6 2 e 4 e- 2) You should have 6 total electrons for Carbon. 3) Only two electrons can fit in the 1 st electron shell. 4) The 2 nd electron shell can hold up to 8 electrons. 5) The 3 rd shell can hold 18, but the elements in the first few periods only use 8 electrons.

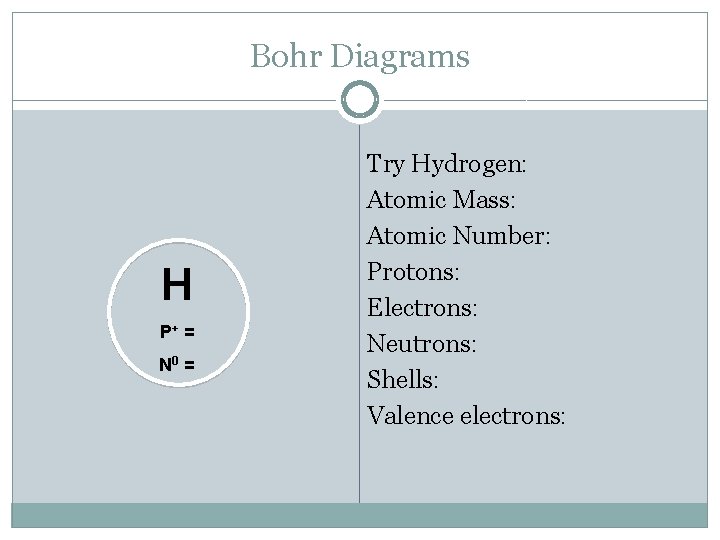
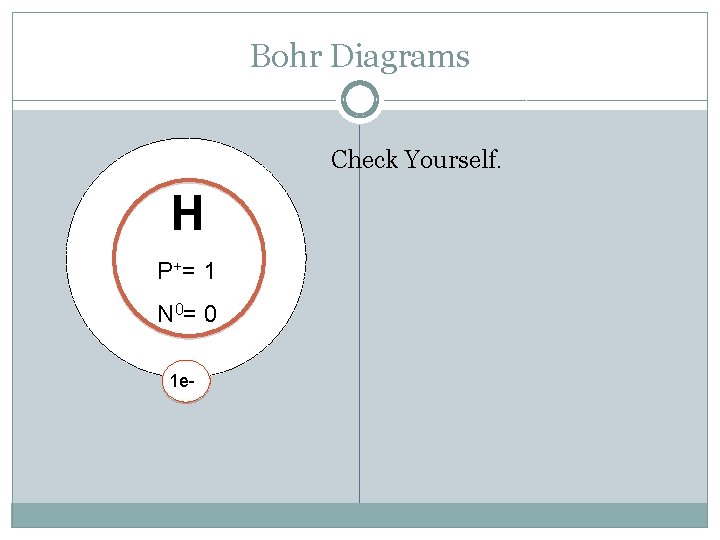
Bohr Diagrams H P+ = N 0 = Try Hydrogen: Atomic Mass: Atomic Number: Protons: Electrons: Neutrons: Shells: Valence electrons:

Bohr Diagrams Check Yourself. H P += 1 N 0= 0 1 e-

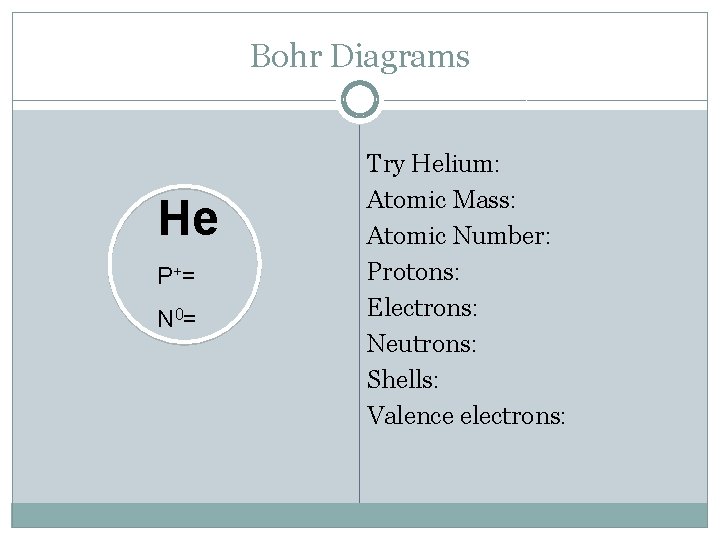
Bohr Diagrams He P += N 0= Try Helium: Helium Atomic Mass: Atomic Number: Protons: Electrons: Neutrons: Shells: Valence electrons:

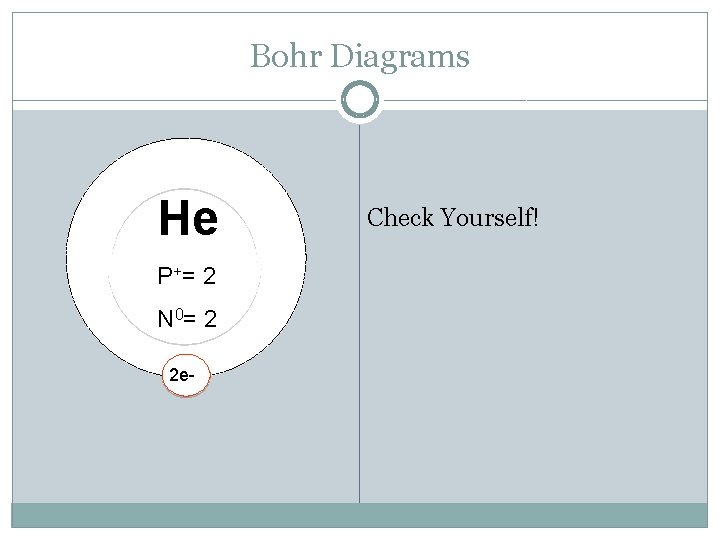
Bohr Diagrams He P += 2 N 0= 2 2 e- Check Yourself!

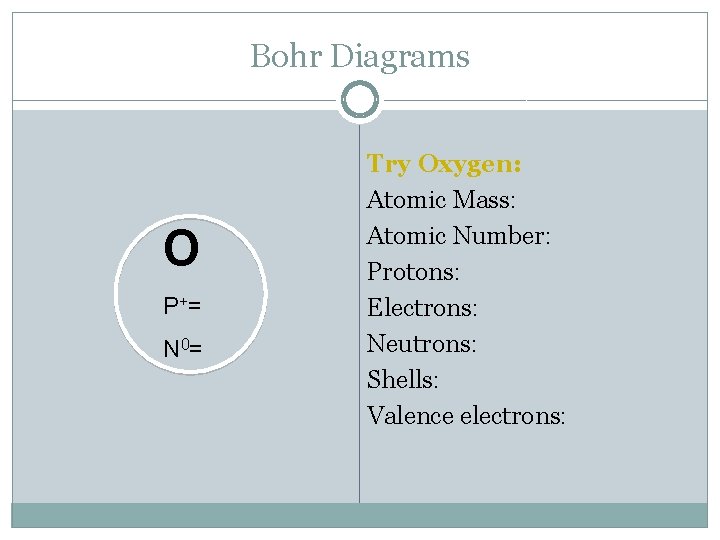
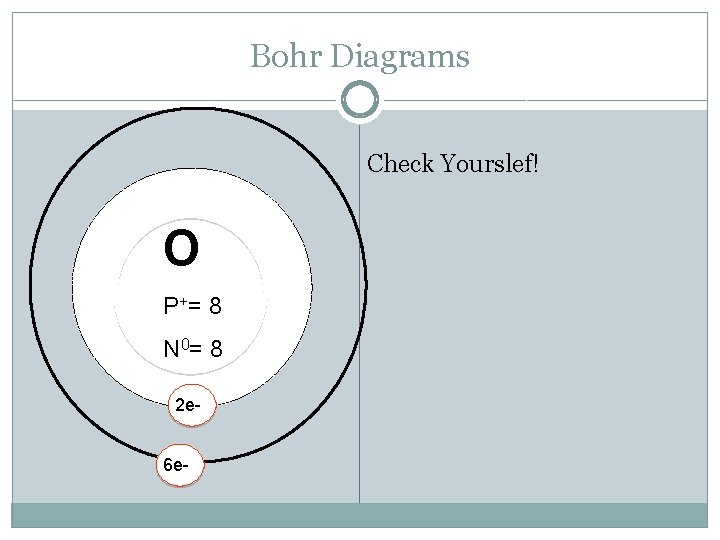
Bohr Diagrams O P += N 0= Try Oxygen: Atomic Mass: Atomic Number: Protons: Electrons: Neutrons: Shells: Valence electrons:

Bohr Diagrams Check Yourslef! O P += 8 N 0= 8 2 e 6 e-

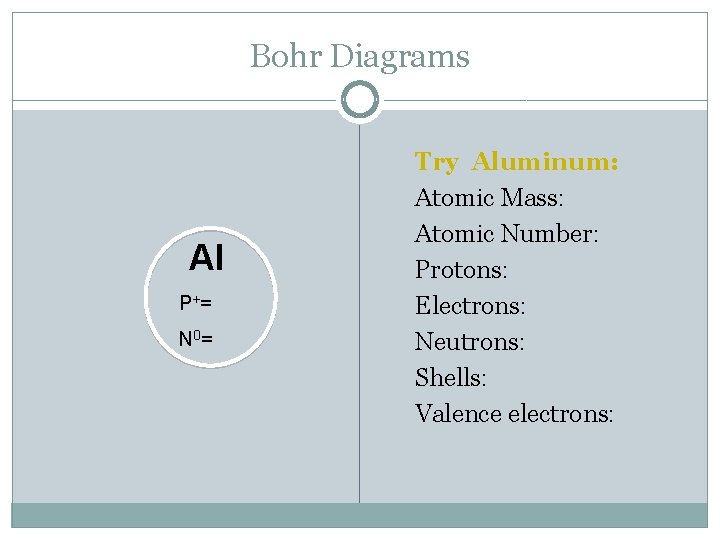
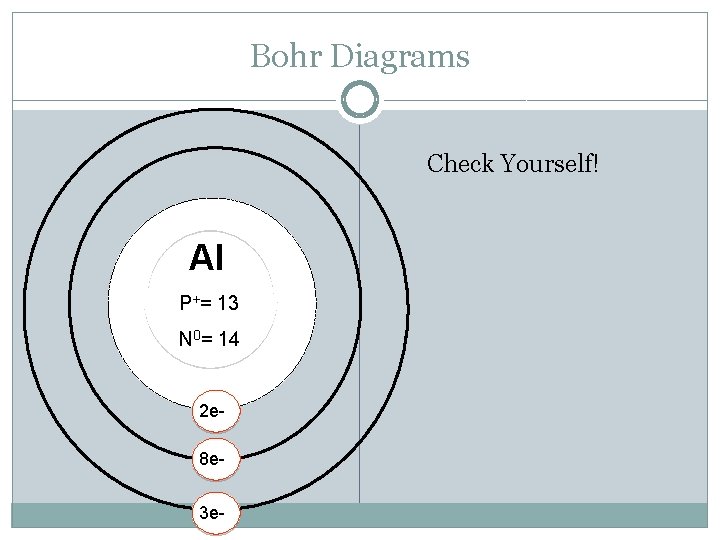
Bohr Diagrams Al P+ = N 0 = Try Aluminum: Atomic Mass: Atomic Number: Protons: Electrons: Neutrons: Shells: Valence electrons:

Bohr Diagrams Check Yourself! Al P+= 13 N 0= 14 2 e 8 e 3 e-

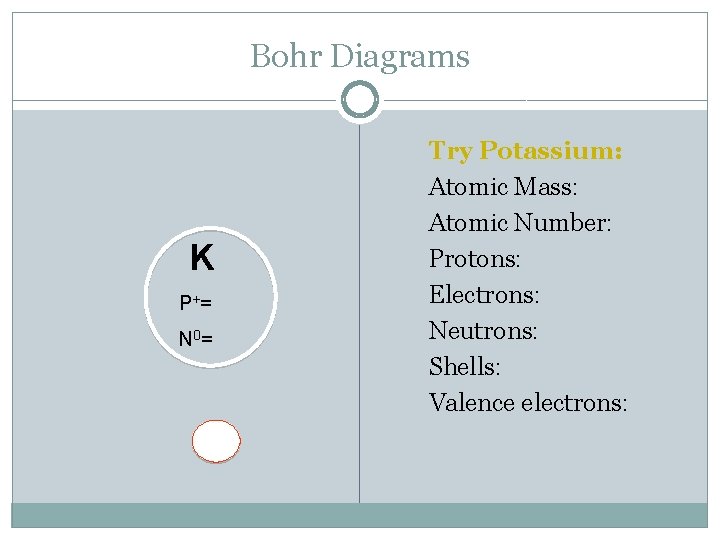
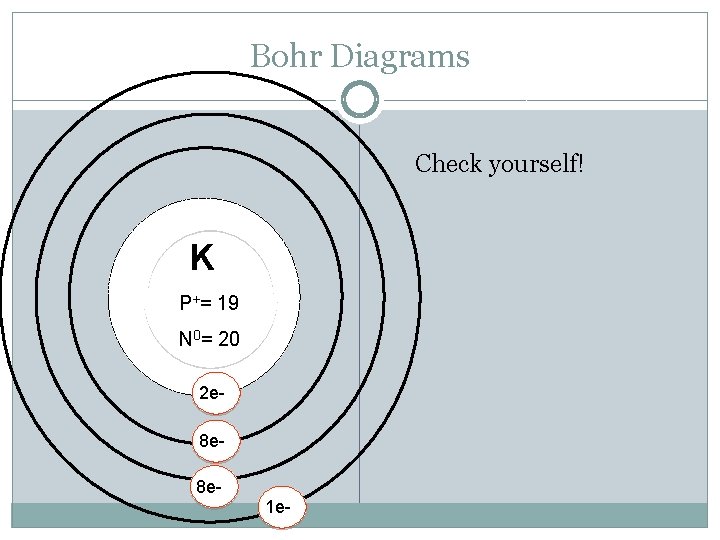
Bohr Diagrams K P+ = N 0 = Try Potassium: Atomic Mass: Atomic Number: Protons: Electrons: Neutrons: Shells: Valence electrons:

Bohr Diagrams Check yourself! K P+= 19 N 0= 20 2 e 8 e 8 e 1 e-

Bohr Diagrams You should know how to draw a Bohr Diagram for the first 20 elements.

Periodic Table Study Guide How to Draw Lewis Structures By Mrs. Hunt and Mrs. La. Rosa www. middleschoolscience. com 2008

Lewis Structures 1) Find the element on the periodic table. 2) Determine the number of valence electrons. 3) This is how many electrons you will draw.

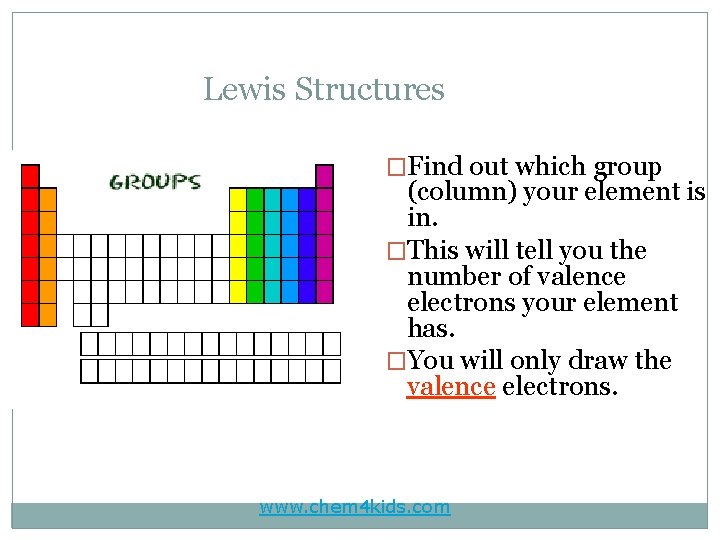
Lewis Structures �Find out which group (column) your element is in. �This will tell you the number of valence electrons your element has. �You will only draw the valence electrons. www. chem 4 kids. com

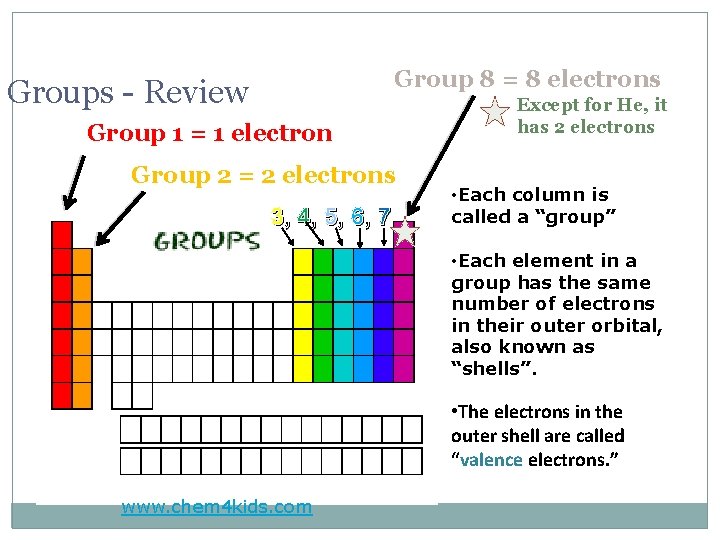
Group 8 = 8 electrons Groups - Review Group 1 = 1 electron Group 2 = 2 electrons 3, 4, 5, 6, 7 Except for He, it has 2 electrons • Each column is called a “group” • Each element in a group has the same number of electrons in their outer orbital, also known as “shells”. • The electrons in the outer shell are called “valence electrons. ” www. chem 4 kids. com

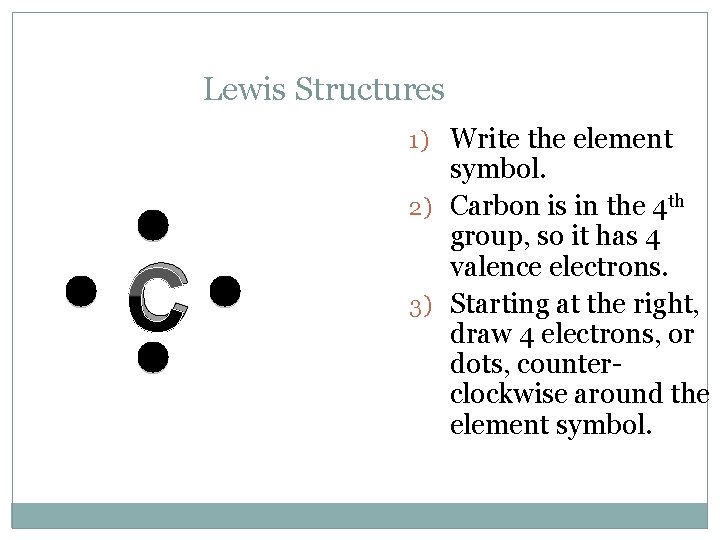
Lewis Structures 1) Write the element C symbol. 2) Carbon is in the 4 th group, so it has 4 valence electrons. 3) Starting at the right, draw 4 electrons, or dots, counterclockwise around the element symbol.


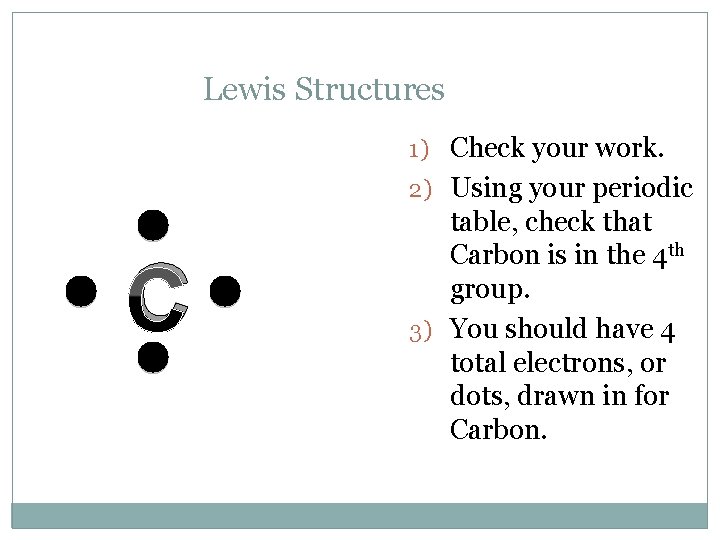
Lewis Structures 1) Check your work. 2) Using your periodic C table, check that Carbon is in the 4 th group. 3) You should have 4 total electrons, or dots, drawn in for Carbon.

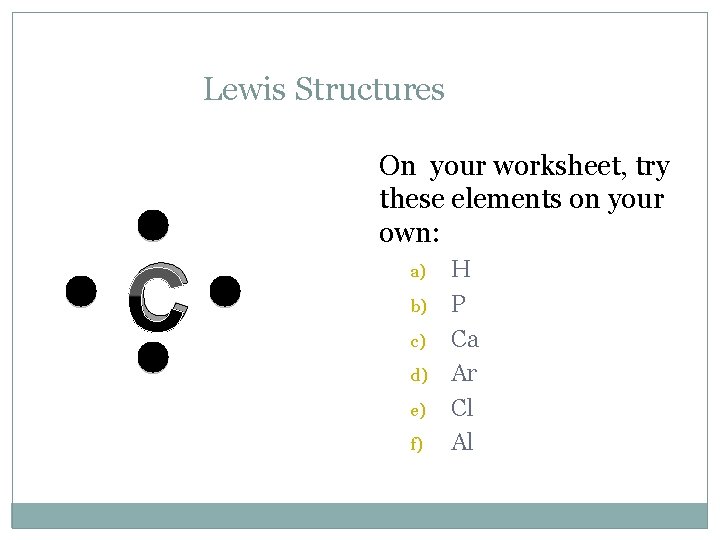
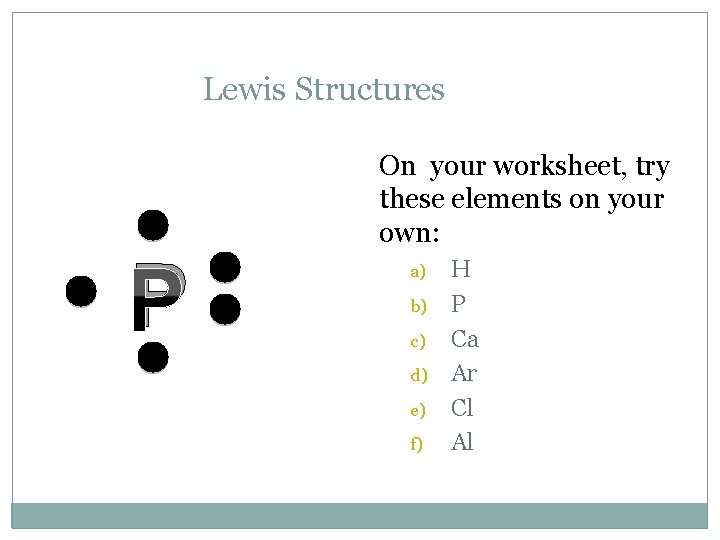
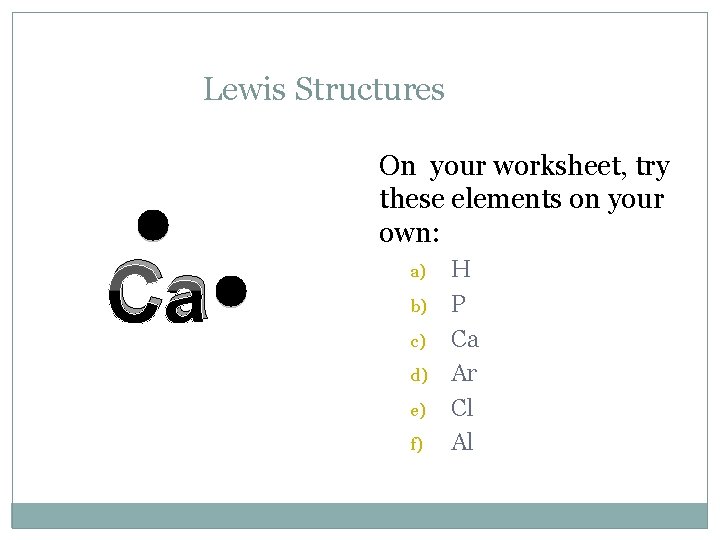
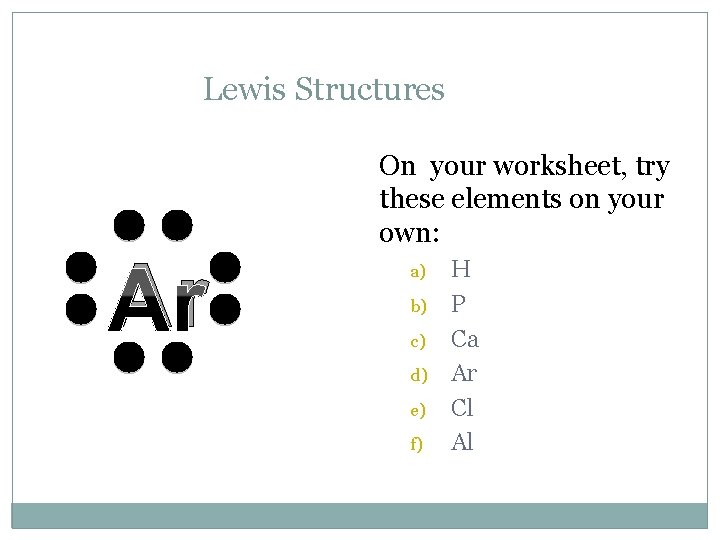
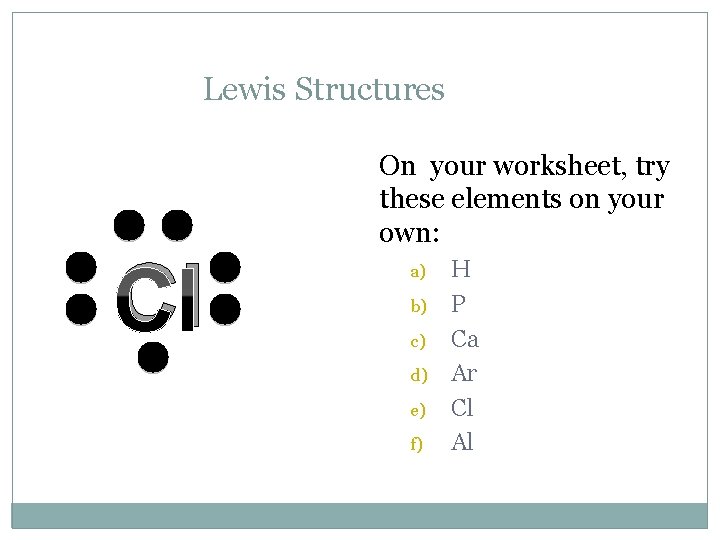
Lewis Structures C On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al

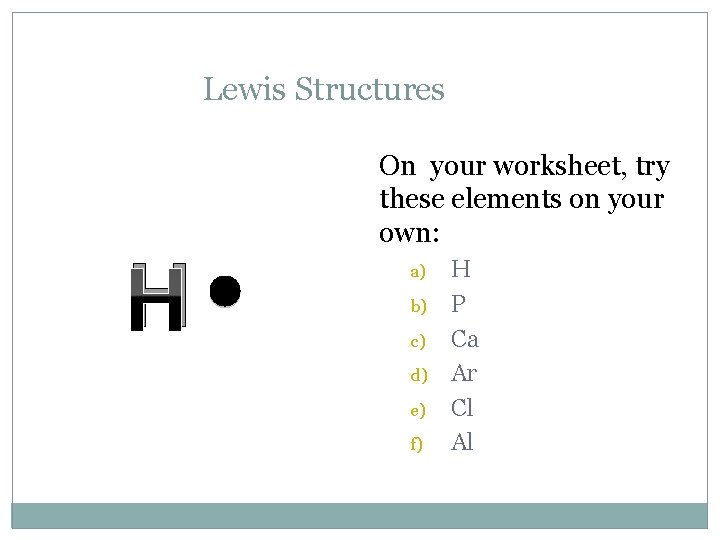
Lewis Structures H On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al

Lewis Structures P On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al

Lewis Structures Ca On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al

Lewis Structures Ar On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al

Lewis Structures Cl On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al

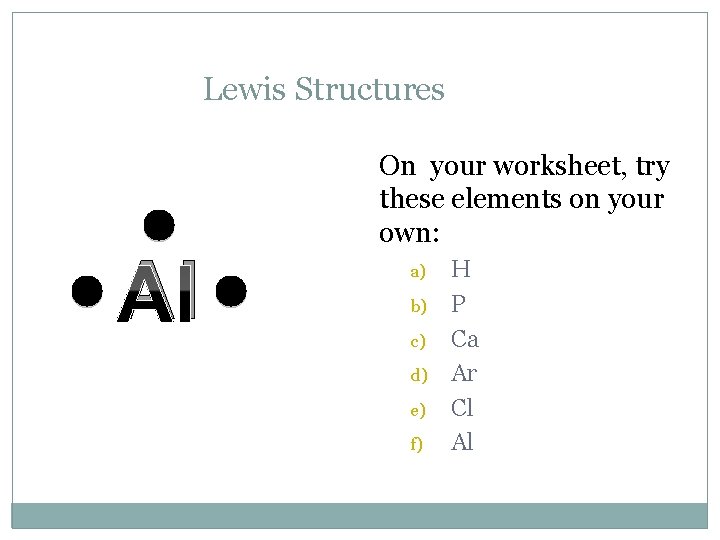
Lewis Structures Al On your worksheet, try these elements on your own: a) b) c) d) e) f) H P Ca Ar Cl Al

End of Study Guide. Complete the Lewis Structure Worksheet You should know how to draw Lewis Structures for the first 20 elements.