Gasses and Kinetic Theory Schedule for Week M
























- Slides: 29

Gasses and Kinetic Theory

Schedule for Week • • • M – define terms/Presentation Sign-up T – Boyles Law Lab W – Lab Discussion R – Charles Law Lab F – Quiz or Presentation

Key terms - Define • • Kinetic Theory Standard Conditions Pressure Temperature – Fahrenheit – Celsius – Kelvin • • Boyles Law Charles Law Dalton's Law Ideal Gas/ Ideal Gas Law

Boyles Law lab • • Equation Variables Constants Artfully construct tool • Gather spectacular data, graph with excel, answer questions • Treat books respectfully

Wed-Thur • Work on completing Boyles law lab – mail or print out lab and turn in. • Begin Charles law lab. . ask questions – Due Friday. . • Assign for Thur 2/9 – Text, Page, 436 Questions; 14 -15 • Assign for Fri 2/10 – Text, Page 436, Questions; 9 -10

Question Before Lab • A balloon is filled with lung gas to a volume of 2. 00 liters (lung temp = body temp). The balloon is placed in a kitchenaide freezer and assumes a new volume of 2500 ml. What is the temperature of the freezer? Would you keep your meet in this freezer?

AChem Friday Part 1 – Either Quiz up front Or Quiet Presentation prep in back Part 2 – Presentation State which law likely explains phenomenon demonstrated Observers, be respectful and appreciative! Part 3 – Homework for weekend Online quiz – Boyles and Charles Law Calcs. Note – Monday We will finish data collection for labs

Quiz 1)A Marshmellow at sea level has a volume of 55 ml (STP). Suppose someone put the marshmellow in their pocket and jumps on a plane. At altitude the cabin pressure is 625 mm Hg. Determine the volume of the marshmellow. . 2) Is a thermometer based on Boyle’s or Charles law? Explain. .

Practice 1 A balloon having a volume of 1. 5 l, is taken from room temperature and placed in a freezer with temperature of -20 C. The balloon now has a volume of. 65 l. Calculate the temperature of the room. Law: Equation:

Practice 2 A balloon having a volume of 25 ml, is taken from room temperature (295 K)and placed in a freezer with temperature of -20 C. Calculate the volume of the balloon. Law: Equation:

Practice 3 A sealed syringe with volume of 75 ml is placed in a vacuum chamber. Air is “sucked” out until the pressure is 125 mm Hg and the syringe has a volume of 15 torr. Determine the pressure while the syringe was sealed. Law: Equation:

Practice 4 A marshmallow at STP has an unknown volume. The mallow is placed in a microwave and heated to 75 C, bloating to a megamallow volume of. 85 l. Determine the initial volume of the (mini)mallo Law: Equation:

Practice 5 See Demonstration Law: Equation:

Homework P 436 Q 23 b=2700 mm. Hg c=334 K Q 24 b=. 334 ATM c=. 0257 moles

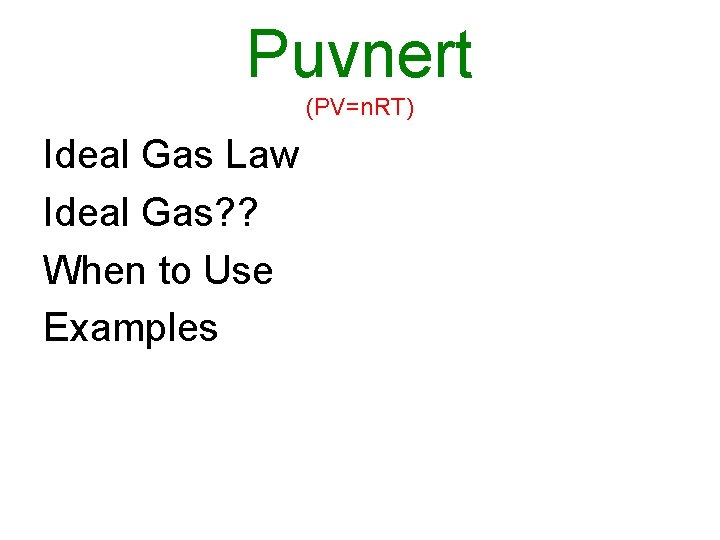
Puvnert (PV=n. RT) Ideal Gas Law Ideal Gas? ? When to Use Examples

0. 08206 Realize: Ideal gas constant units are liters, ATM, moles, kelvin !!!!

0. 08206 Example; A balloon contains 2. 50 l of ethyne gas at a pressure of 1. 75 ATM and a temperature of 321 K. Determine how many moles of gas are contained in the balloon. . . 166 moles How many grams of ethylene? 4. 32

0. 08206 Example; Say we have the same balloon, this time, we know there are 15 grams of ethyne, the temperature is 25. 0 C, and the volume is 1250 ml, determine the pressure of the balloon. . 577 11. 3

0. 08206 OK, make up your own exciting balloon problem and hand in.

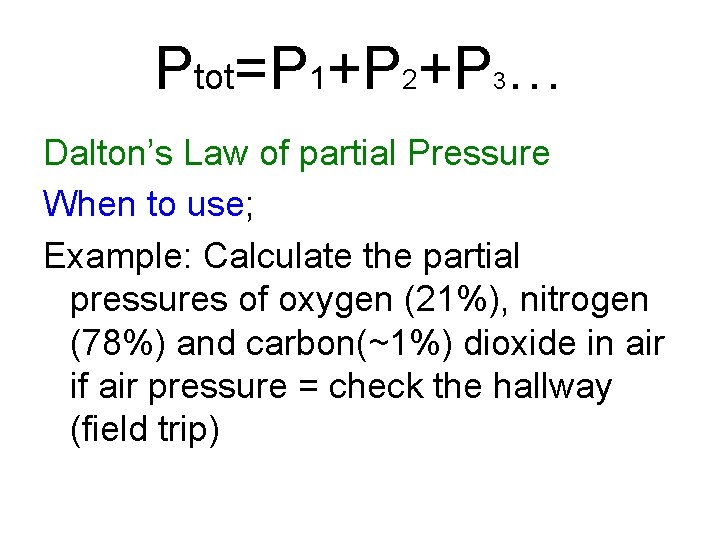
Monday – Another Law and stoichwgas… Dalton’s Law of partial Pressure


Ptot=P 1+P 2+P … 3 Dalton’s Law of partial Pressure When to use; Example: Calculate the partial pressures of oxygen (21%), nitrogen (78%) and carbon(~1%) dioxide in air if air pressure = check the hallway (field trip)

Chapter 13 – Final Topic Gas stoichiometry Real life chemistry Combines stoichiometry from ch 9 with ideal gas law from ch 13 Examples:

Example #1 10. 0 g HCl, with excess aluminum. Determine the volume of gas produced (assuming STP)… 3. 07

Example #2 10. 0 g HCl, with excess aluminum. Determine the volume of gas produced (At typical lab conditions T=22. 0 C, P=0. 985 ATM)… . 1370 3. 37

Example #3 If 2. 00 liters of methane are to be burned, determine the volume of oxygen required (assuming STP).

Example #4 5. 00 liters of propane are reacted with 5. 00 liters of oxygen. Determine the volume of Carbon dioxide produced (Check across hall for current conditions)

Thrstdy Make sure gas stoichiometry lab ready to hand in Fri. Think about barometer for < $1 Demonstration Question Draw a diagram illustrating your answer…

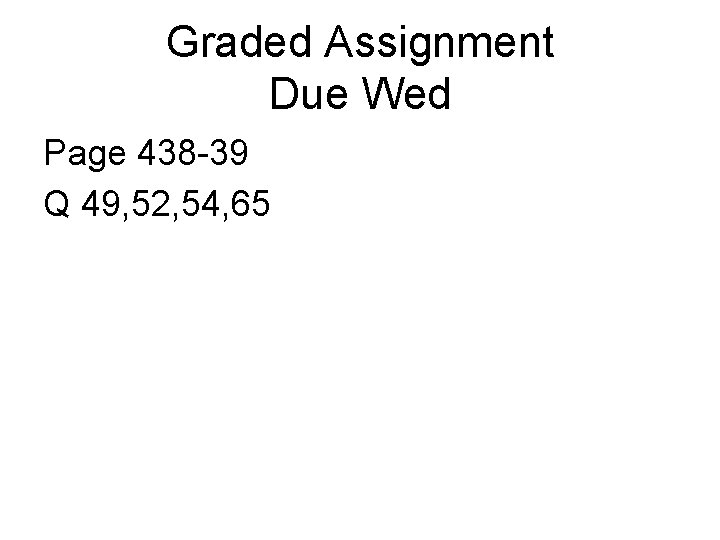
Graded Assignment Due Wed Page 438 -39 Q 49, 52, 54, 65
 Characteristics of gases
Characteristics of gases How many elements are gasses at room temperature
How many elements are gasses at room temperature Noble gas block
Noble gas block Gasses or gases
Gasses or gases Gasses
Gasses Gasses
Gasses Blue gasses
Blue gasses State avogadro's law
State avogadro's law Magma volatile gasses definition
Magma volatile gasses definition Week by week plans for documenting children's development
Week by week plans for documenting children's development 40-hour work week schedule examples
40-hour work week schedule examples 10 hour workday schedule
10 hour workday schedule Contoh kertas kerja pemeriksaan
Contoh kertas kerja pemeriksaan Kinetic molecular theory of solids
Kinetic molecular theory of solids Adhesive force
Adhesive force The kinetic theory of matter states that
The kinetic theory of matter states that Kinetic theory of matter definition
Kinetic theory of matter definition Kinetic theory of matter
Kinetic theory of matter Buoyancyability
Buoyancyability Kinetic molecular model of gases
Kinetic molecular model of gases The kinetic molecular theory
The kinetic molecular theory Kinetic molecular theory of liquids
Kinetic molecular theory of liquids Kinetic molecular theory
Kinetic molecular theory Kinetic molecular theory def
Kinetic molecular theory def Kinetic particle theory o level questions
Kinetic particle theory o level questions Timeline of kinetic molecular theory
Timeline of kinetic molecular theory The attraction between particles gives solids a definite
The attraction between particles gives solids a definite Kinetic theory of gases
Kinetic theory of gases Charles law in terms of kinetic molecular theory
Charles law in terms of kinetic molecular theory Postulates of kinetic theory of gas
Postulates of kinetic theory of gas