NIKAM N D M Sc NET DEPARTMENT OF













- Slides: 22

NIKAM N. D. M. Sc. NET DEPARTMENT OF CHEMISTRY

Sulfuric Acid H 2 SO 4 • commercially important raw material

Sulfuric Acid H 2 SO 4 • commercially important raw material • more sulfuric acid made than any other compound

Sulfuric Acid H 2 SO 4 • commercially important raw material • more sulfuric acid made than any other compound • fertilisers, paints and pigments, detergents, car batteries, laboratory acid

Sulfuric Acid H 2 SO 4 • manufacture • properties • uses

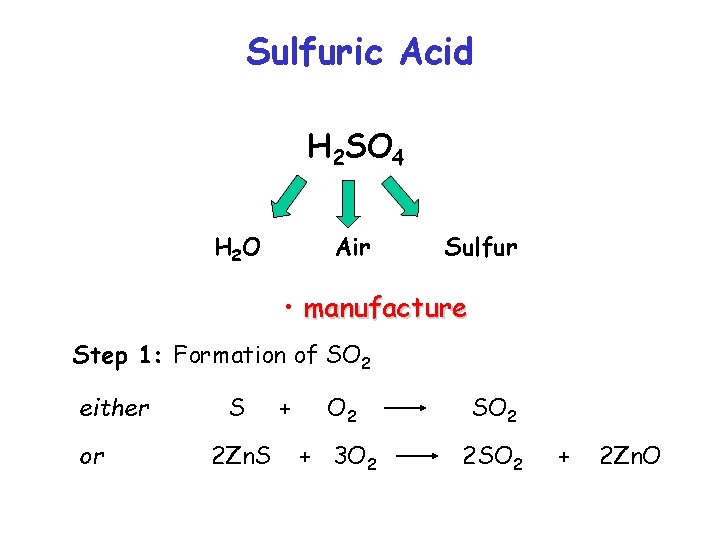
Sulfuric Acid H 2 SO 4 H 2 O Air Sulfur • manufacture • properties • uses

Sulfuric Acid H 2 SO 4 H 2 O Air Sulfur • manufacture Step 1: Formation of SO 2 either or S 2 Zn. S + O 2 SO 2 + 3 O 2 2 SO 2 + 2 Zn. O

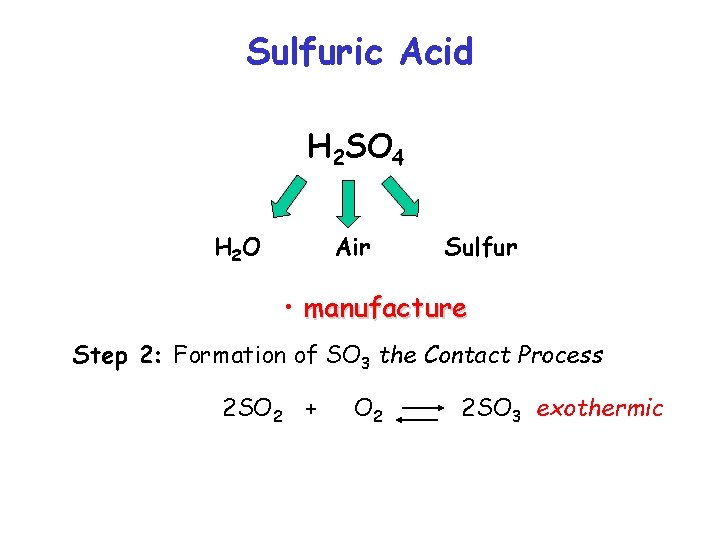
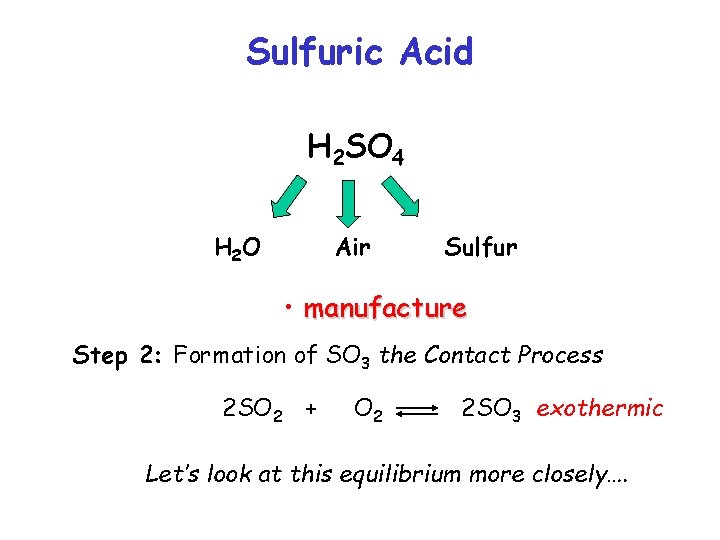
Sulfuric Acid H 2 SO 4 H 2 O Air Sulfur • manufacture Step 2: Formation of SO 3 the Contact Process 2 SO 2 + O 2 2 SO 3 exothermic

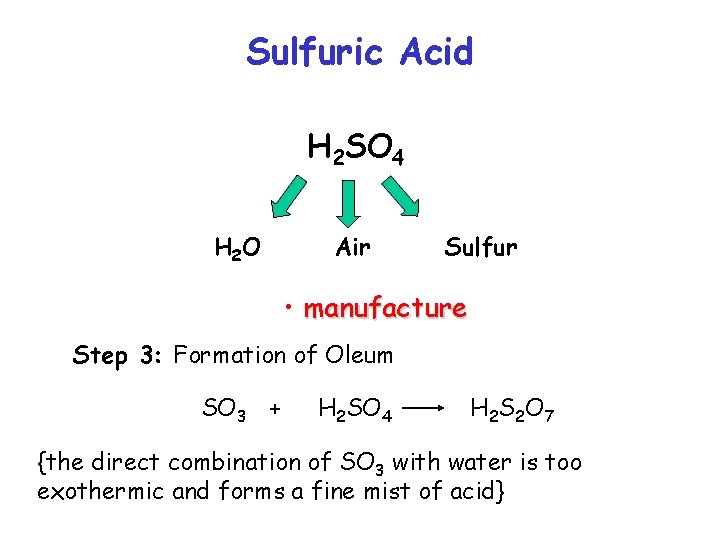
Sulfuric Acid H 2 SO 4 H 2 O Air Sulfur • manufacture Step 3: Formation of Oleum SO 3 + H 2 SO 4 H 2 S 2 O 7 {the direct combination of SO 3 with water is too exothermic and forms a fine mist of acid}

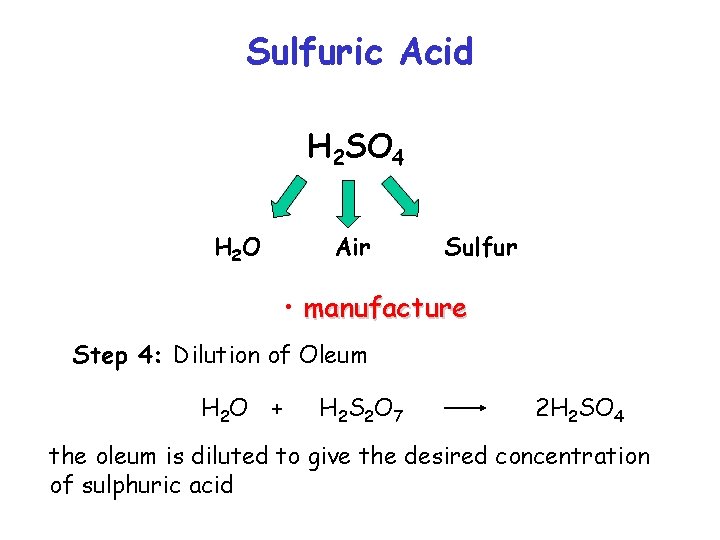
Sulfuric Acid H 2 SO 4 H 2 O Air Sulfur • manufacture Step 4: Dilution of Oleum H 2 O + H 2 S 2 O 7 2 H 2 SO 4 the oleum is diluted to give the desired concentration of sulphuric acid

Sulfuric Acid H 2 SO 4 H 2 O Air Sulfur • manufacture Step 2: Formation of SO 3 the Contact Process 2 SO 2 + O 2 2 SO 3 exothermic Let’s look at this equilibrium more closely….

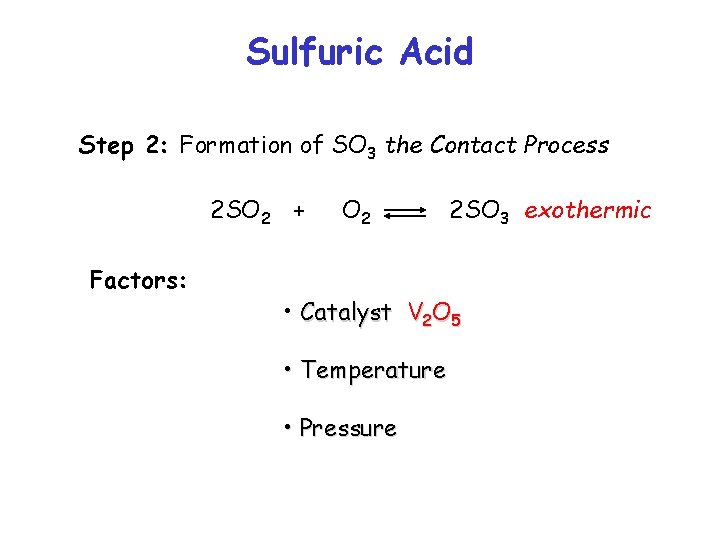
Sulfuric Acid Step 2: Formation of SO 3 the Contact Process 2 SO 2 + Factors: O 2 • Catalyst • Temperature • Pressure 2 SO 3 exothermic

Sulfuric Acid Step 2: Formation of SO 3 the Contact Process 2 SO 2 + Factors: O 2 2 SO 3 exothermic • Catalyst V 2 O 5 • Temperature • Pressure

Sulfuric Acid Step 2: Formation of SO 3 the Contact Process 2 SO 2 + Factors: O 2 2 SO 3 exothermic • Catalyst V 2 O 5 • Temperature 450 o. C • Pressure

Sulfuric Acid Step 2: Formation of SO 3 the Contact Process 2 SO 2 + Factors: O 2 2 SO 3 exothermic • Catalyst V 2 O 5 • Temperature 450 o. C • Pressure 1 -2 atmospheres ***These are compromise conditions***

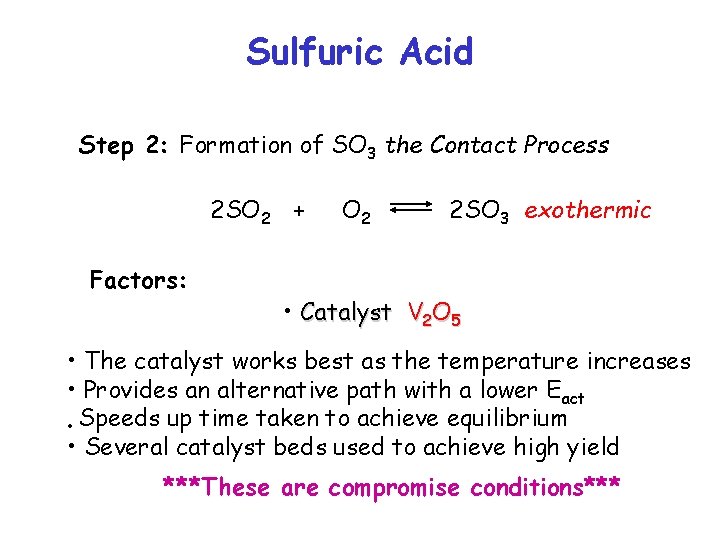
Sulfuric Acid Step 2: Formation of SO 3 the Contact Process 2 SO 2 + Factors: O 2 2 SO 3 exothermic • Catalyst V 2 O 5 • The catalyst works best as the temperature increases • Provides an alternative path with a lower Eact • Speeds up time taken to achieve equilibrium • Several catalyst beds used to achieve high yield ***These are compromise conditions***

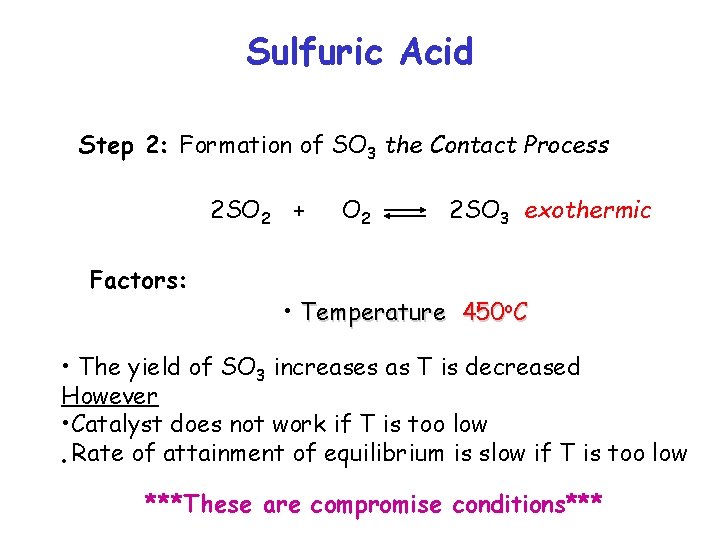
Sulfuric Acid Step 2: Formation of SO 3 the Contact Process 2 SO 2 + Factors: O 2 2 SO 3 exothermic • Temperature 450 o. C • The yield of SO 3 increases as T is decreased However • Catalyst does not work if T is too low • Rate of attainment of equilibrium is slow if T is too low ***These are compromise conditions***

Sulfuric Acid Step 2: Formation of SO 3 the Contact Process 2 SO 2 + Factors: O 2 2 SO 3 exothermic • Pressure 1 -2 atmospheres • The yield of SO 3 increases as P is increased • High pressure is costly £££ ***These are compromise conditions***

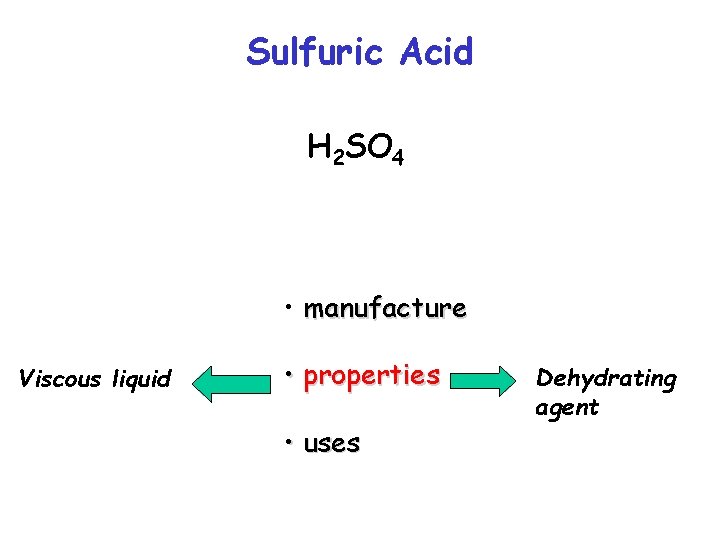
Sulfuric Acid H 2 SO 4 • manufacture • properties • uses

Sulfuric Acid H 2 SO 4 • manufacture Viscous liquid • properties • uses Dehydrating agent

Sulfuric Acid H 2 SO 4 • manufacture Viscous liquid • properties • uses Dehydrating agent Conc H 2 SO 4 will dehydrated copper sulphate and carbohydrates

Sulfuric Acid H 2 SO 4 Describe briefly what you observe when the following are treated with concentrated sulphuric acid. • sugar (a carbohydrate) • blue copper sulphate crystals
 Achmed lach net
Achmed lach net Ado.net vb.net
Ado.net vb.net Department of materials science and engineering iit delhi
Department of materials science and engineering iit delhi Tennessee department of health licensure
Tennessee department of health licensure Electrical engineering department
Electrical engineering department Sparks police report
Sparks police report Republic of the philippines department of education
Republic of the philippines department of education Food laws in pakistan
Food laws in pakistan Computer science tutor bridgeport
Computer science tutor bridgeport Department of corrections wisconsin
Department of corrections wisconsin Sales and marketing department in hotel
Sales and marketing department in hotel Department management services dms
Department management services dms Illinois department of rehabilitation
Illinois department of rehabilitation Usf chemistry
Usf chemistry Operational plan
Operational plan Leather quality control
Leather quality control Guthrie health department
Guthrie health department Njit physics department
Njit physics department Erie county department of mental health
Erie county department of mental health It department
It department Washington state department of social and health services
Washington state department of social and health services 5 responsibilities of the maintenance department
5 responsibilities of the maintenance department Maricopa county department of transportation
Maricopa county department of transportation