Chapter 4 Part 2 Protons neutrons and electrons






- Slides: 17

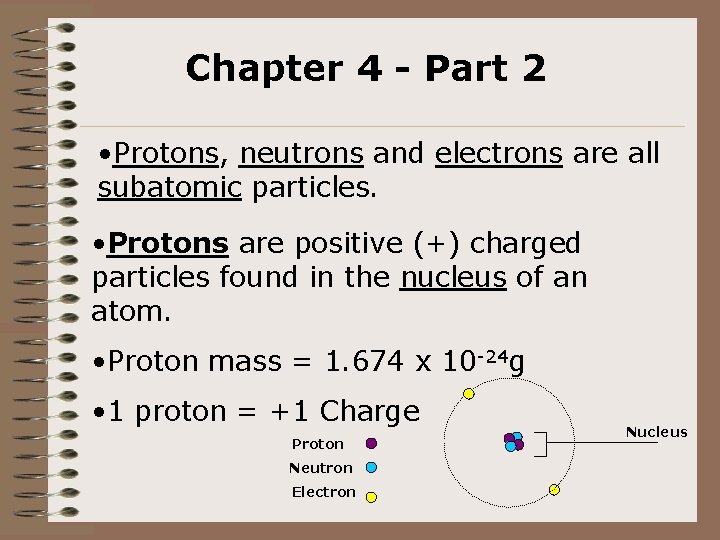
Chapter 4 - Part 2 • Protons, neutrons and electrons are all subatomic particles. • Protons are positive (+) charged particles found in the nucleus of an atom. • Proton mass = 1. 674 x 10 -24 g • 1 proton = +1 Charge Proton Neutron Electron Nucleus

The Neutron • The neutron is a subatomic particle found in the nucleus. • 1 neutron = no charge • The neutron was discovered in 1932 by James Chadwick. • Its mass is almost the same as a proton.

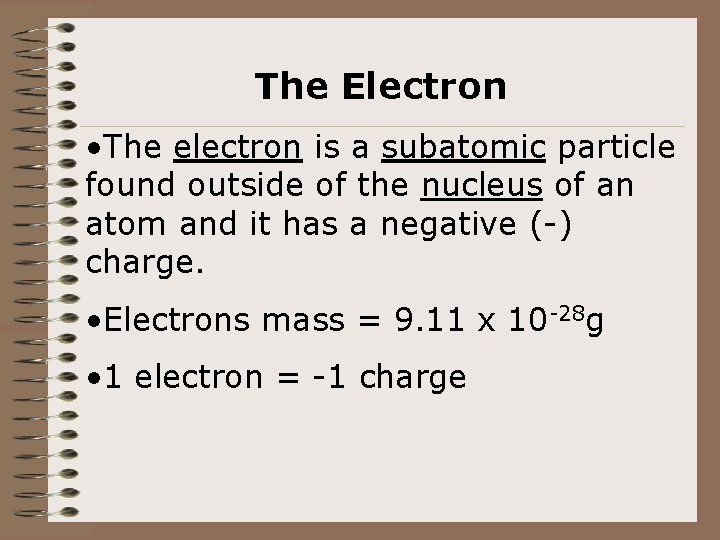
The Electron • The electron is a subatomic particle found outside of the nucleus of an atom and it has a negative (-) charge. • Electrons mass = 9. 11 x 10 -28 g • 1 electron = -1 charge

Charge 4 A proton has a positive charge +1 4 An electron has a negative charge -1 4 A neutron is neutral. It won’t affect overall charge. 4 One positive proton will cancel out one negative electron leaving an overall charge of Zero 0 +1 -1 Overall Charge = 0

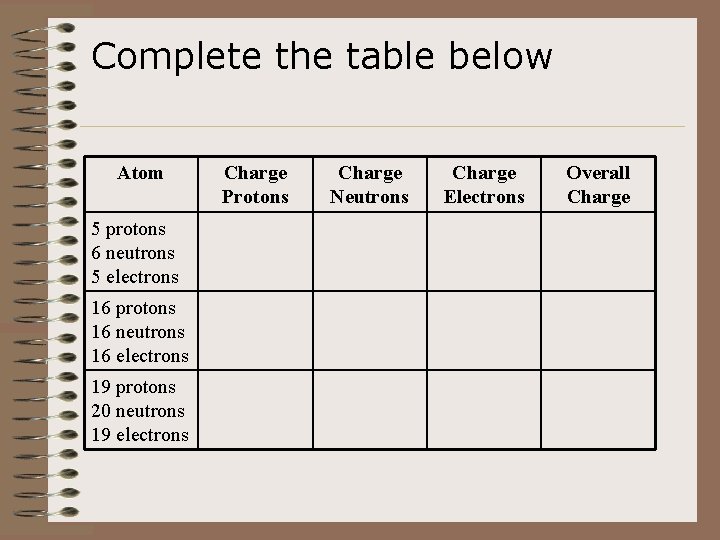
Complete the table below Atom 5 protons 6 neutrons 5 electrons 16 protons 16 neutrons 16 electrons 19 protons 20 neutrons 19 electrons Charge Protons Charge Neutrons Charge Electrons Overall Charge

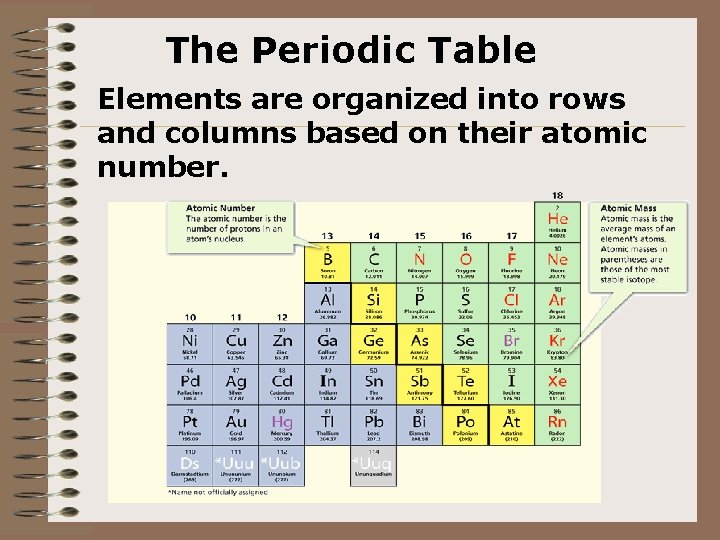
The Periodic Table Elements are organized into rows and columns based on their atomic number.

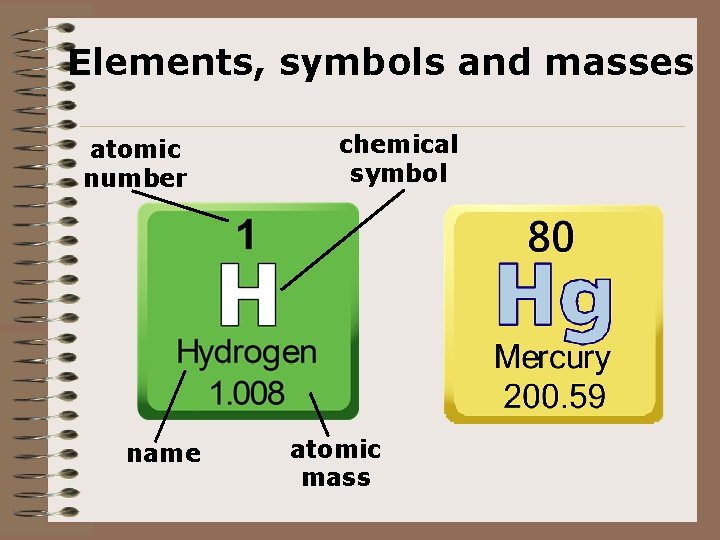
Elements, symbols and masses atomic number name chemical symbol atomic mass

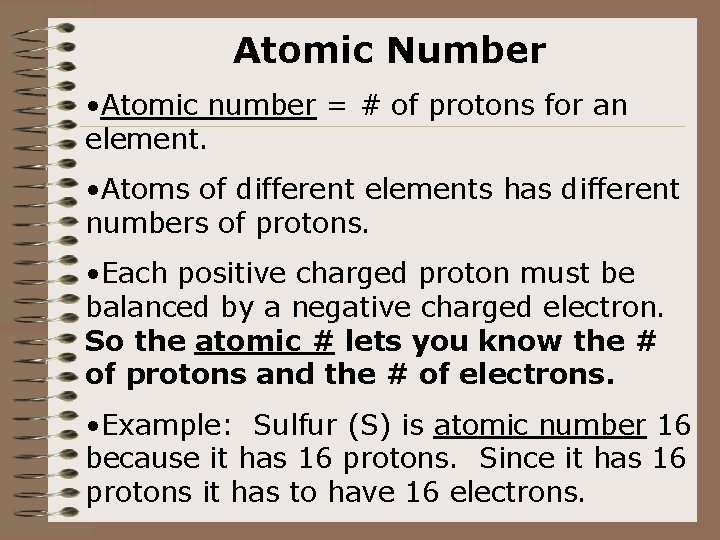
Atomic Number • Atomic number = # of protons for an element. • Atoms of different elements has different numbers of protons. • Each positive charged proton must be balanced by a negative charged electron. So the atomic # lets you know the # of protons and the # of electrons. • Example: Sulfur (S) is atomic number 16 because it has 16 protons. Since it has 16 protons it has to have 16 electrons.

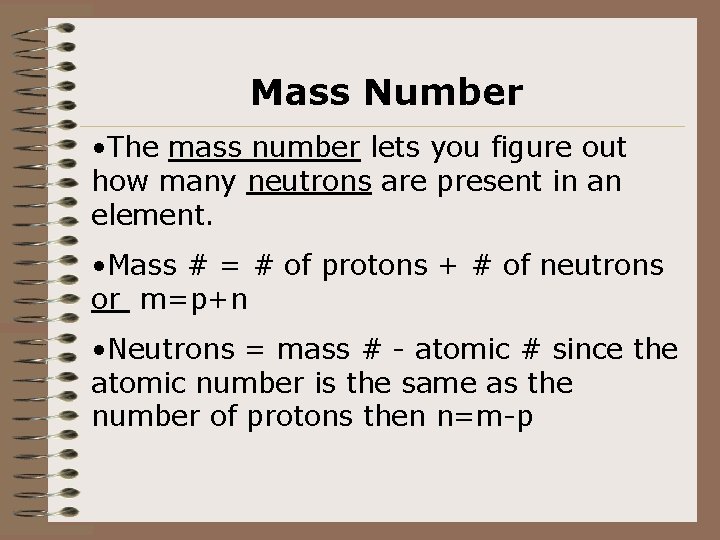
Mass Number • The mass number lets you figure out how many neutrons are present in an element. • Mass # = # of protons + # of neutrons or m=p+n • Neutrons = mass # - atomic # since the atomic number is the same as the number of protons then n=m-p

Mass number continued… • Example: Aluminum (Al) has 13 protons. Its mass number is 27. So, 27 -13=14 and 14 is the number of neutrons found in an atom of Aluminum.

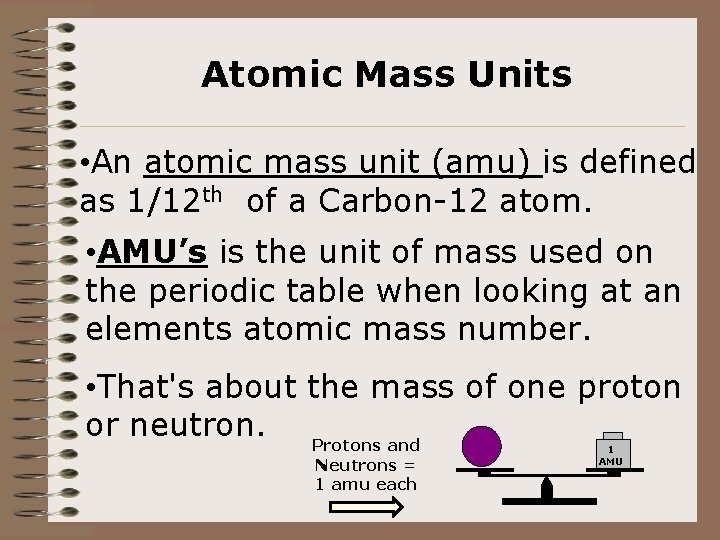
Atomic Mass Units • An atomic mass unit (amu) is defined as 1/12 th of a Carbon-12 atom. • AMU’s is the unit of mass used on the periodic table when looking at an elements atomic mass number. • That's about the mass of one proton or neutron. Protons and Neutrons = 1 amu each 1 AMU U

AMU’s continued… • 1 atomic mass unit = 1. 66053886 × 10 -24 kg • One gram is about 600, 000, 000, 000 amu. • Ex: Fluorine’s (F) atomic mass is 19 amu, but it’s mass in grams is 3. 155 x 10 -28 grams. • Wow--I can see why you'd want to use amu's instead of grams.

? What is the atomic mass of an atom with… 4 protons and 5 neutrons = 24 protons and 28 neutrons = 7 protons and 7 neutrons = 91 protons and 140 neutrons =

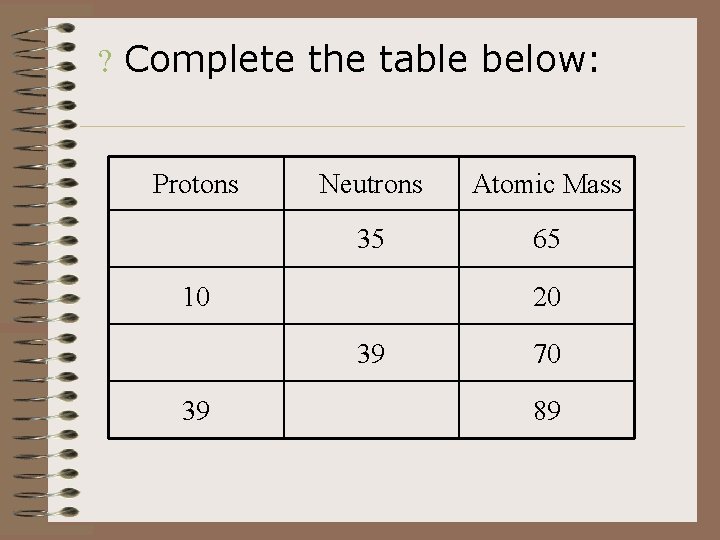
? Complete the table below: Protons Neutrons Atomic Mass 35 65 10 20 39 39 70 89

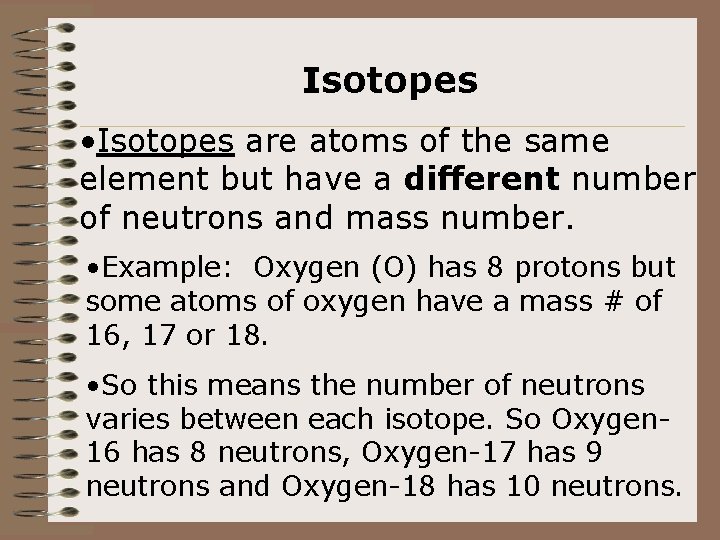
Isotopes • Isotopes are atoms of the same element but have a different number of neutrons and mass number. • Example: Oxygen (O) has 8 protons but some atoms of oxygen have a mass # of 16, 17 or 18. • So this means the number of neutrons varies between each isotope. So Oxygen 16 has 8 neutrons, Oxygen-17 has 9 neutrons and Oxygen-18 has 10 neutrons.

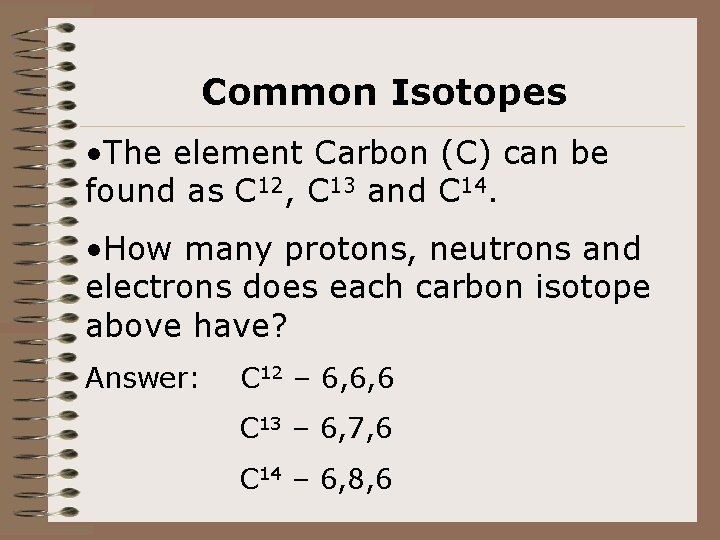
Common Isotopes • The element Carbon (C) can be found as C 12, C 13 and C 14. • How many protons, neutrons and electrons does each carbon isotope above have? Answer: C 12 – 6, 6, 6 C 13 – 6, 7, 6 C 14 – 6, 8, 6

Hydrogen Isotopes • Hydrogen-1 doesn’t have any neutrons. • Hydrogen-2 has 1 neutron • Hydrogen-3 has 2 neutrons