Definition Gases in magmas are called volatile components












































- Slides: 82

Definition Gases in magmas are called volatile components, magmatic volatiles, volatile species “Fugitive elements”

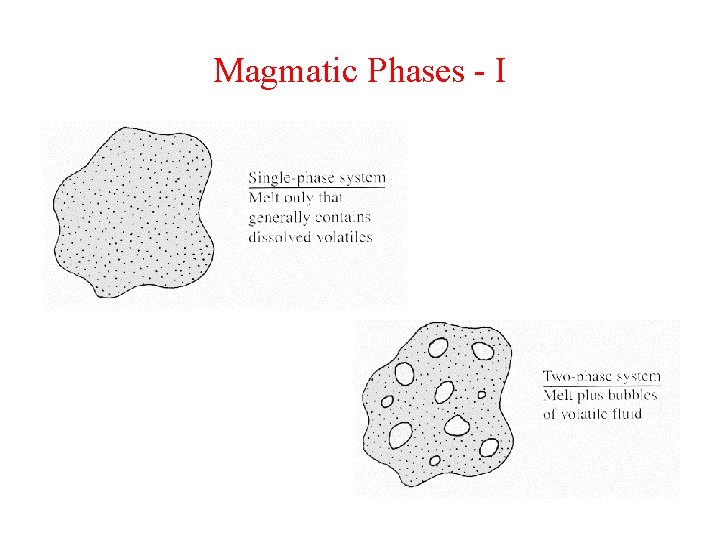
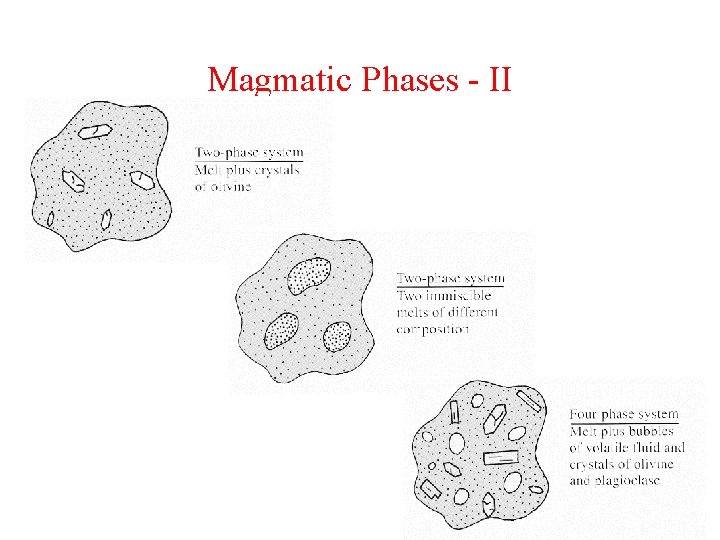
Key Concept Volatile species can be dissolved in melt (accommodated in melt structure) Or They can be present as exsolved species (bubbles)

Magmatic Phases - I

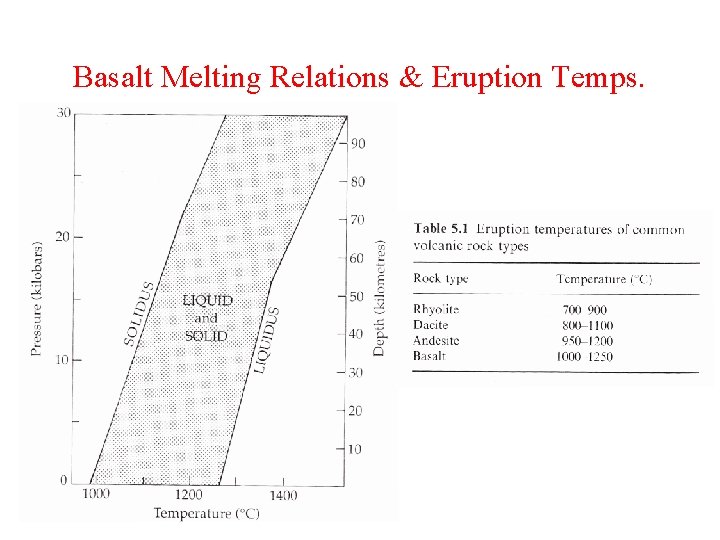
Basalt Melting Relations & Eruption Temps.

Magmatic Phases - II

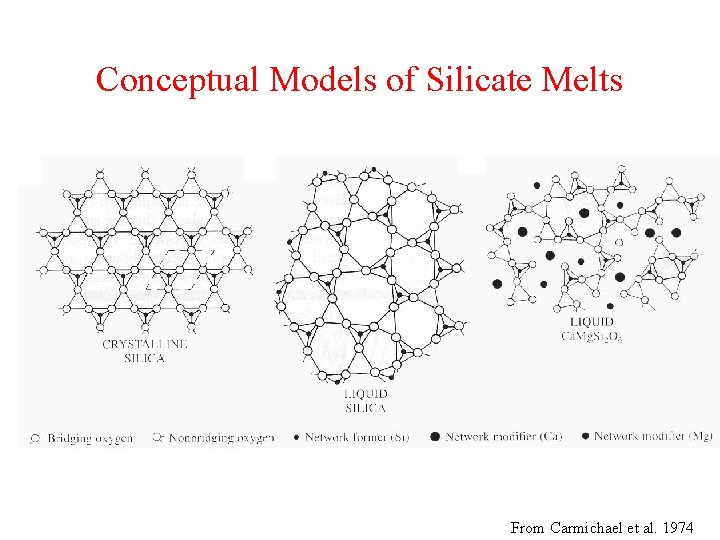
Conceptual Models of Silicate Melts From Carmichael et al. 1974

Masaya, Nicaragua; 1972

Quantifying Volcanic Emissions of Trace Elements to the Atmosphere: Ideas Based on Past Studies William I Rose Geological Engineering & Sciences Michigan Tech University Fall AGU, San Francisco 8 December 2003, paper V 12 E-08 15: 25

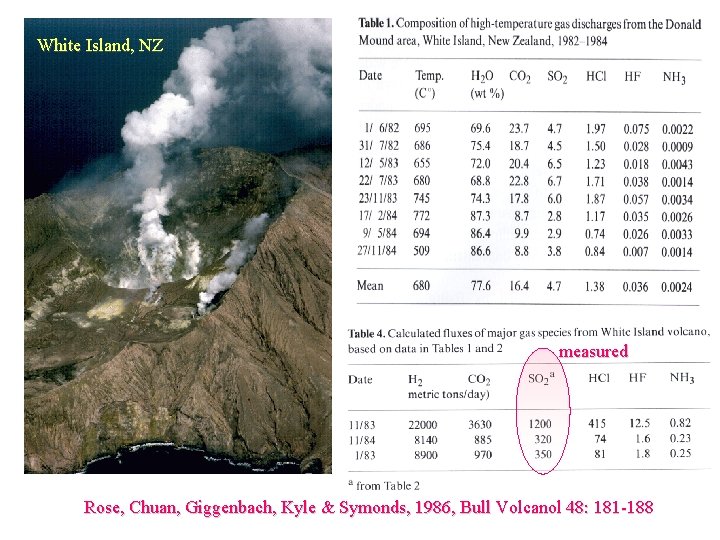
White Island, NZ measured Rose, Chuan, Giggenbach, Kyle & Symonds, 1986, Bull Volcanol 48: 181 -188

Selected Direct samples of volcanic gases from rift volcanoes.

Common Magmatic Volatile Species • Volatiles are defined as those chemical species that at near atmospheric P and high T appropriate for magmas, exist in a gas or vapor phase. • Common chemical species include: H 2 O (steam), CO 2, HCl, HF, F, Cl, SO 2, H 2 S, CO, CH 4, O 2, NH 3, S 2, and noble gases He and Ar. H 2 O and CO 2 dominate! • Most volatile species consist of only six low-atomic weight elements: H, C, O, S, Cl, and F. Small but measurable amounts of these elements can be dissolved in both the coexisting melt and crystalline phases. • Oxygen is the major ion in all three phases in magmatic systems: solid, liquid, and volatile.

State of Volatiles in Magmas • Critical Point: for a volatile species is the T, P at which there is no physical distinction between liquid and gas • Exsolved volatiles are above the critical point. Called supercritical fluids.

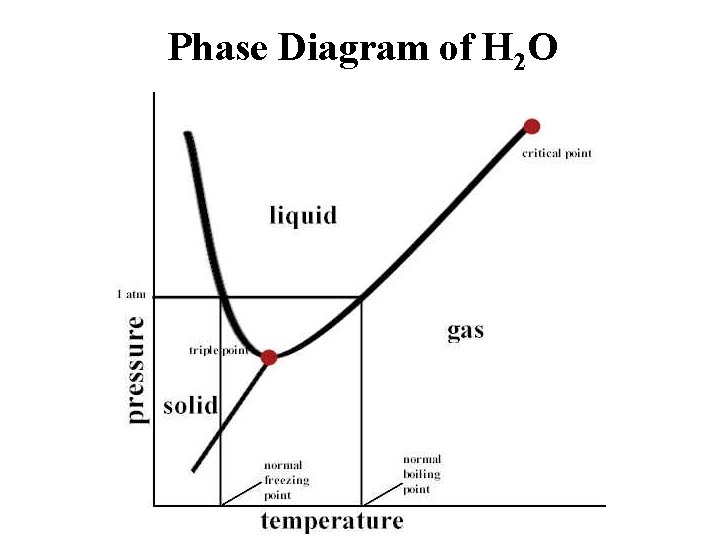
Phase Diagram of H 2 O

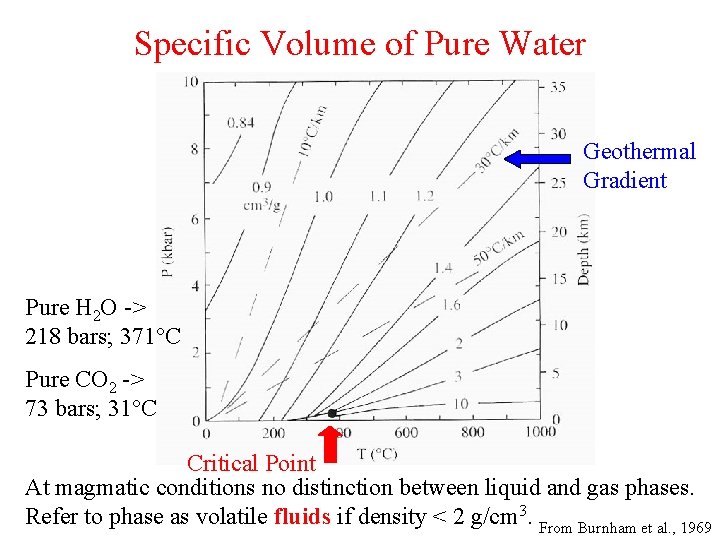
Specific Volume of Pure Water Geothermal Gradient Pure H 2 O -> 218 bars; 371°C Pure CO 2 -> 73 bars; 31°C Critical Point At magmatic conditions no distinction between liquid and gas phases. Refer to phase as volatile fluids if density < 2 g/cm 3. From Burnham et al. , 1969

Supercritical Fluids……. Characteristics include: • Density more like liquid • Solubility like those of liquid • Diffusivities like those of gas • Viscosity like those of gas Keep in mind that these are still P, T dependent

Supercritical Fluids in Magmas • Density of supercritical fluid very LOW • Means the specific volume (volume/mass) very LARGE • 10 -10, 000 cm 3/gm • Why is this important?

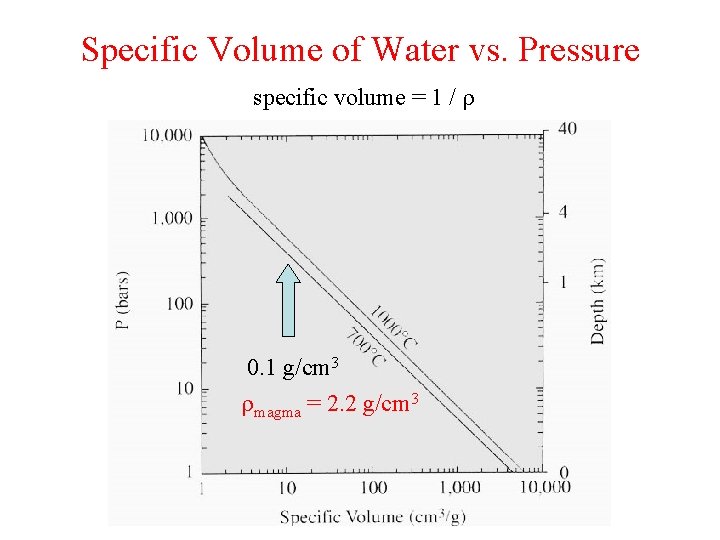
Specific Volume of Water vs. Pressure specific volume = 1 / r 0. 1 g/cm 3 rmagma = 2. 2 g/cm 3

What are the most important volatile species? • Most important are H 2 O and CO 2 • Secondary importance are S in the form of SO 2 and H 2 S • Additional importance are the halides--Cl, F

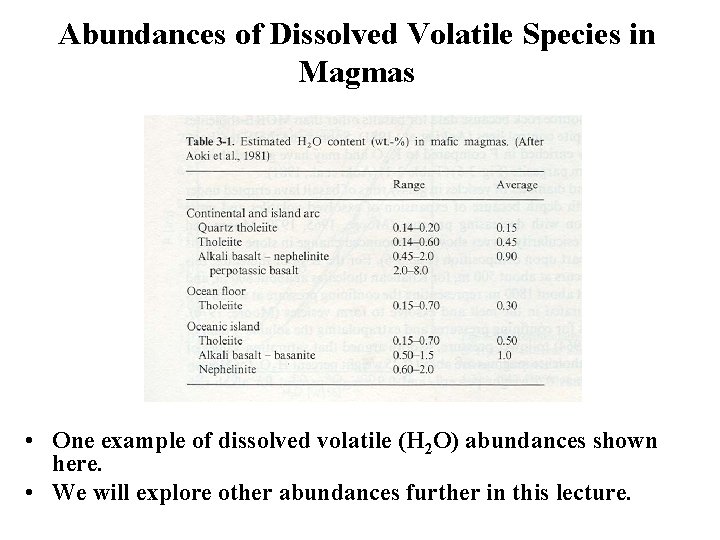
Abundances of Dissolved Volatile Species in Magmas • One example of dissolved volatile (H 2 O) abundances shown here. • We will explore other abundances further in this lecture.

Example of Volatile Discharge at an Active Volcano: Merapi, Indonesia In tons • 3000 CO 2 • 400 SO 2 • 250 HCl • 50 HF

Why study volatile species? • Play a fundamental role in forcing magma to ascend, and erupt • For example, typical percentage by mass might be 0. 1%; equivalent to 90% bubbles in magma!

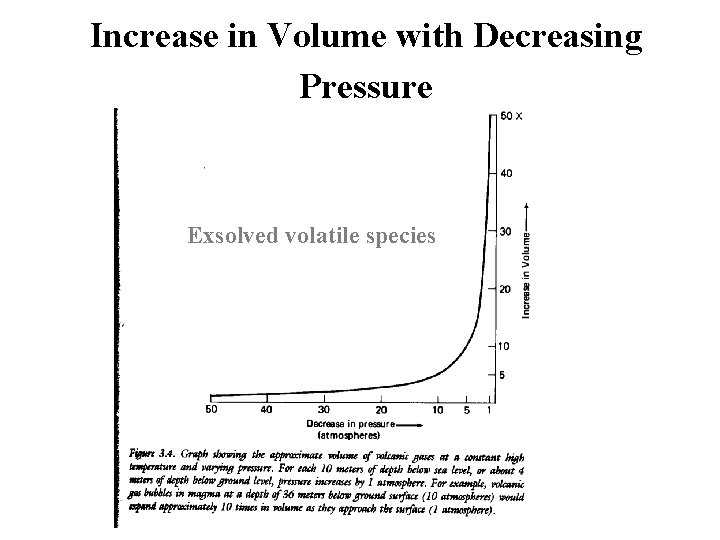
Increase in Volume with Decreasing Pressure Exsolved volatile species

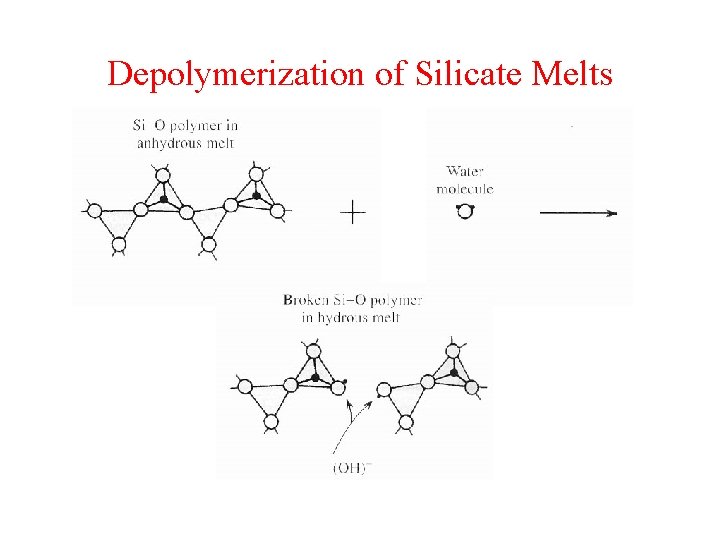
Depolymerization of Silicate Melts

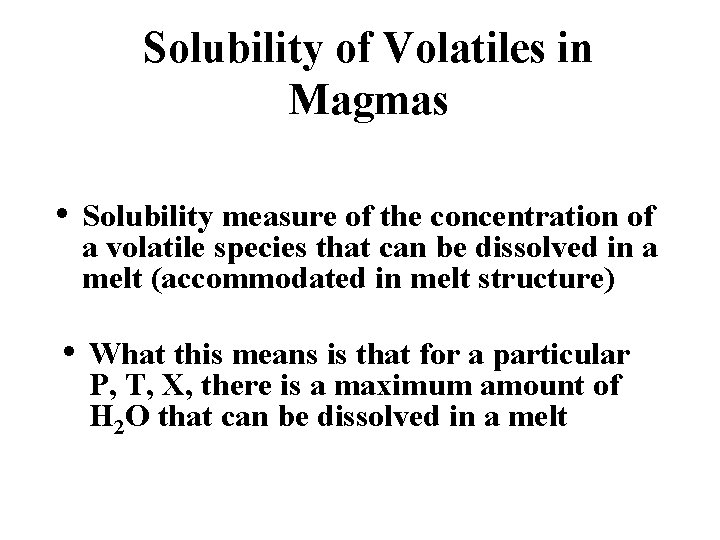
Solubility of Volatiles in Magmas • Solubility measure of the concentration of a volatile species that can be dissolved in a melt (accommodated in melt structure) • What this means is that for a particular P, T, X, there is a maximum amount of H 2 O that can be dissolved in a melt

Solubility is a function of: • P, T, X--most important are composition and pressure

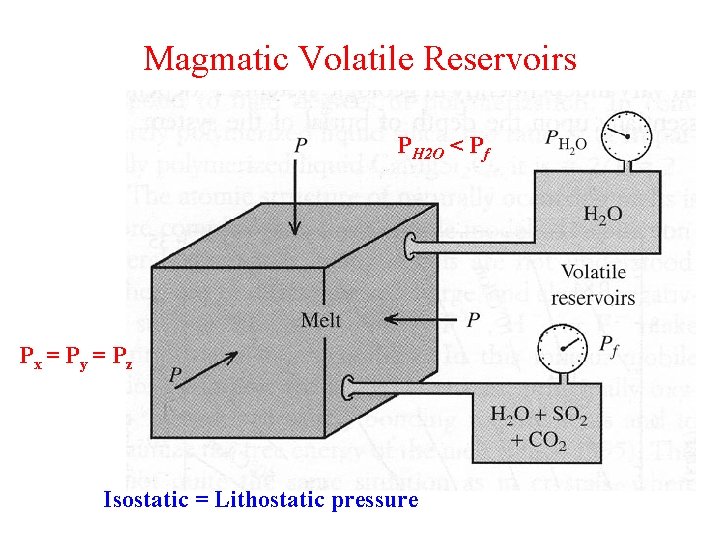
Magmatic Volatile Reservoirs PH 2 O < Pf Px = Py = Pz Isostatic = Lithostatic pressure

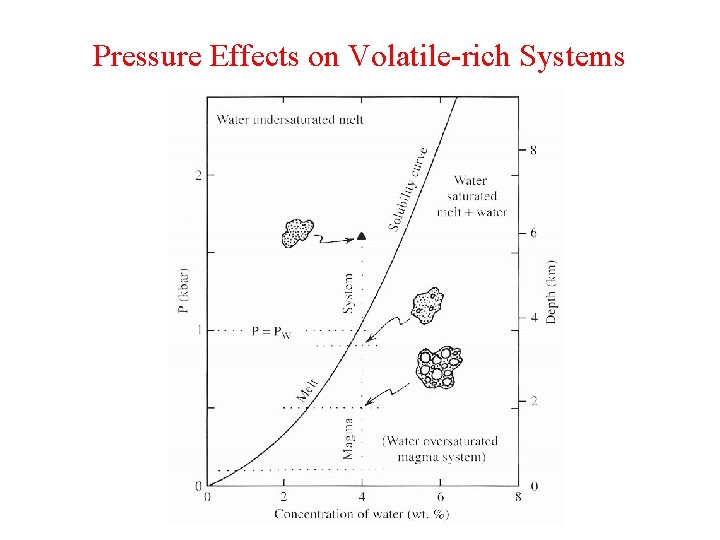
Solubility as a Function of Pressure Volume of volatile-rich melt << volatile-absent melt + free volatile phase (bubbles) • What happens at increasing P? • Push reaction to side with smaller volume • This means that solubility increases with pressure

Solubility as a Function of Pressure Volume of volatile-rich melt << volatile-absent melt + free volatile phase (bubbles) • Easy way to remember this: soda analogy

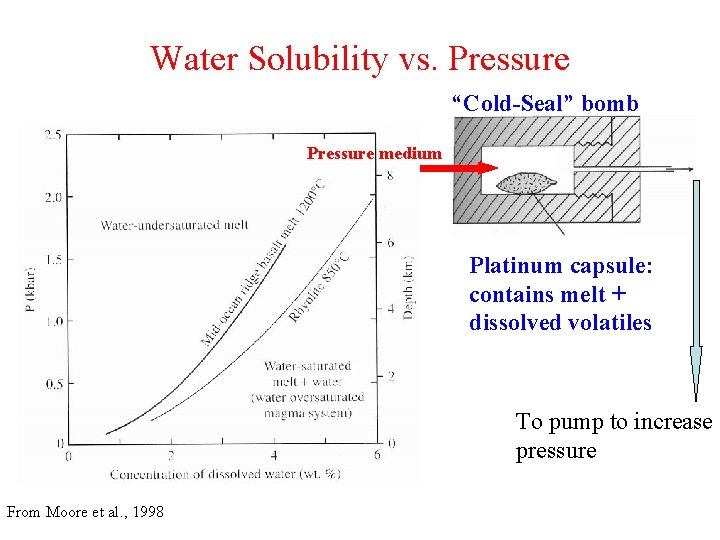
Water Solubility vs. Pressure “Cold-Seal” bomb Pressure medium Platinum capsule: contains melt + dissolved volatiles To pump to increase pressure From Moore et al. , 1998

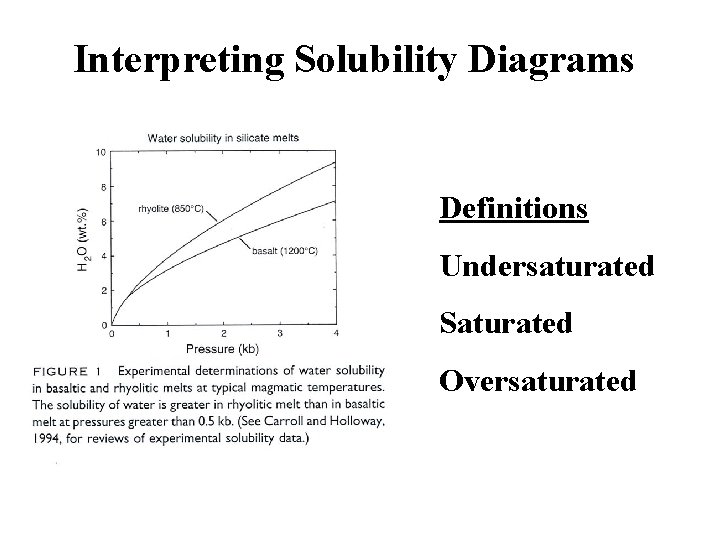
Interpreting Solubility Diagrams Definitions Undersaturated Saturated Oversaturated

Pressure Effects on Volatile-rich Systems

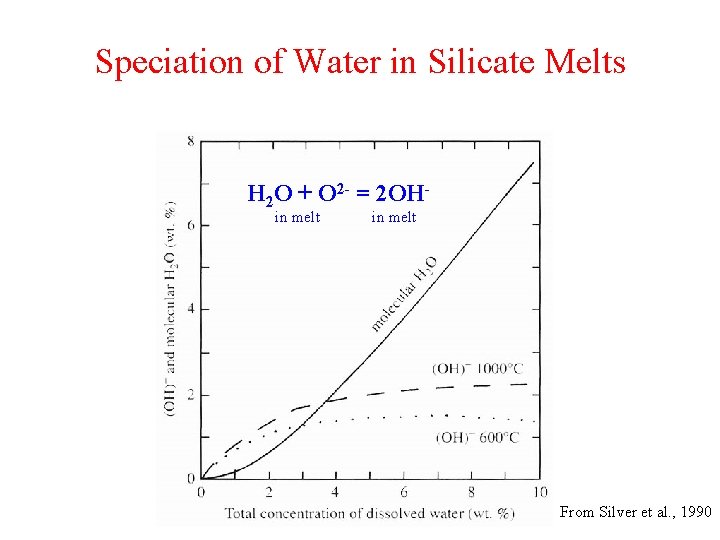
Speciation of Water in Silicate Melts H 2 O + O 2 - = 2 OH in melt From Silver et al. , 1990

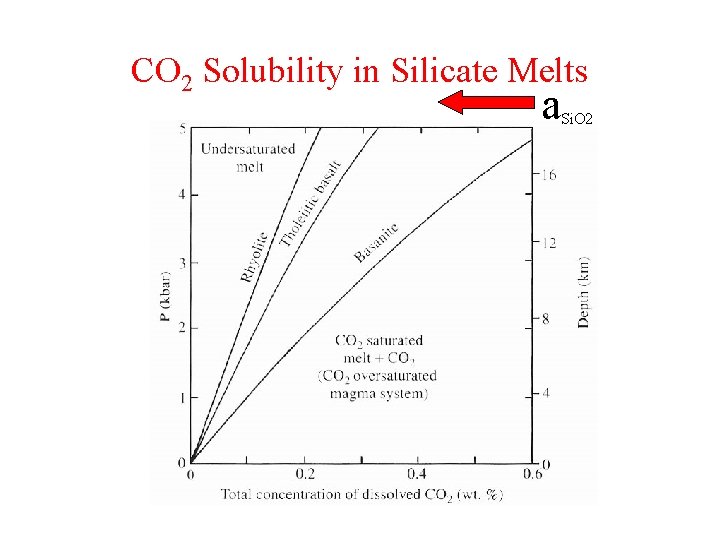
CO 2 Solubility in Silicate Melts a Si. O 2

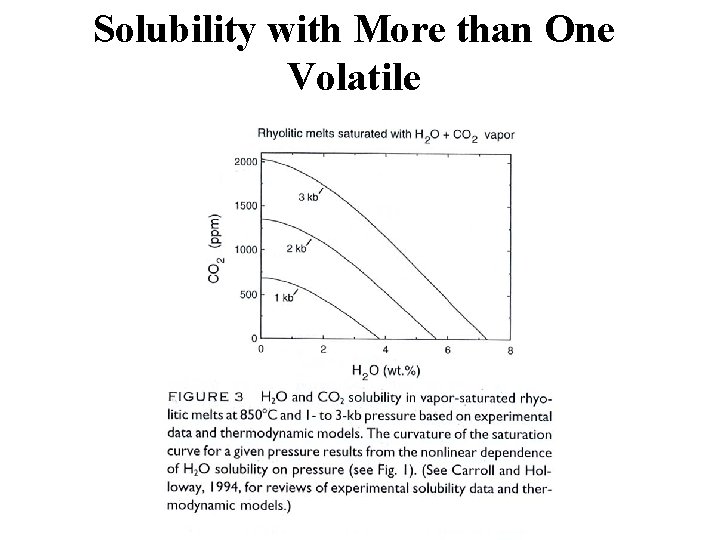
Solubility with More than One Volatile

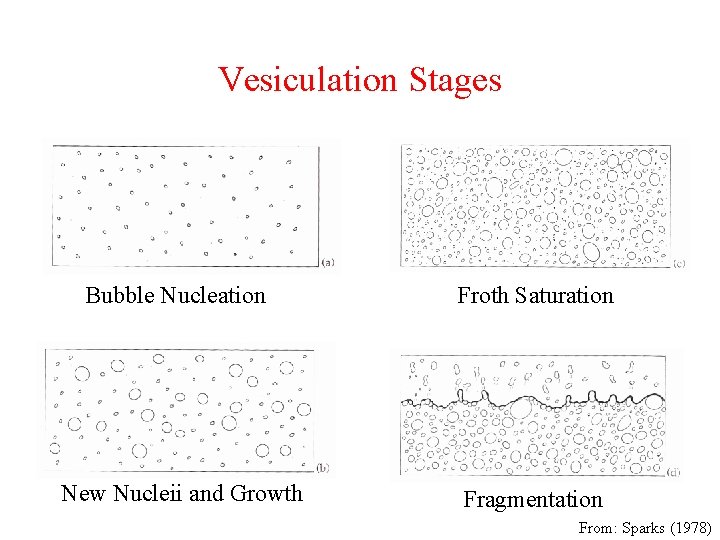
Vesiculation Stages Bubble Nucleation Froth Saturation New Nucleii and Growth Fragmentation From: Sparks (1978)

Summary of ascent issues Cooling of magma Saturation and supersaturation Formation of bubble nuclei Bubble growth Formation of solid phase nuclei Growth of crystals Interpretation of textures

Cooling of magma As magma rises from mantle levels to the surface, the wallrock changes from ductile to brittle. Diapirs are thought to exist in ductile regions, and above there are dikes and cylindrical conduits. With rise of magma, there is a cooling of the wallrock temperature and an increase in the temp contrast between the magma and the wallrock. Geometry of feeder system influences rate of heat loss. The strong relationship of viscosity with T is important in causing magma viscosity to increase as the magma approaches the surface.

Saturation and supersaturation The availability of H 2 O and other volatiles in the source region and the degree of partial melting gives rise to an unsaturated magma with perhaps <1 -7 % volatiles. The effect of dissolving volatiles in silicate magma is depolymerization--resulting in a dramatic viscosity decrease. With rise, the solubility of volatiles decreases and eventually the magma is saturated. This threshold is academic and barely noticable, because volatiles do not begin to escape. Why? If the magma continues to rise and pressure decreases, the magma enters a state of supersaturation, which does lead eventually to bubbles.

Formation of bubble nuclei The most important activation energy barrier for bubble formation is the formation of a nucleus---the accumulation of many molecules of gas that can sustain enough pressure to avoid being resorbed by the magma that surrounds them. There is probably a critical minimum size of nuclei that can survive, perhaps around one micron in diameter. In a probabilistic sense the evolution of a nucleus of critical size, one that has a >50% chance of growing rather than shrinking, is quite unlikely so most nuclei get resorbed. Only when a nuclei reach critical sizes can volatiles escape--this is why supersaturation is needed. An analogous concept is undercooling.

Growth of bubbles If the magma is low in viscosity, bubbles will rise in the liquid after growing to some critical size. Thus they may reach the top of a magma chamber or even burst in an open vent, releasing gas passively. In a viscous magma bubbles never grow large enough to rise by gravity, because resistence of the magma to flow is too great. Diffusion of gas through a viscous liquid is slow--slowest in very viscous ones. Each growing bubble has a gradient around it. Overpressure develops in bubbles as bubbles grow, but as the bubbles “feel” each other’s presence, their growth is inhibited. Thus a particular vesicle size is reached when a foam is formed.

Why do Bubbles Grow • Bubbles nucleate and grow when magma reaches super-saturation. • Equivalent to when vapor pressure equals or more typically exceeds confining P. This allows critical fluid to separate (equal to formation of bubble)

Why do Bubbles Grow? • Super-saturation can occur via: v. Decompression = first boiling v. Crystallization = second boiling

Bubble Growth • Once bubbles successfully nucleate, they grow! • Rate of growth function of a number of variables: vconcentration of volatiles vrate of diffusion (diffusivity) vdensity and viscosity of magma vsurface tension of bubble • Size of bubble in part result of competition between nucleation and growth

Idealized Bubble Growth • Defined as growth of a bubble at constant pressure, in a stable (nonmoving) melt • Two stages: v Growth by diffusion (viscosity limited) v Growth by expansion (diffusion limited)

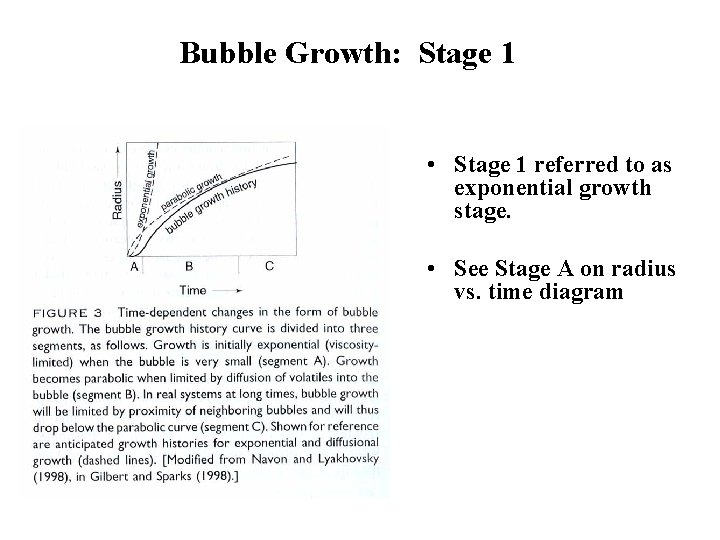
Bubble Growth: Stage 1 • Early in bubble growth history, diffusion efficient. • Bubbles grow by addition of volatile(s) by diffusion. • Bubbles can commonly maintain equilibrium between volatile(s) in bubble and volatile(s) in melt. • Growth is viscosity-limited. That is, although volatiles are diffusing into bubble, bubble still has to expend energy to grow against surrounding melt. • Higher viscosity makes bubble growth more difficult.

Bubble Growth: Stage 1 • Stage 1 referred to as exponential growth stage. • See Stage A on radius vs. time diagram

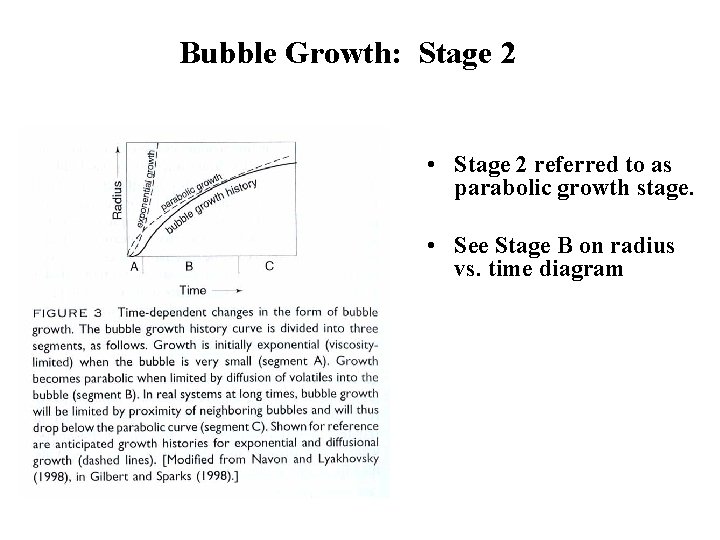
Bubble Growth: Stage 2 • Later in bubble growth, diffusion can not keep pace with growth of bubble. • Bubble may get too big for diffusion to maintain equilibrium with melt. • Thus, diffusive flux of H 2 O or CO 2 into bubble can not maintain equilibrium (saturation) pressure. • Consequence is rate of growth slows. • This stage limited by diffusion.

Bubble Growth: Stage 2 • Stage 2 referred to as parabolic growth stage. • See Stage B on radius vs. time diagram

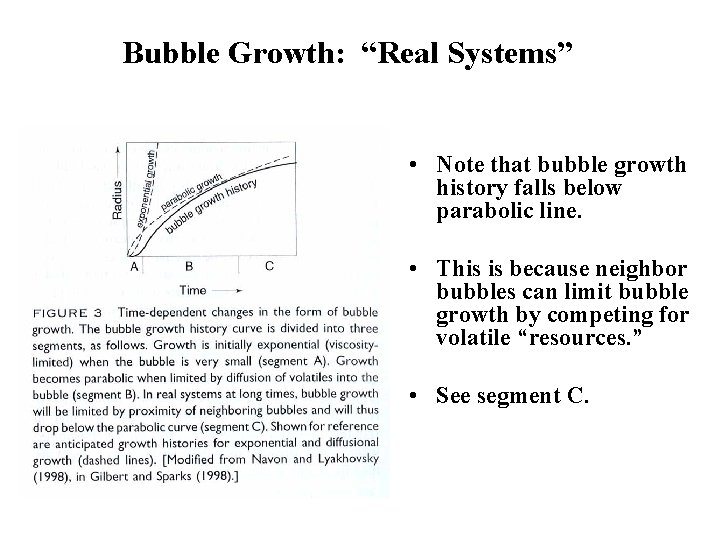
Bubble Growth: In “Real Systems” • During “real” bubble growth, other factors contribute to bubble growth. • What might they be? • Proximity to neighboring bubbles • Decompression

Bubble Growth: “Real Systems” • Note that bubble growth history falls below parabolic line. • This is because neighbor bubbles can limit bubble growth by competing for volatile “resources. ” • See segment C.

Bubble Growth during Decompression • Rate of decompression also very important: • At depth in conduit, bubble grows initially by diffusion. • As magma accelerates upward in conduit, bubbles grow by expansion. • That is critical fluid/gas phase is expanding against melt. • Expansion limited by viscous resistance of melt and neighboring bubbles. • Thus “excess” pressure develops.

Bubble Growth during Decompression • “Excess” pressure may lead to fragmentation. • In general, fragmentation is favored by rapid rates of decompression AND • High viscosity (because difficult for bubbles to maintain equilibrium, and thus more common for bubbles to become over-pressured). • More on exact mechanisms of fragmentation when we talk about Plinian eruptions.

Bubble Growth: Basaltic vs. Silicic Systems • In general, silicic magmas form smaller bubbles (0. 001 -0. 1 cm) compared to basaltic (0. 1 -5 cm). • WHY? • Diffusivity is slower in silicic magmas • Viscosity is higher, so more resistance to bubble growth.

Formation of solid phase nuclei Crystallization is also driven by cooling and volatile loss from the magma. Undercooling, or cooling at a rate faster that crystallization can keep pace with, creates impetus for overcoming activation energy barriers. In the same way as vesicle formation happens, critical crystal nuclei must attain a size which ensures their survival from reabsorption by the magma. At some critical undercooling there is a peak nucleation rate for each mineral in each magma. If the magma is appropriately cooled it will nucleate some phase readily (and others perhaps not).

Growth of Crystals Phenocrysts form mostly long before eruption. They can record tidal effects, mixing effects and magma movements of other sorts. Rapid growth features include skeletal or bow-tie crystals, elongate spinifex crystals and spherulites. Textures in the groundmass of many lavas record events that occur near the surface before eruption. Examination of these requires high magnification--back scattered xray images using SEM or microprobe.

Interpretation of textures Petrographic interpretation of volcanic rocks can be used to interpret subsurface events. What happened before eruption? What was the temp, pressure and p. H 2 O of phenocryst formation? When did saturation and volatile loss events occur? Are mixing events recorded? What happened in the weeks and days before eruption?

Measuring Magmatic Volatiles What is the challenge in accurately measuring/estimating amount of volatiles in magmas? • When gas samples taken at surface, they can become contaminated with atmosphere • If magma saturated and bubbles formed, lost some of its volatile supply prior to eruption

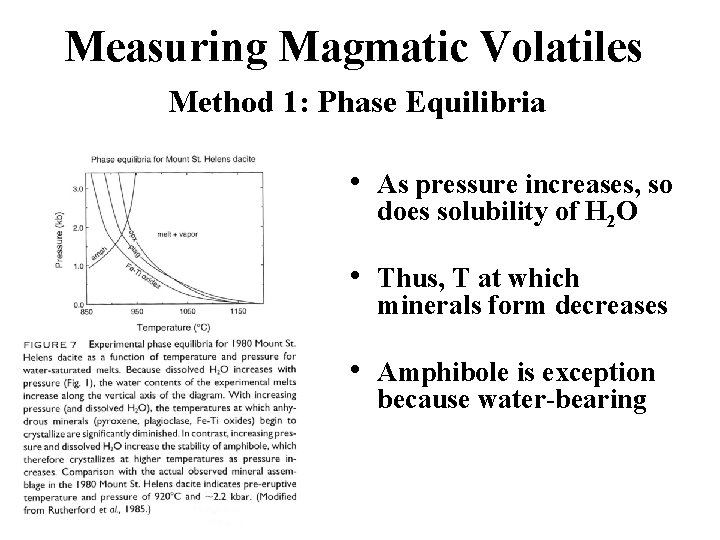
Measuring Magmatic Volatiles Method 1: Phase Equilibria • As pressure increases, so does solubility of H 2 O • Thus, T at which minerals form decreases • Amphibole is exception because water-bearing

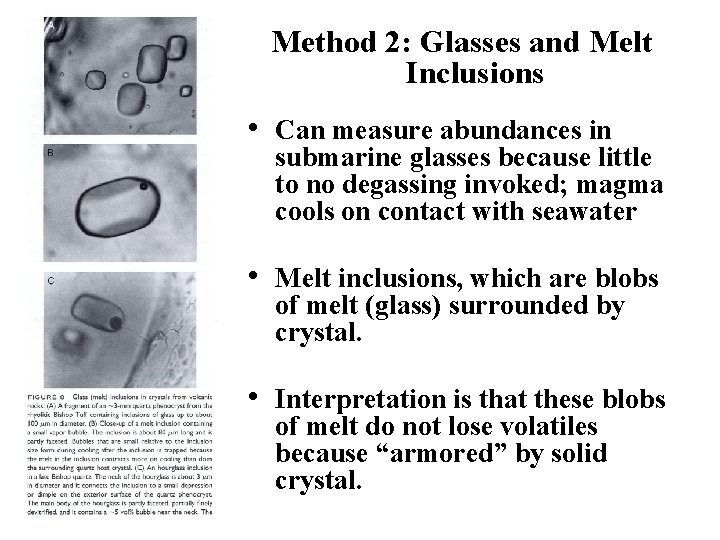
Method 2: Glasses and Melt Inclusions • Can measure abundances in submarine glasses because little to no degassing invoked; magma cools on contact with seawater • Melt inclusions, which are blobs of melt (glass) surrounded by crystal. • Interpretation is that these blobs of melt do not lose volatiles because “armored” by solid crystal.

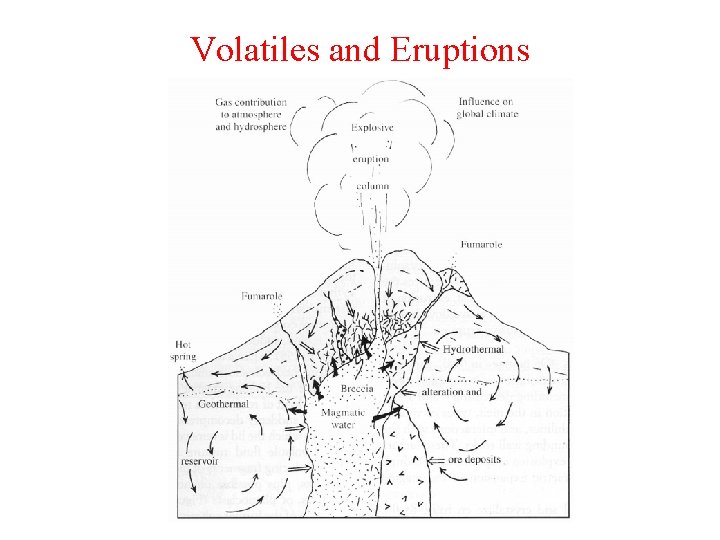
Volatiles and Eruptions


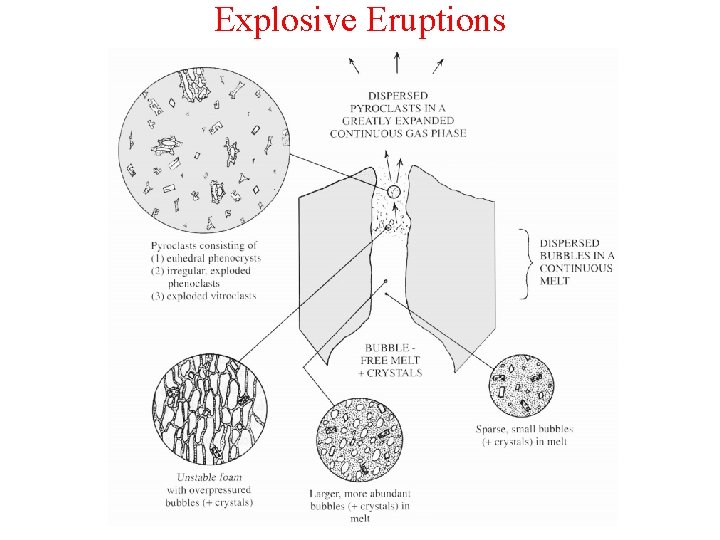
Explosive Eruptions

Types of volcanic eruptions Explosive Gas-particle dispersion flows out of the vent Extrusive Lava flows or domes

Explosive volcanic eruptions Strombolian Vulcanian Plinian

Eruption size distribution

The Volcanic Explosivity Index

VEI damages ýVEI 0: quiet, effusive eruptions of lava; typically a threat to local property only ýVEI 1 -3: progressively more violent explosive eruptions capable of local damage ýVEI 4 -5: moderate explosive eruptions capable of regional damage and disruption ýVEI 6 -7: large to gigantic explosive eruptions capable of global impact through climate modification ýVEI 8: super-eruptions capable of severe global climate modification

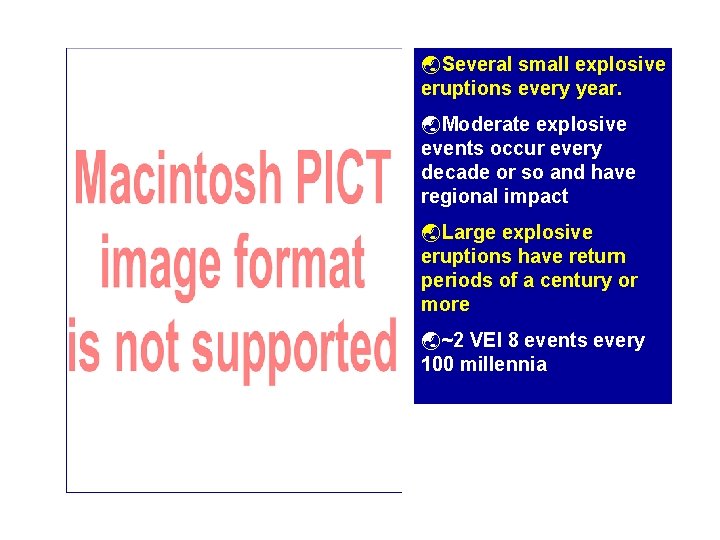
ýSeveral small explosive eruptions every year. ýModerate explosive events occur every decade or so and have regional impact ýLarge explosive eruptions have return periods of a century or more ý~2 VEI 8 events every 100 millennia

Extrusive eruptions • Lava flows • Lava domes Photo: Copyright Marco Fulle - Stromboli On-Line - http: //stromboli. net

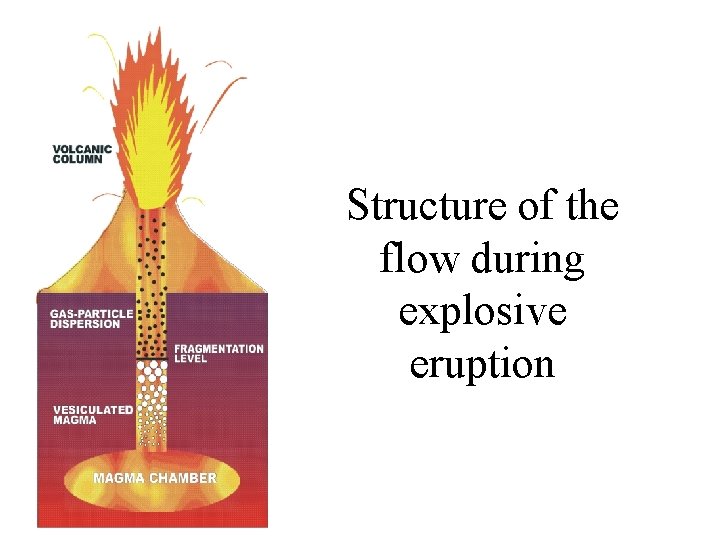
Structure of the flow during explosive eruption

Structure of the flow during extrusive eruption

Physical properties of magma • Magma: melt + crystals + gas. • Melt: Temperature 800 -1300 о. С, pressure 103 -10 -1 MPa, Viscosity 102 -1012 Pa • s. • Crystals: size 10 -7 -10 -1 m, number density up to 1017 m-3, fraction up to 95 %. • Gas: H 2 O - 60 -95, CO 2 - 0 -35%, mass fraction 0. 1 -7 %. • Ascent velocity: V =10 -4 - 500 м/c. Special features: high viscosity, strongly dependent on chemical composition and temperature, gas solubility and diffusion, complicated crystal growth kinetics. !

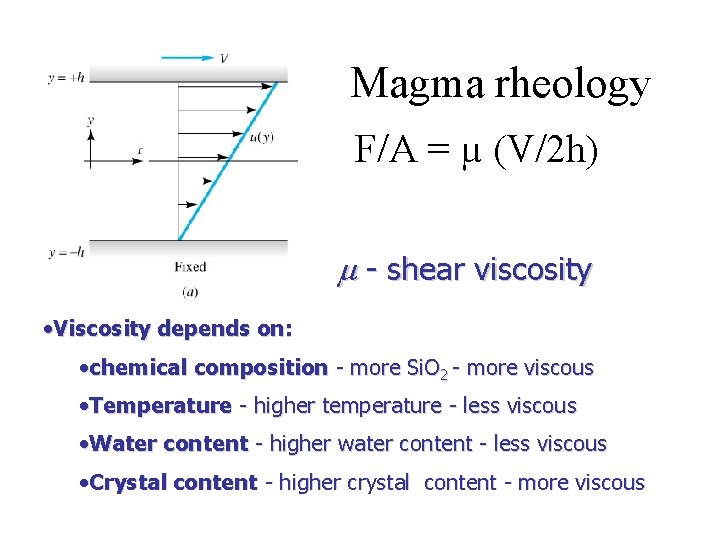
Magma rheology F/A = µ (V/2 h) m - shear viscosity • Viscosity depends on: • chemical composition - more Si. O 2 - more viscous • Temperature - higher temperature - less viscous • Water content - higher water content - less viscous • Crystal content - higher crystal content - more viscous

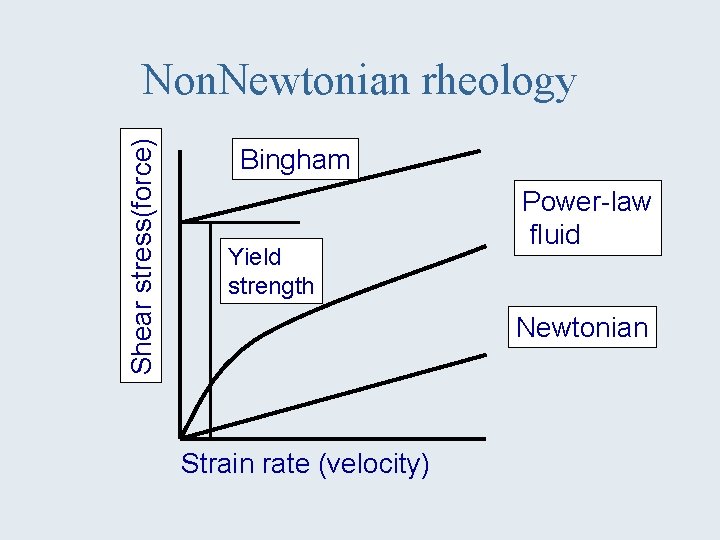
Shear stress(force) Non. Newtonian rheology Bingham Yield strength Power-law fluid Newtonian Strain rate (velocity)

Questions--As magma ascends: A. Will bubbles rise or not? B. Can gas escape through a foam? C. If both A and B are no, and gas escape continues, overpressure will build. Will it explode? D. As magma cools, degasses and crystallizes will it continue to flow? E. If flow stagnates, what happens below? F. Flow of magma is unsteady because it does stagnate, and pressurizes. Then what?

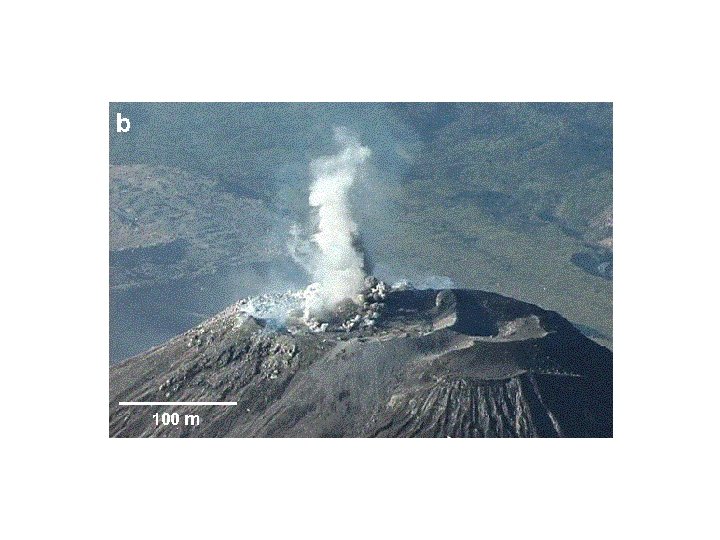
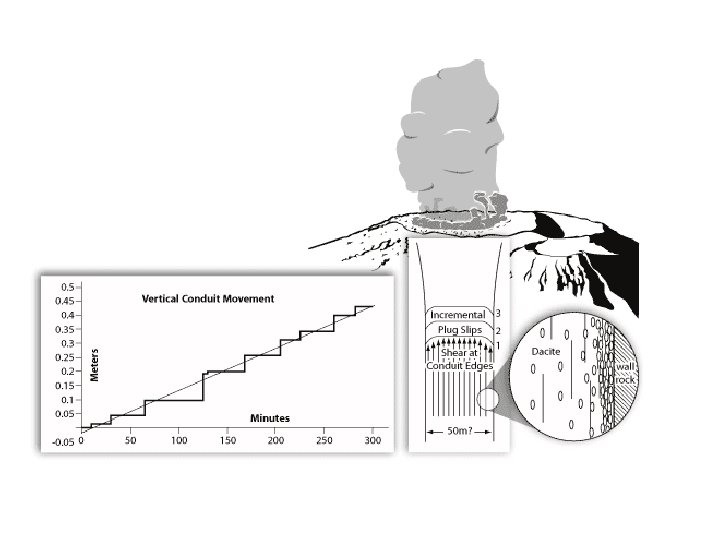
Vertical explosions of ash and bombs occur every 20 -100 minutes from a vertical conduit of viscous dacite magma. This has been true for decades and it happens during both slow and rapid conduit flow. 3770 m 2500 m





