GEOMETRY OF MOLECULES NIKAM N D DEPARTMENT OF












- Slides: 38

GEOMETRY OF MOLECULES NIKAM N. D. DEPARTMENT OF CHEMISTRY










Rules for Predicting Molecular Geometry 1. Sketch the Lewis structure of the molecule or ion 2. Count the electron pairs and arrange them in the way that minimizes electron-pair repulsion. 3. Determine the position of the atoms from the way the electron pairs are shared. 4. Determine the name of the molecular structure from the position of the atoms. 5. Double or triple bonds are counted as one bonding pair when predicting geometry.


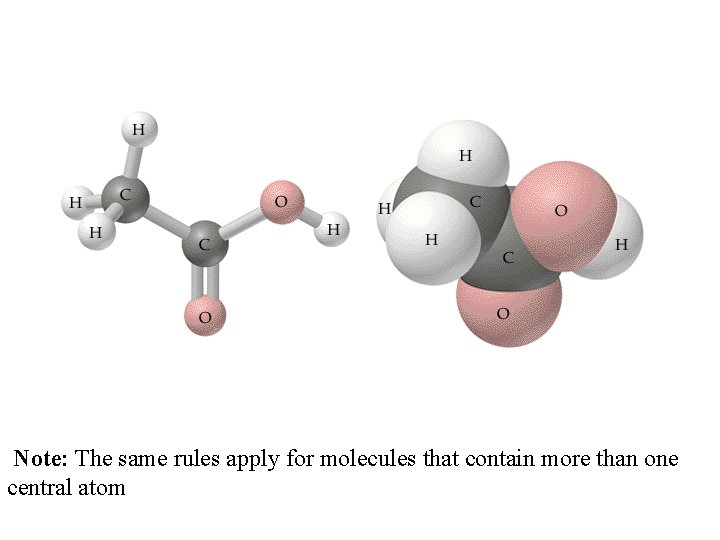
Note: The same rules apply for molecules that contain more than one central atom

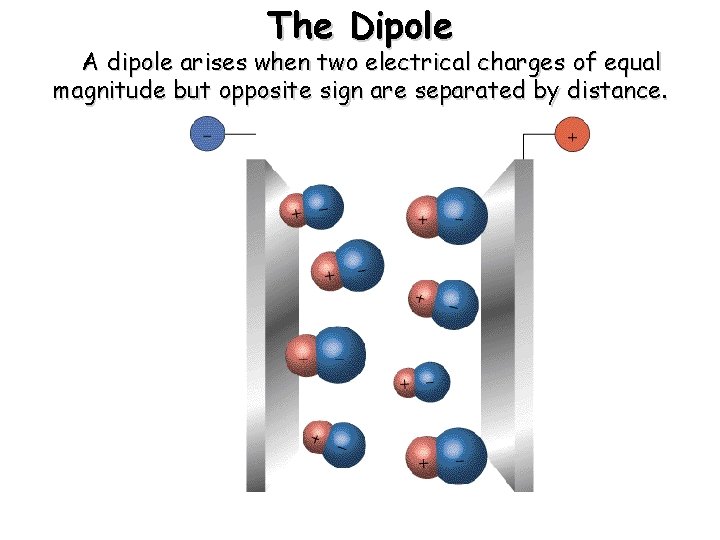
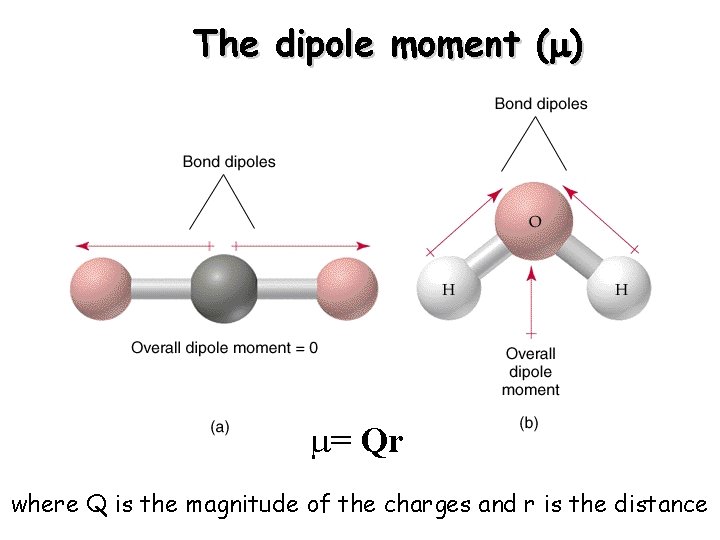
The Dipole A dipole arises when two electrical charges of equal magnitude but opposite sign are separated by distance.

The dipole moment (m) m= Qr where Q is the magnitude of the charges and r is the distance

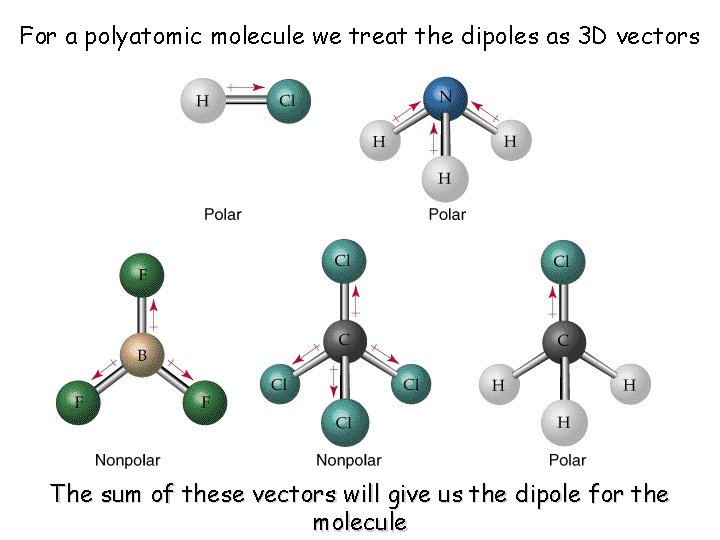
For a polyatomic molecule we treat the dipoles as 3 D vectors The sum of these vectors will give us the dipole for the molecule

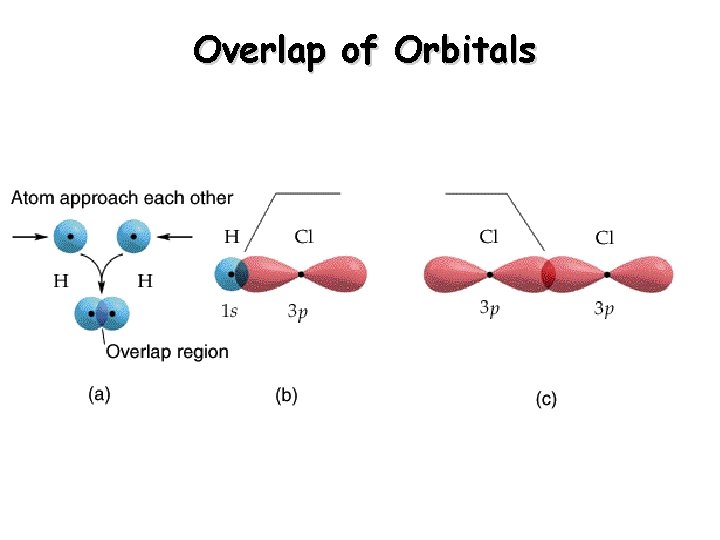
Overlap of Orbitals

The degree of overlap is determined by the system’s potential energy equilibrium bond distance The point at which the potential energy is a minimum is called the equilibrium bond distance

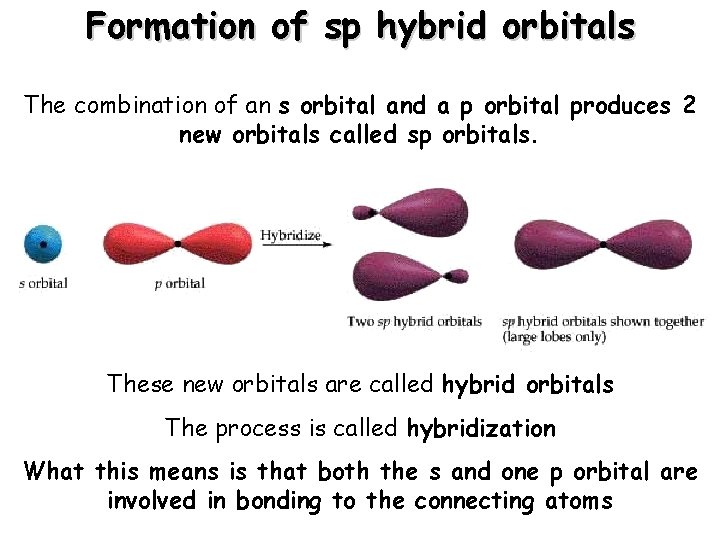
Formation of sp hybrid orbitals The combination 2 s of an s orbital and a p orbital produces 2 new orbitals called sp orbitals. These new orbitals are called hybrid orbitals The process is called hybridization What this means is that both the s and one p orbital are involved in bonding to the connecting atoms

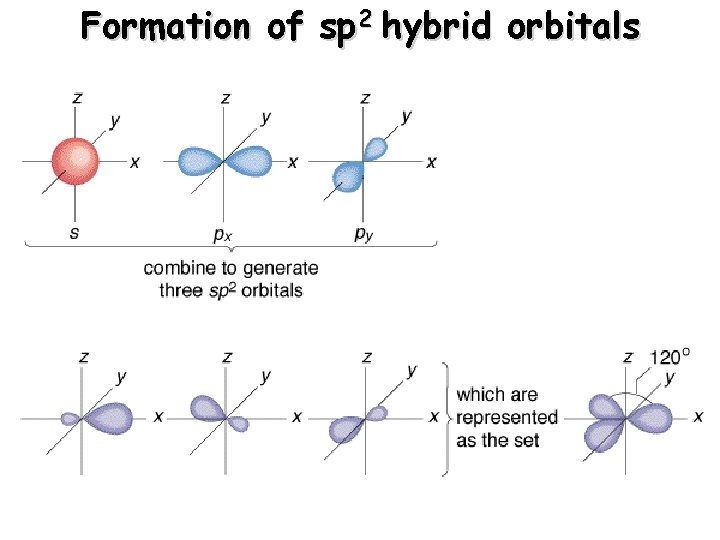
Formation of sp 2 hybrid orbitals

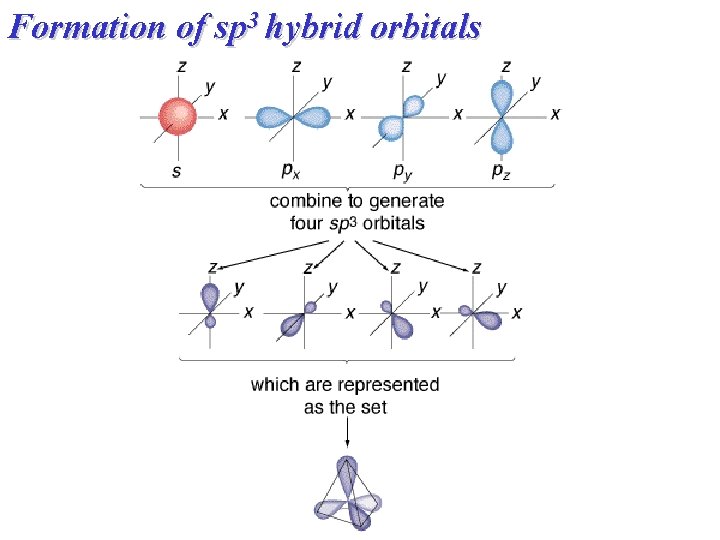
Formation of sp 3 hybrid orbitals

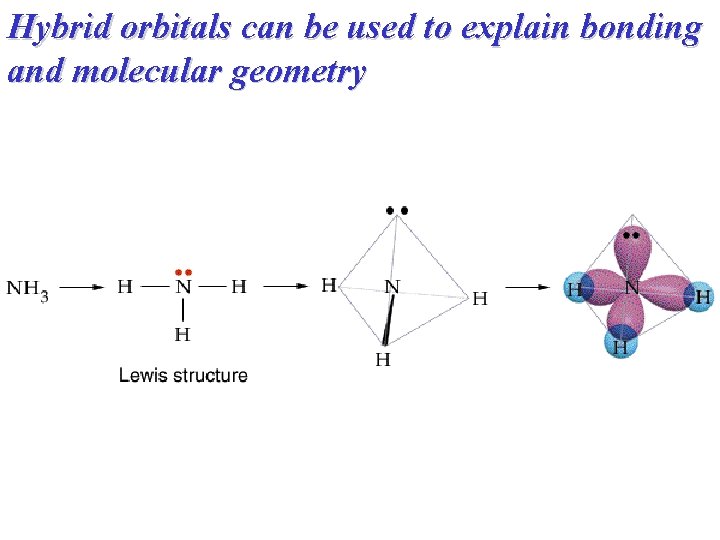
Hybrid orbitals can be used to explain bonding and molecular geometry

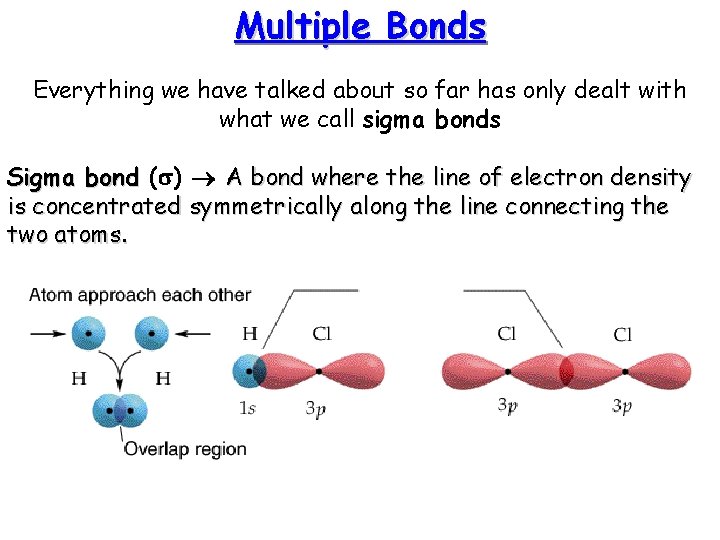
Multiple Bonds Everything we have talked about so far has only dealt with what we call sigma bonds Sigma bond (s) A bond where the line of electron density is concentrated symmetrically along the line connecting the two atoms.

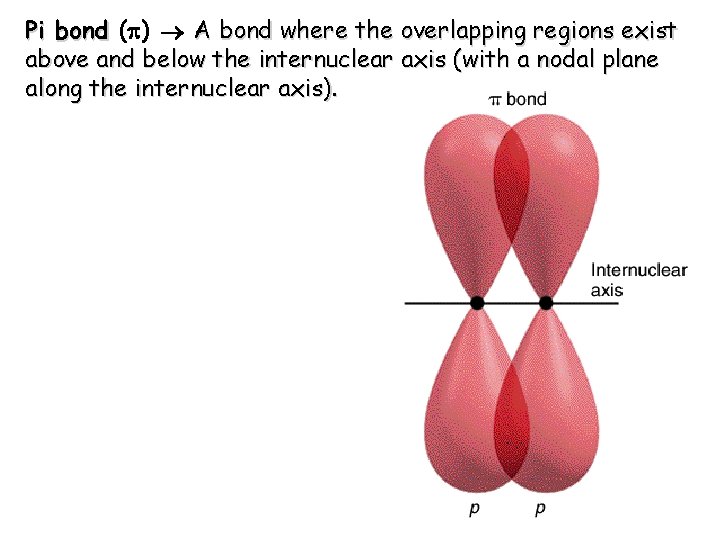
Pi bond (p) A bond where the overlapping regions exist above and below the internuclear axis (with a nodal plane along the internuclear axis).

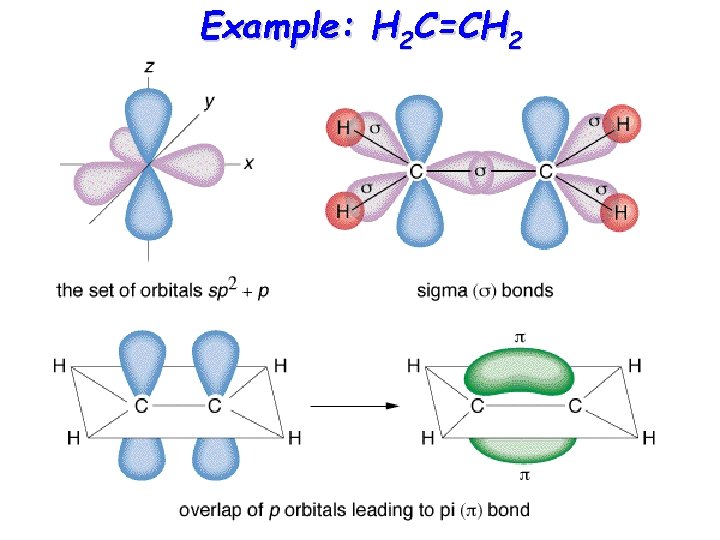
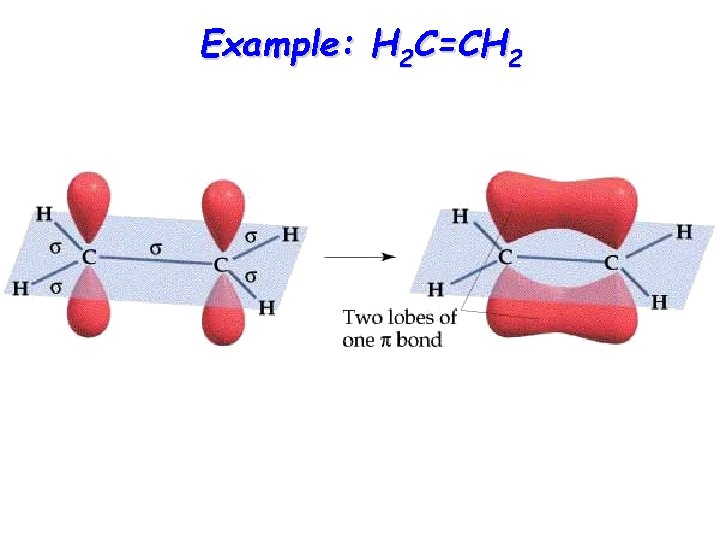
Example: H 2 C=CH 2

Example: H 2 C=CH 2

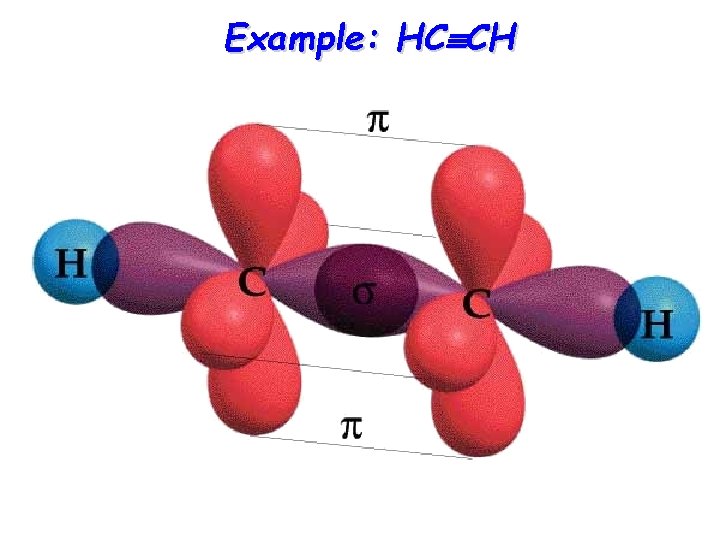
Example: HC CH

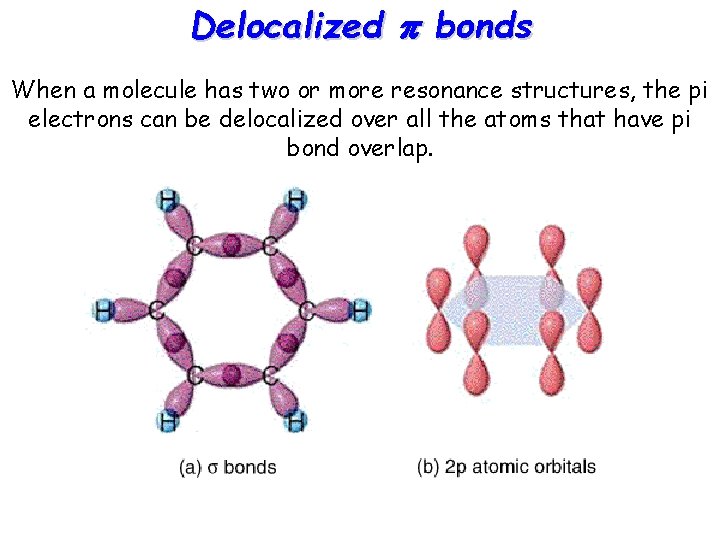
Delocalized p bonds When a molecule has two or more resonance structures, the pi electrons can be delocalized over all the atoms that have pi bond overlap.

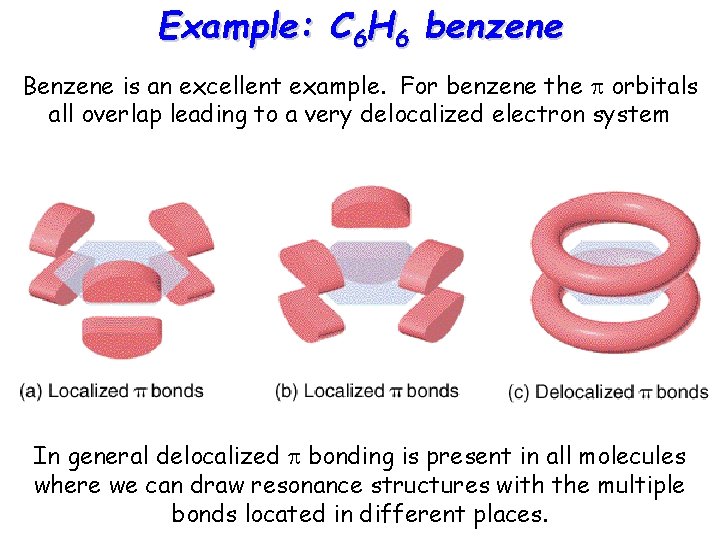
Example: C 6 H 6 benzene Benzene is an excellent example. For benzene the p orbitals all overlap leading to a very delocalized electron system In general delocalized p bonding is present in all molecules where we can draw resonance structures with the multiple bonds located in different places.

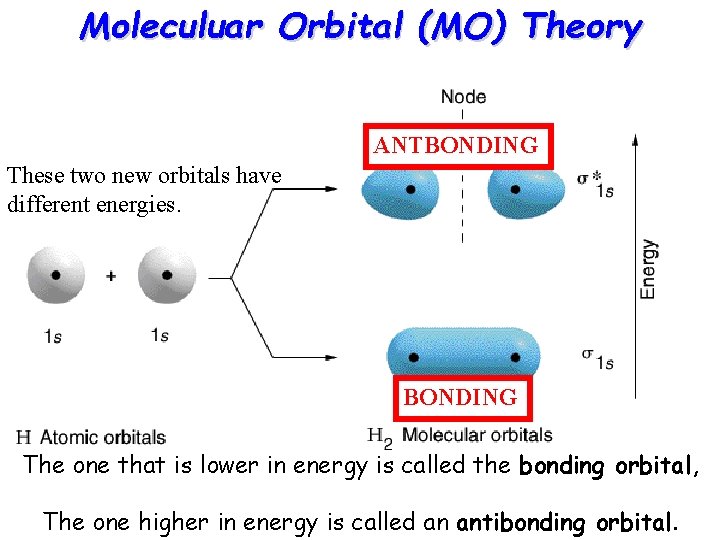
Moleculuar Orbital (MO) Theory ANTBONDING These two new orbitals have different energies. BONDING The one that is lower in energy is called the bonding orbital, The one higher in energy is called an antibonding orbital.

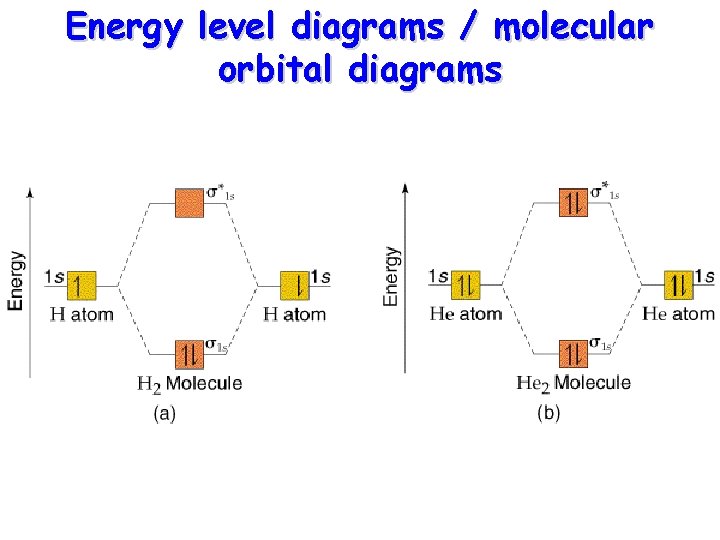
Energy level diagrams / molecular orbital diagrams

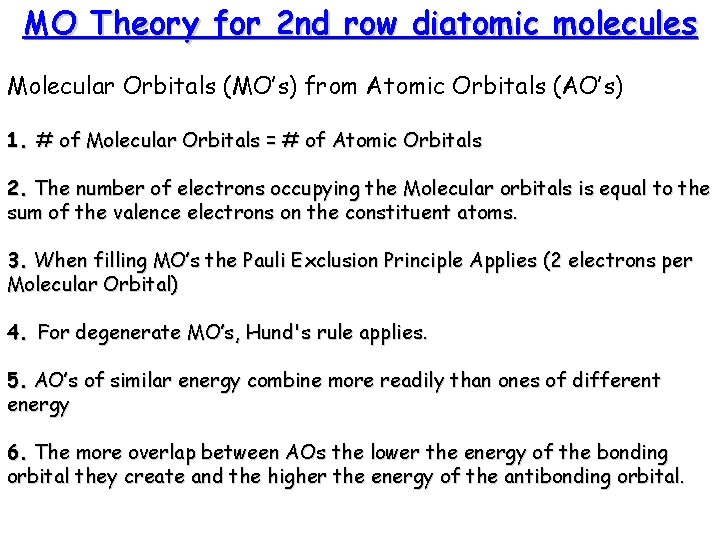
MO Theory for 2 nd row diatomic molecules Molecular Orbitals (MO’s) from Atomic Orbitals (AO’s) 1. # of Molecular Orbitals = # of Atomic Orbitals 2. The number of electrons occupying the Molecular orbitals is equal to the sum of the valence electrons on the constituent atoms. 3. When filling MO’s the Pauli Exclusion Principle Applies (2 electrons per Molecular Orbital) 4. For degenerate MO’s, Hund's rule applies. 5. AO’s of similar energy combine more readily than ones of different energy 6. The more overlap between AOs the lower the energy of the bonding orbital they create and the higher the energy of the antibonding orbital.

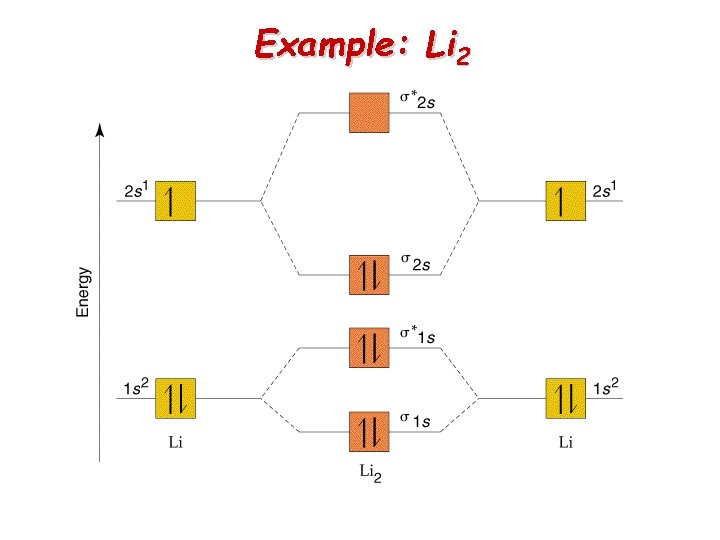
Example: Li 2

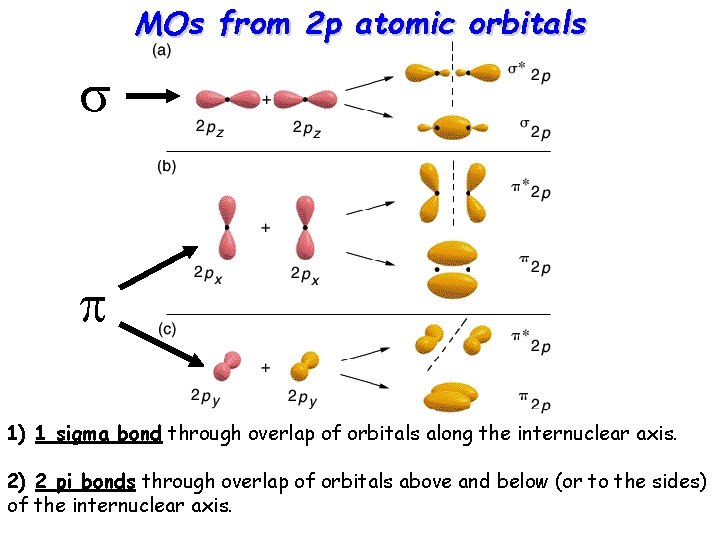
MOs from 2 p atomic orbitals s p 1) 1 sigma bond through overlap of orbitals along the internuclear axis. 2) 2 pi bonds through overlap of orbitals above and below (or to the sides) of the internuclear axis.


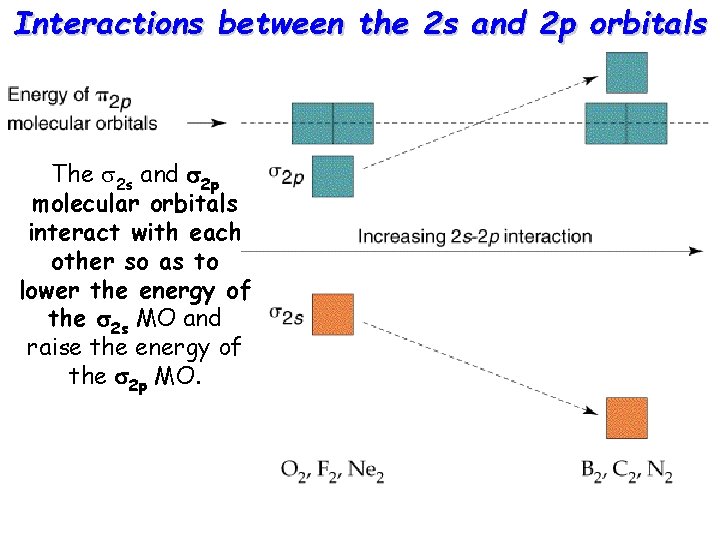
Interactions between the 2 s and 2 p orbitals The s 2 s and s 2 p molecular orbitals interact with each other so as to lower the energy of the s 2 s MO and raise the energy of the s 2 p MO.

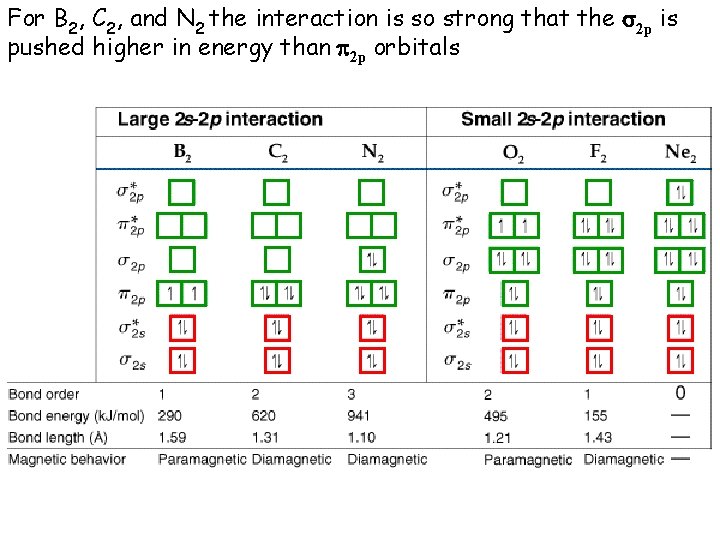
For B 2, C 2, and N 2 the interaction is so strong that the s 2 p is pushed higher in energy than p 2 p orbitals

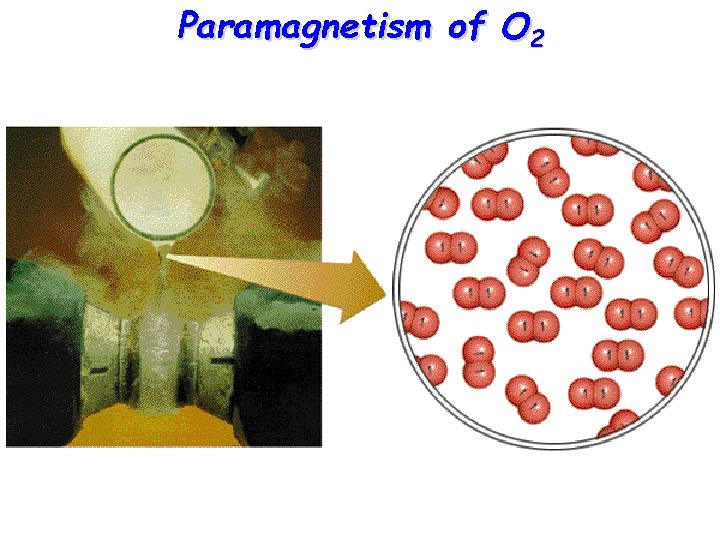
Paramagnetism of O 2
 Organic molecules vs inorganic molecules
Organic molecules vs inorganic molecules Theory of structures
Theory of structures Electron domain geometry vs molecular geometry
Electron domain geometry vs molecular geometry The basis of the vsepr model of molecular bonding is _____.
The basis of the vsepr model of molecular bonding is _____. Its molecules are closely packed together.
Its molecules are closely packed together. What is radiation examples
What is radiation examples Four main types of carbon based molecules
Four main types of carbon based molecules Atoms elements molecules and compounds worksheet
Atoms elements molecules and compounds worksheet Cecil writes the equation for the reaction of hydrogen
Cecil writes the equation for the reaction of hydrogen Chapter 3 molecules of life
Chapter 3 molecules of life Shiva amiri
Shiva amiri Atoms molecules and ions
Atoms molecules and ions Molecules to grams
Molecules to grams Biological molecules
Biological molecules Kinetic energy of gas molecules
Kinetic energy of gas molecules Number of oxygen molecules
Number of oxygen molecules Bcl2 molecular geometry
Bcl2 molecular geometry Permanent dipole moment
Permanent dipole moment A solid compound that contains water molecules
A solid compound that contains water molecules Electronic spectra of polyatomic molecules
Electronic spectra of polyatomic molecules Carbon based molecules
Carbon based molecules Solid molecules
Solid molecules Carbonbased molecules in
Carbonbased molecules in All molecule shapes
All molecule shapes Siof2 lewis structure
Siof2 lewis structure Gram to liter conversion
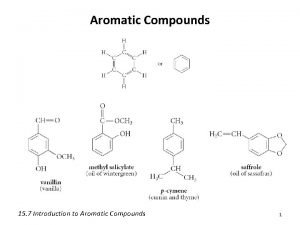
Gram to liter conversion Which of the following molecules are aromatic hydrocarbons
Which of the following molecules are aromatic hydrocarbons Building molecules lab answers
Building molecules lab answers Chapter 2 atoms molecules and ions
Chapter 2 atoms molecules and ions Chiral molecule
Chiral molecule Intermolecular and intramolecular forces
Intermolecular and intramolecular forces Are water molecules polar
Are water molecules polar Non polar molecules that include fats oils and cholesterol
Non polar molecules that include fats oils and cholesterol Liquid molecules
Liquid molecules Molecules biology definition
Molecules biology definition Polarity arrows
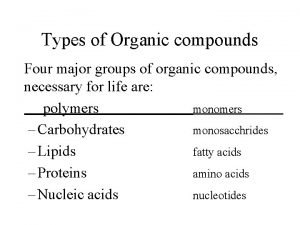
Polarity arrows Four types of organic compound
Four types of organic compound Target molecules
Target molecules Which molecule is polar
Which molecule is polar