GASES Paul Gilletti Ph D Mesa Community College






- Slides: 44

GASES Paul Gilletti, Ph. D. Mesa Community College 1

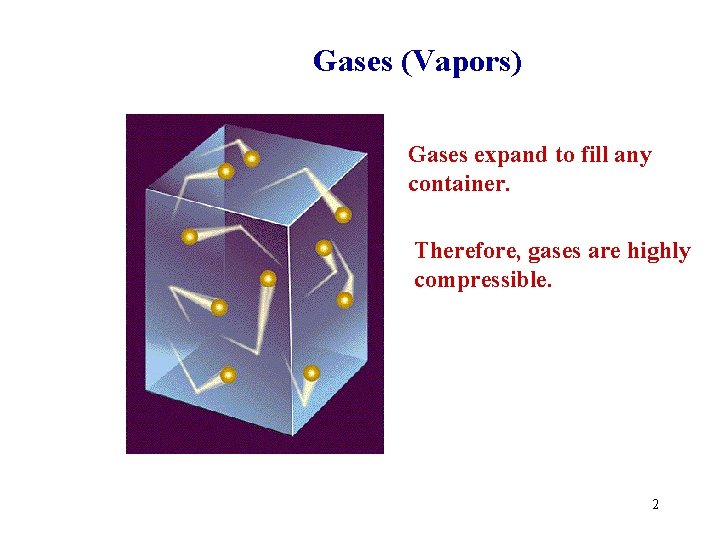
Gases (Vapors) Gases expand to fill any container. Therefore, gases are highly compressible. 2

Kinetic Molecular Theory (of an Ideal Gas): 1. Gases are composed of molecules or atoms whose size is negligible compared to the average distance between them. (Most of the space in the gas container is empty. ) 2. Gas molecules move randomly in straight lines in all directions at various speeds. 3. The forces of attraction or repulsion between gas molecules are very weak or negligible (except during collisions) 4. Collisions between gas molecules are considered elastic. 5. The average kinetic energy of a molecule is proportional to the absolute temperature. 3

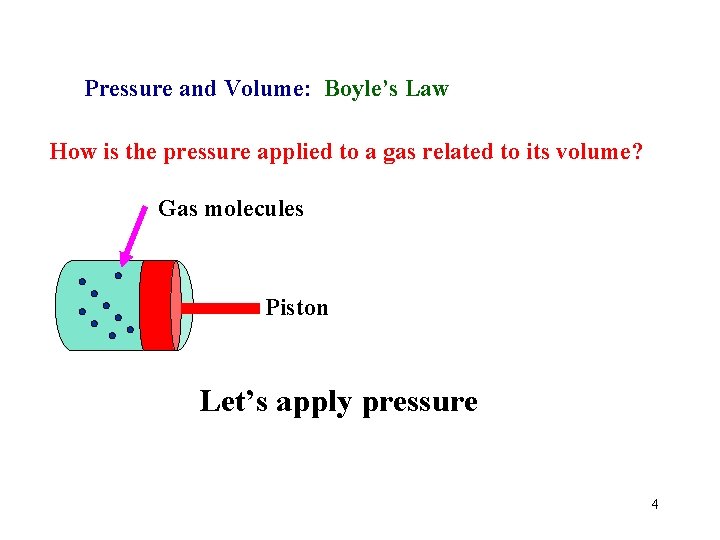
Pressure and Volume: Boyle’s Law How is the pressure applied to a gas related to its volume? Gas molecules Piston Let’s apply pressure 4

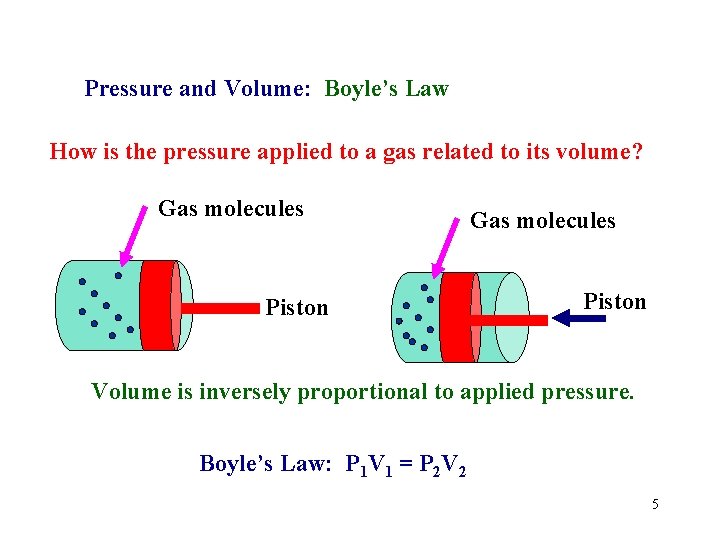
Pressure and Volume: Boyle’s Law How is the pressure applied to a gas related to its volume? Gas molecules Piston Volume is inversely proportional to applied pressure. Boyle’s Law: P 1 V 1 = P 2 V 2 5

The Harder we Push the smaller the gas volume gets! Boyle’s Law: P 1 V 1 = P 2 V 2 6

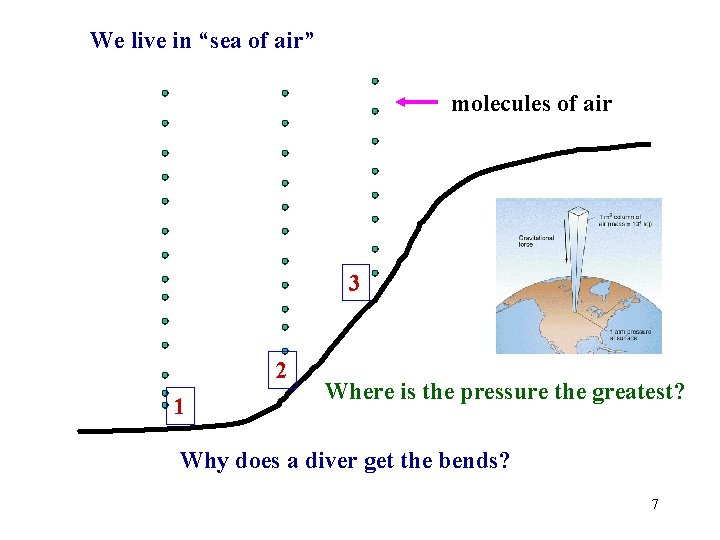
We live in “sea of air” molecules of air 3 2 1 Where is the pressure the greatest? Why does a diver get the bends? 7

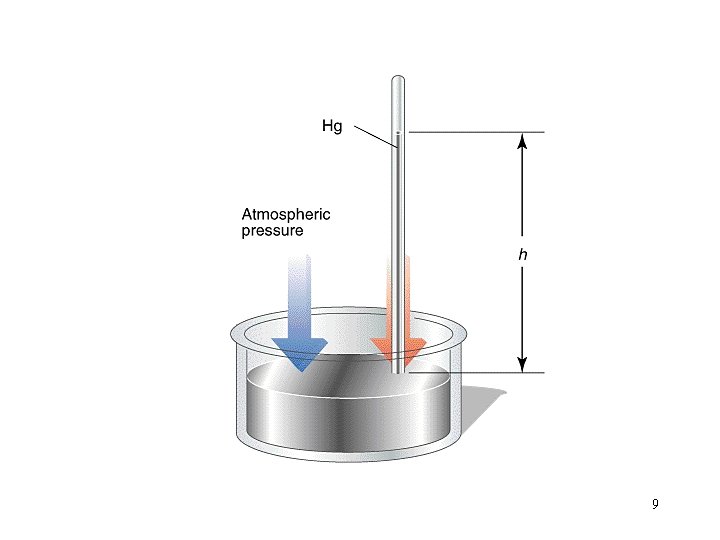
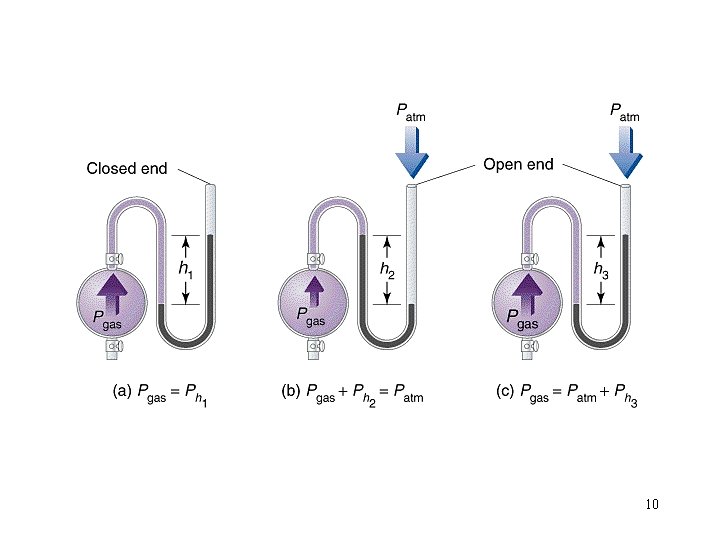
Pressure: force per unit area of surface Units lbs per in 2 (psi) mm of Hg (torr) atmospheres (atm) Pascal (Pa) 1 atm = 760 mm of Hg =760 torr = 14. 70 psi = 101. 325 k. Pa Pairs of these can be used as conversion factors. 8

9

10

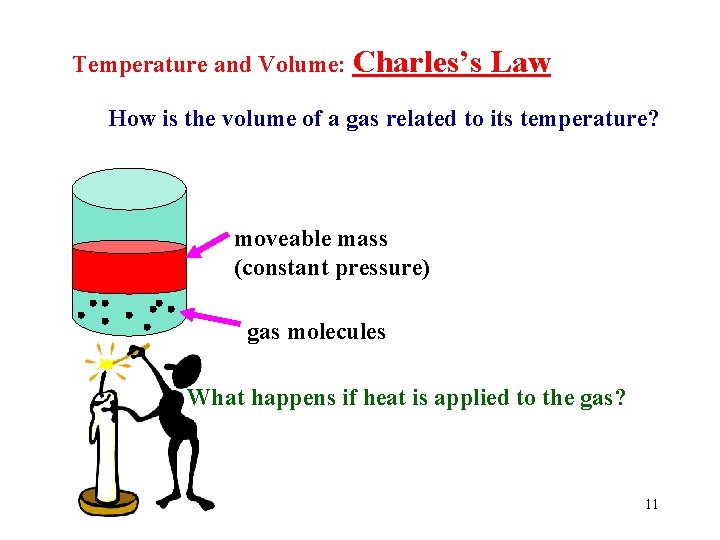
Temperature and Volume: Charles’s Law How is the volume of a gas related to its temperature? moveable mass (constant pressure) gas molecules What happens if heat is applied to the gas? 11

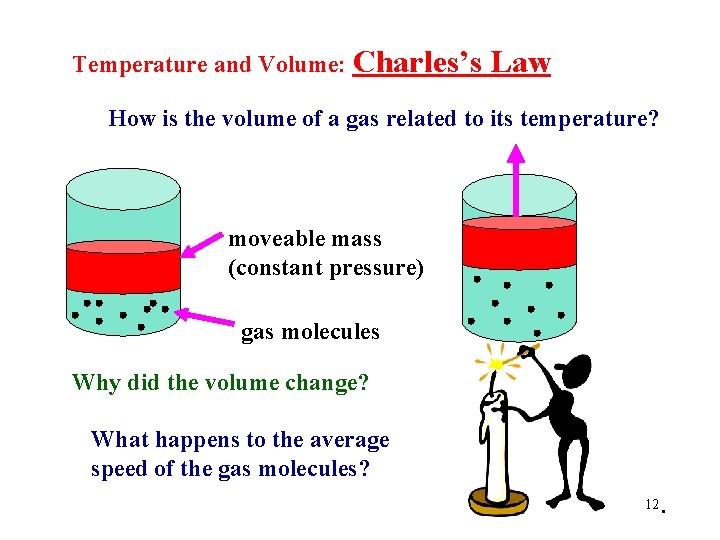
Temperature and Volume: Charles’s Law How is the volume of a gas related to its temperature? moveable mass (constant pressure) gas molecules Why did the volume change? What happens to the average speed of the gas molecules? 12 .

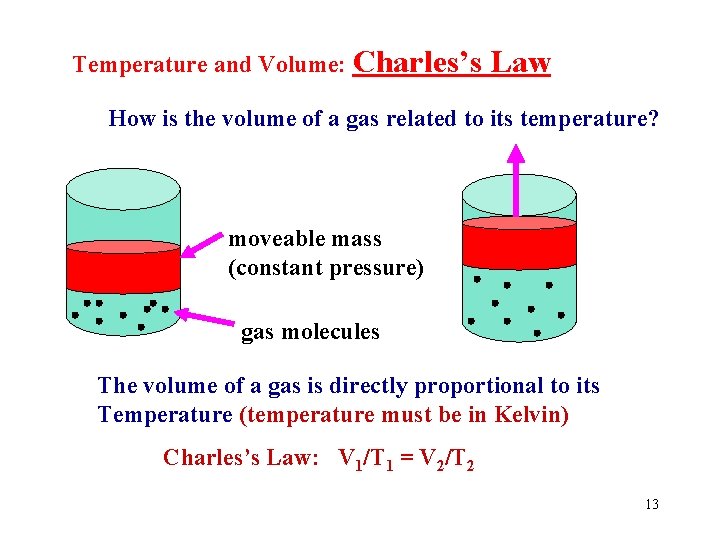
Temperature and Volume: Charles’s Law How is the volume of a gas related to its temperature? moveable mass (constant pressure) gas molecules The volume of a gas is directly proportional to its Temperature (temperature must be in Kelvin) Charles’s Law: V 1/T 1 = V 2/T 2 13

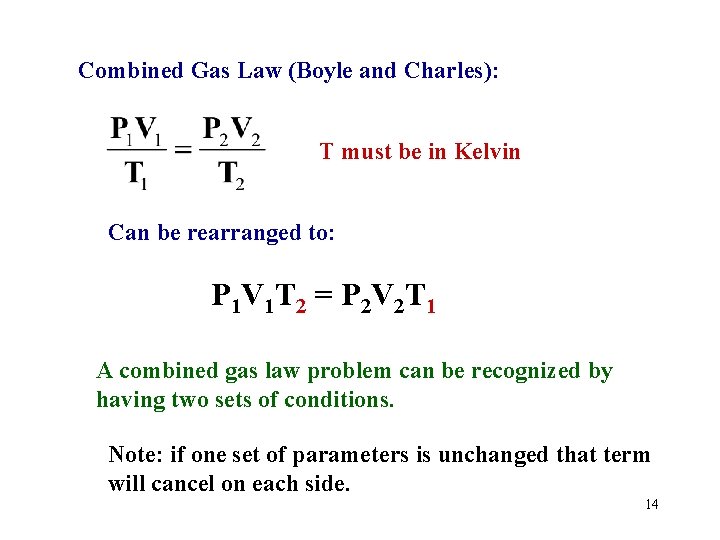
Combined Gas Law (Boyle and Charles): T must be in Kelvin Can be rearranged to: P 1 V 1 T 2 = P 2 V 2 T 1 A combined gas law problem can be recognized by having two sets of conditions. Note: if one set of parameters is unchanged that term will cancel on each side. 14

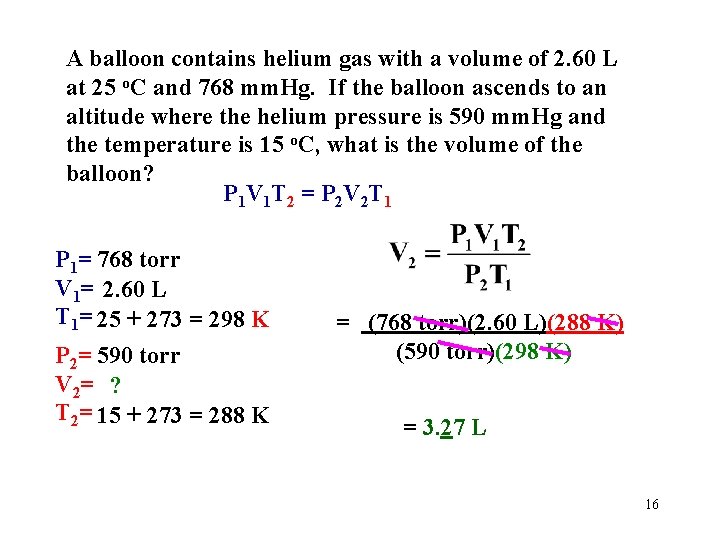
A balloon contains helium gas with a volume of 2. 60 L at 25 o. C and 768 mm. Hg. If the balloon ascends to an altitude where the helium pressure is 590 mm. Hg and the temperature is 15 o. C, what is the volume of the balloon? What type of problem is this? There are 2 sets of conditions. 15

A balloon contains helium gas with a volume of 2. 60 L at 25 o. C and 768 mm. Hg. If the balloon ascends to an altitude where the helium pressure is 590 mm. Hg and the temperature is 15 o. C, what is the volume of the balloon? P 1 V 1 T 2 = P 2 V 2 T 1 P 1= 768 torr V 1= 2. 60 L T 1= 25 + 273 = 298 K P 2= 590 torr V 2= ? T 2= 15 + 273 = 288 K = (768 torr)(2. 60 L)(288 K) (590 torr)(298 K) = 3. 27 L 16

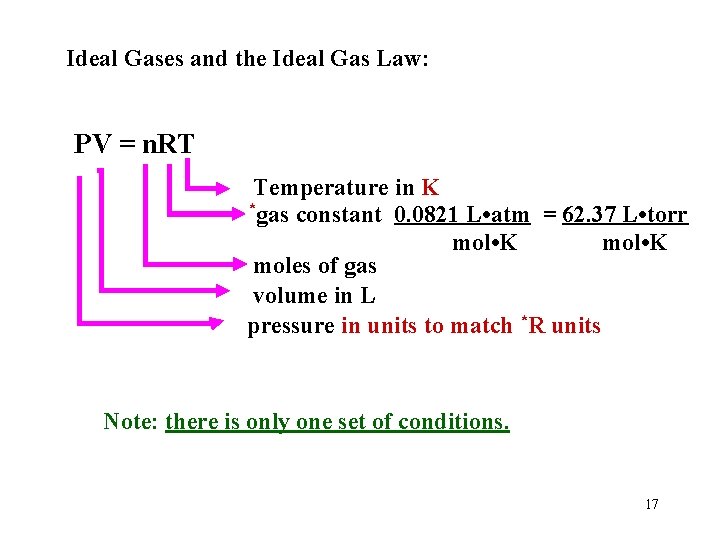
Ideal Gases and the Ideal Gas Law: PV = n. RT Temperature in K *gas constant 0. 0821 L • atm = 62. 37 L • torr mol • K moles of gas volume in L pressure in units to match *R units Note: there is only one set of conditions. 17

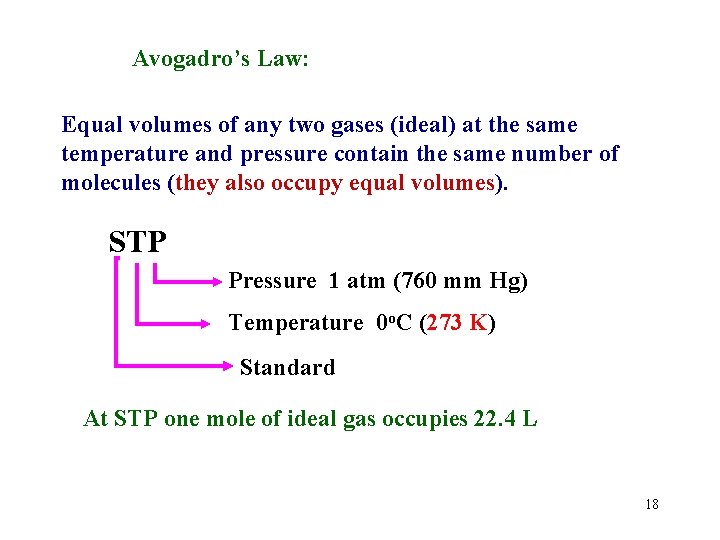
Avogadro’s Law: Equal volumes of any two gases (ideal) at the same temperature and pressure contain the same number of molecules (they also occupy equal volumes). STP Pressure 1 atm (760 mm Hg) Temperature 0 o. C (273 K) Standard At STP one mole of ideal gas occupies 22. 4 L 18

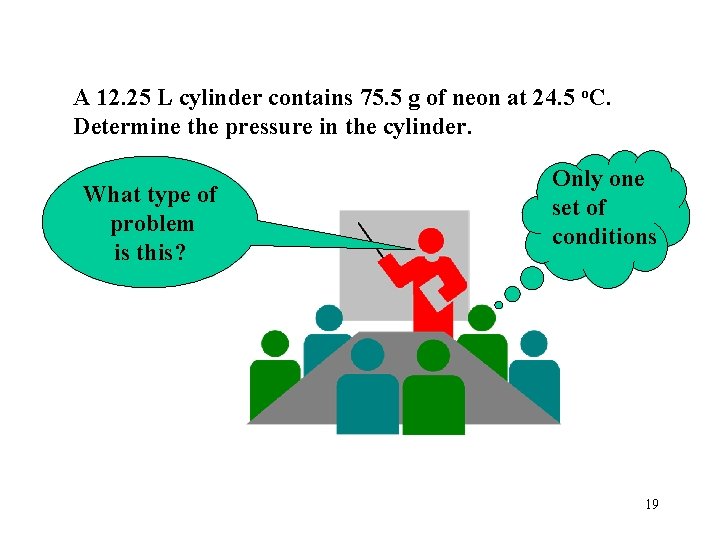
A 12. 25 L cylinder contains 75. 5 g of neon at 24. 5 o. C. Determine the pressure in the cylinder. What type of problem is this? Only one set of conditions 19

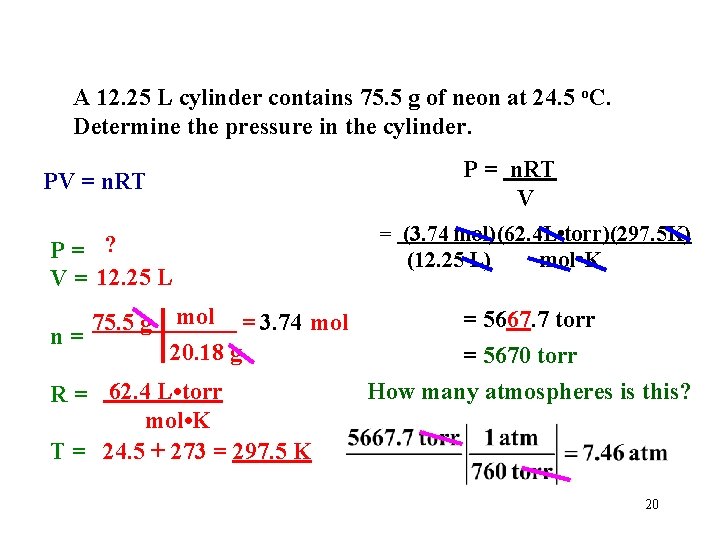
A 12. 25 L cylinder contains 75. 5 g of neon at 24. 5 o. C. Determine the pressure in the cylinder. P = n. RT V PV = n. RT P= ? V = 12. 25 L n= 75. 5 g mol = 3. 74 mol 20. 18 g R = 62. 4 L • torr mol • K T = 24. 5 + 273 = 297. 5 K = (3. 74 mol)(62. 4 L • torr)(297. 5 K) (12. 25 L) mol • K = 5667. 7 torr = 5670 torr How many atmospheres is this? 20

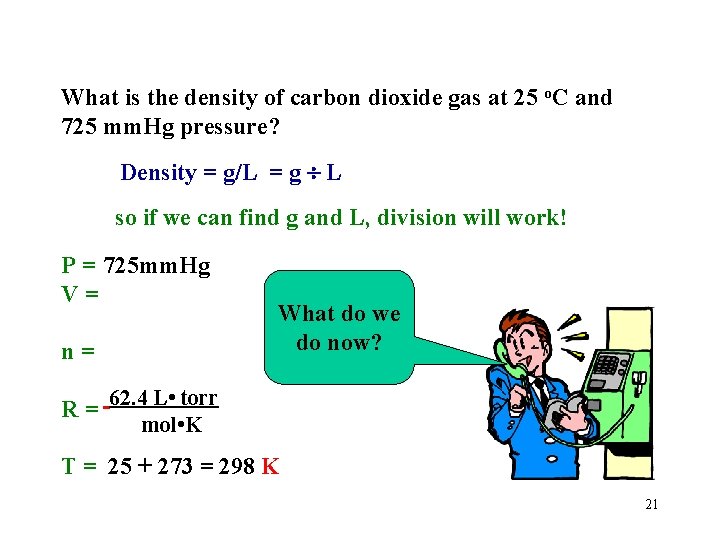
What is the density of carbon dioxide gas at 25 o. C and 725 mm. Hg pressure? Density = g/L = g L so if we can find g and L, division will work! P = 725 mm. Hg V= n= R= What do we do now? 62. 4 L • torr mol • K T = 25 + 273 = 298 K 21

What is the density of carbon dioxide gas at 25 o. C and 725 mm. Hg pressure? Density = g/L = g L so if we can find g and L division will work! P = 725 mm. Hg V= Two variables! Let’s pick an amount for one and calculate the other! n= R= 62. 4 L • torr mol • K Let’s choose 1 mol of CO 2 and find the number of Liters. T = 25 + 273 = 298 K 22

What is the density of carbon dioxide gas at 25 o. C and 725 mm. Hg pressure? Density = g/L = g L so if we can find g and L division will work! V = n. RT P = 725 mm. Hg P V= = (1 mol) (62. 4 L • torr) (298 K) ( mol • K ) (725 torr) n = 1. 0 mol (44. 0 g) R= 62. 4 L • torr mol • K T = 25 + 273 = 298 K = 25. 6 L 44. 0 g = 1. 72 g NOW: ______ L 25. 6 L 23

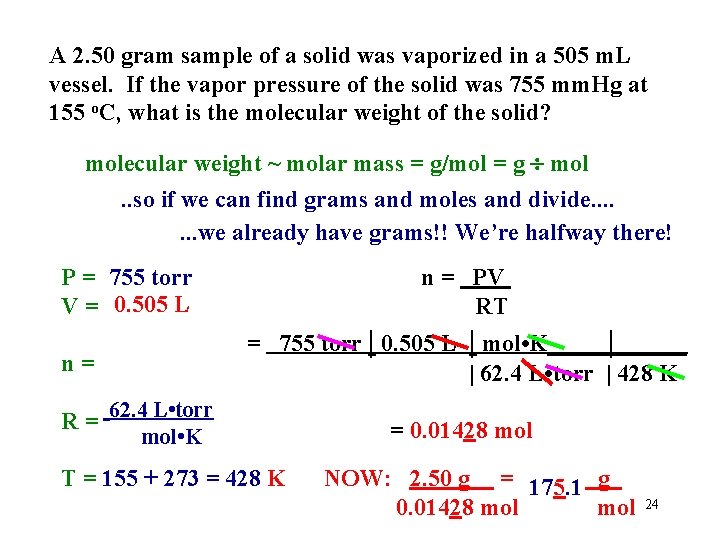
A 2. 50 gram sample of a solid was vaporized in a 505 m. L vessel. If the vapor pressure of the solid was 755 mm. Hg at 155 o. C, what is the molecular weight of the solid? molecular weight ~ molar mass = g/mol = g mol. . so if we can find grams and moles and divide. . . . we already have grams!! We’re halfway there! P = 755 torr V = 0. 505 L = 755 torr | 0. 505 L n= R= n = PV RT | mol • K_____|______ | 62. 4 L • torr | 428 K 62. 4 L • torr mol • K T = 155 + 273 = 428 K = 0. 01428 mol NOW: 2. 50 g = 175. 1 g 0. 01428 mol 24

So Density is g/L (g ÷ L) and molar mass is g/mol (g ÷ mol). 25

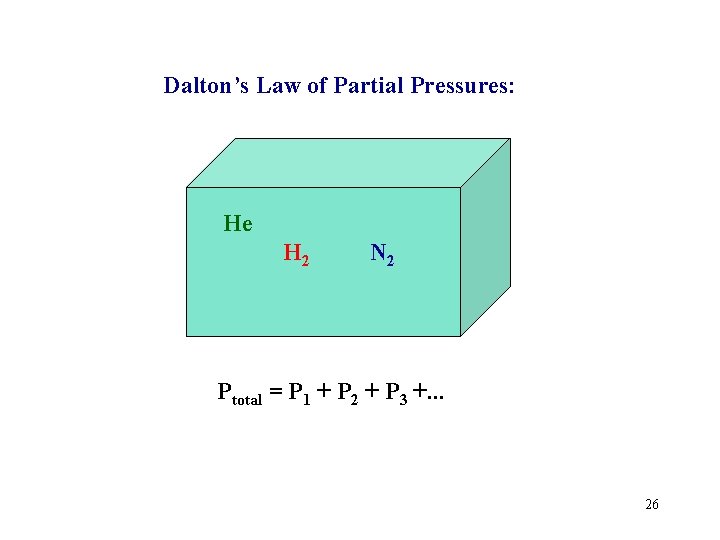
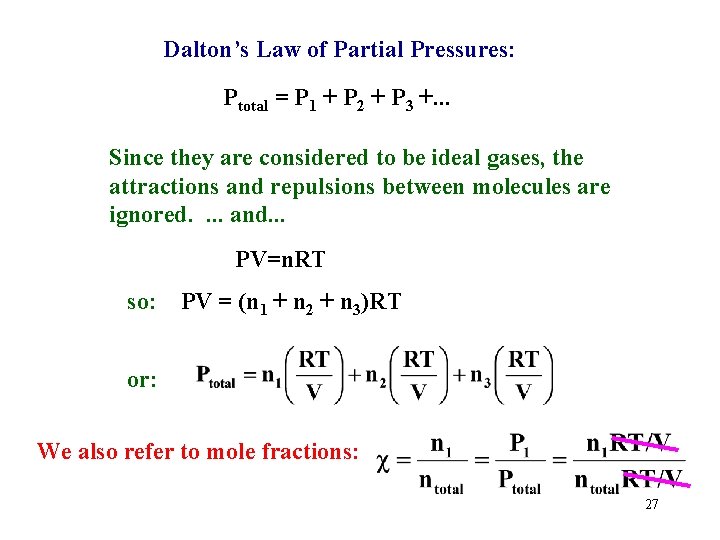
Dalton’s Law of Partial Pressures: He H 2 N 2 Ptotal = P 1 + P 2 + P 3 +. . . 26

Dalton’s Law of Partial Pressures: Ptotal = P 1 + P 2 + P 3 +. . . Since they are considered to be ideal gases, the attractions and repulsions between molecules are ignored. . and. . . PV=n. RT so: PV = (n 1 + n 2 + n 3)RT or: We also refer to mole fractions: 27

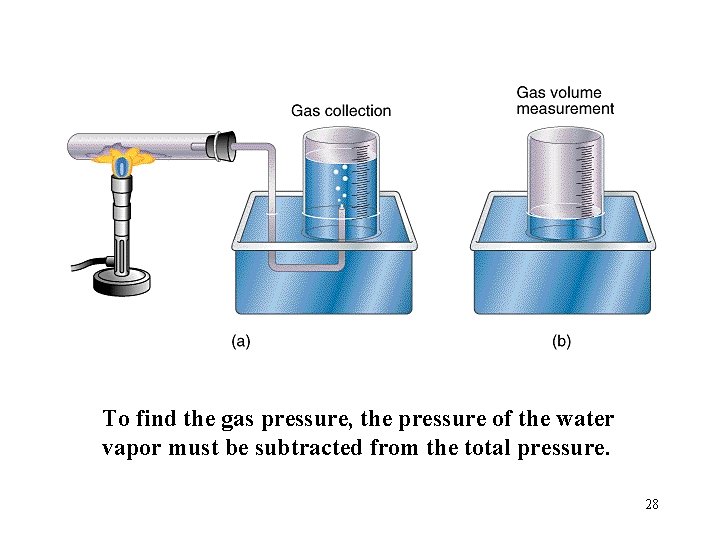
To find the gas pressure, the pressure of the water vapor must be subtracted from the total pressure. 28

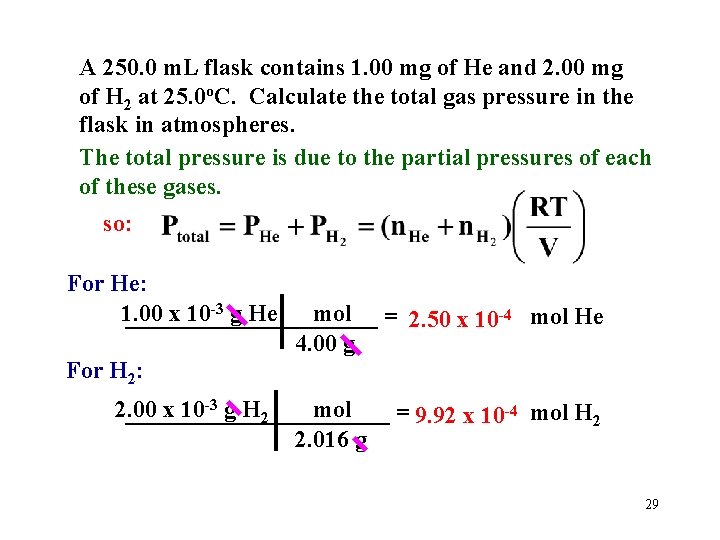
A 250. 0 m. L flask contains 1. 00 mg of He and 2. 00 mg of H 2 at 25. 0 o. C. Calculate the total gas pressure in the flask in atmospheres. The total pressure is due to the partial pressures of each of these gases. so: For He: 1. 00 x 10 -3 g He mol = 2. 50 x 10 -4 mol He ___________ 4. 00 g For H 2: 2. 00 x 10 -3 g H 2 mol ___________ = 9. 92 x 10 -4 mol H 2 2. 016 g 29

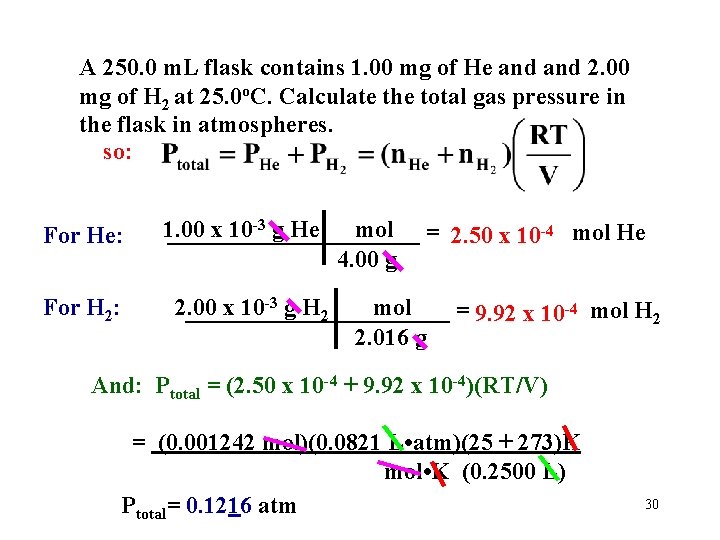
A 250. 0 m. L flask contains 1. 00 mg of He and 2. 00 mg of H 2 at 25. 0 o. C. Calculate the total gas pressure in the flask in atmospheres. so: For He: For H 2: 1. 00 x 10 -3 g He mol = 2. 50 x 10 -4 mol He ___________ 4. 00 g 2. 00 x 10 -3 g H 2 mol ___________ = 9. 92 x 10 -4 mol H 2 2. 016 g And: Ptotal = (2. 50 x 10 -4 + 9. 92 x 10 -4)(RT/V) = (0. 001242 mol)(0. 0821 L • atm)(25 + 273)K mol • K (0. 2500 L) Ptotal= 0. 1216 atm 30

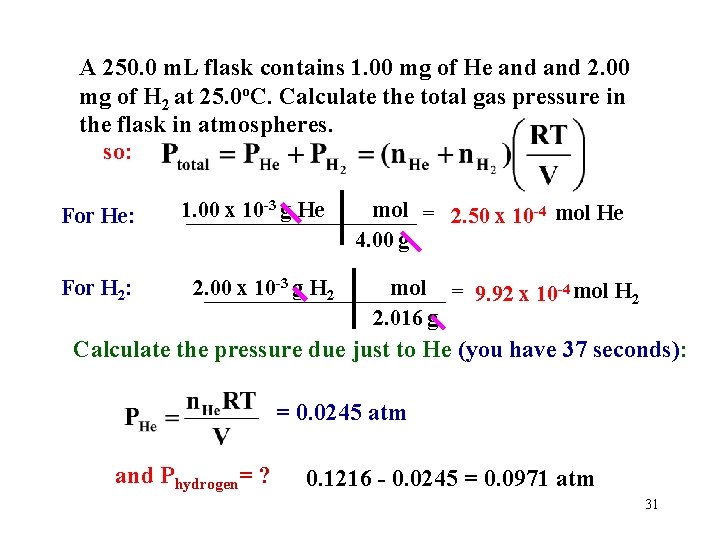
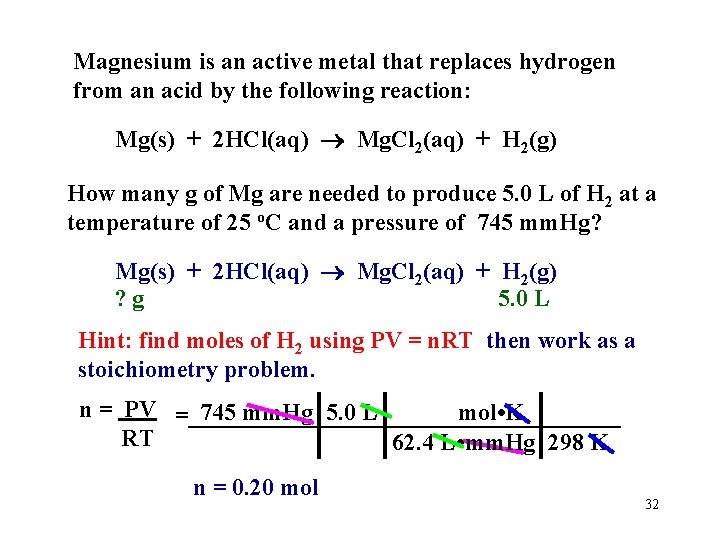
A 250. 0 m. L flask contains 1. 00 mg of He and 2. 00 mg of H 2 at 25. 0 o. C. Calculate the total gas pressure in the flask in atmospheres. so: For He: For H 2: 1. 00 x 10 -3 g He mol = 2. 50 x 10 -4 mol He ___________ 4. 00 g 2. 00 x 10 -3 g H 2 mol = 9. 92 x 10 -4 mol H 2 ___________ 2. 016 g Calculate the pressure due just to He (you have 37 seconds): = 0. 0245 atm and Phydrogen= ? 0. 1216 - 0. 0245 = 0. 0971 atm 31

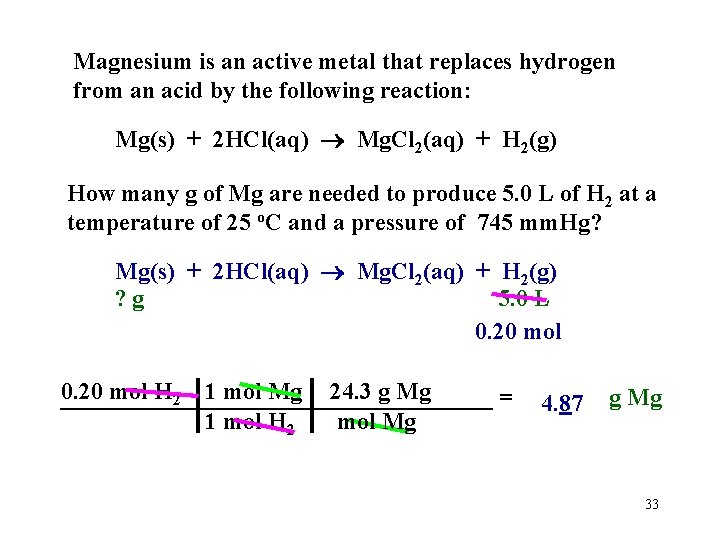
Magnesium is an active metal that replaces hydrogen from an acid by the following reaction: Mg(s) + 2 HCl(aq) Mg. Cl 2(aq) + H 2(g) How many g of Mg are needed to produce 5. 0 L of H 2 at a temperature of 25 o. C and a pressure of 745 mm. Hg? Mg(s) + 2 HCl(aq) Mg. Cl 2(aq) + H 2(g) ? g 5. 0 L Hint: find moles of H 2 using PV = n. RT then work as a stoichiometry problem. n = PV =__________________ 745 mm. Hg 5. 0 L mol • K RT 62. 4 L • mm. Hg 298 K n = 0. 20 mol 32

Magnesium is an active metal that replaces hydrogen from an acid by the following reaction: Mg(s) + 2 HCl(aq) Mg. Cl 2(aq) + H 2(g) How many g of Mg are needed to produce 5. 0 L of H 2 at a temperature of 25 o. C and a pressure of 745 mm. Hg? Mg(s) + 2 HCl(aq) Mg. Cl 2(aq) + H 2(g) ? g 5. 0 L 0. 20 mol H 2 1 mol Mg 24. 3 g Mg __________________ = 1 mol H 2 mol Mg 4. 87 g Mg 33

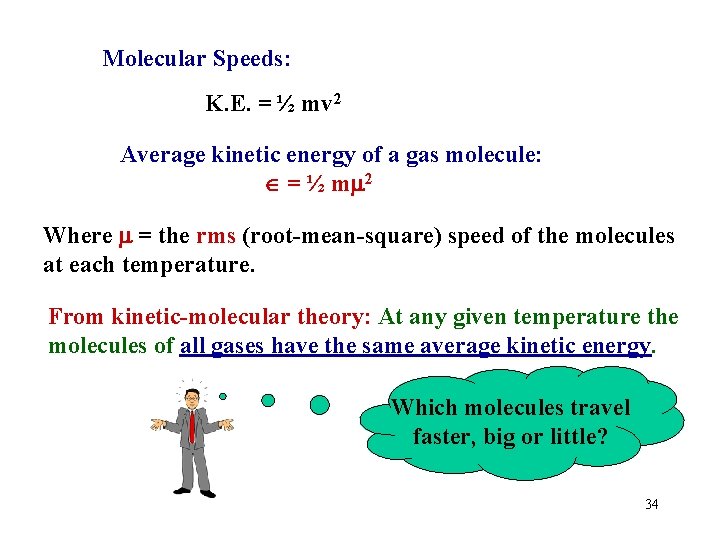
Molecular Speeds: K. E. = ½ mv 2 Average kinetic energy of a gas molecule: = ½ m 2 Where = the rms (root-mean-square) speed of the molecules at each temperature. From kinetic-molecular theory: At any given temperature the molecules of all gases have the same average kinetic energy. Which molecules travel faster, big or little? 34

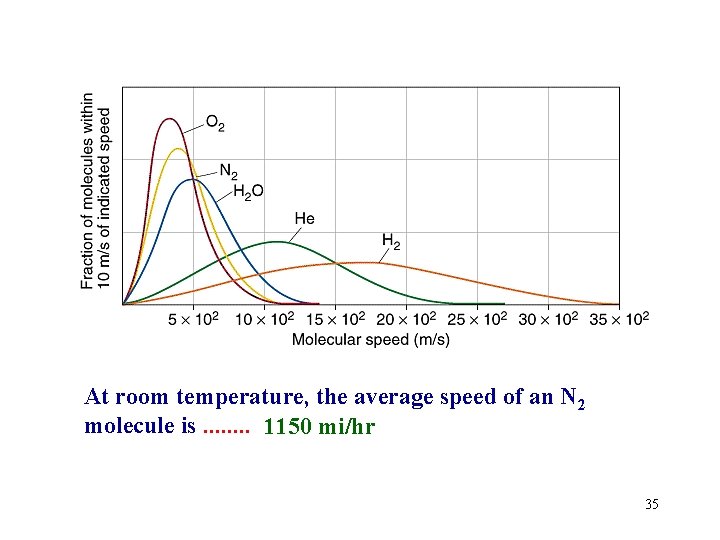
At room temperature, the average speed of an N 2 molecule is. . . . 1150 mi/hr 35

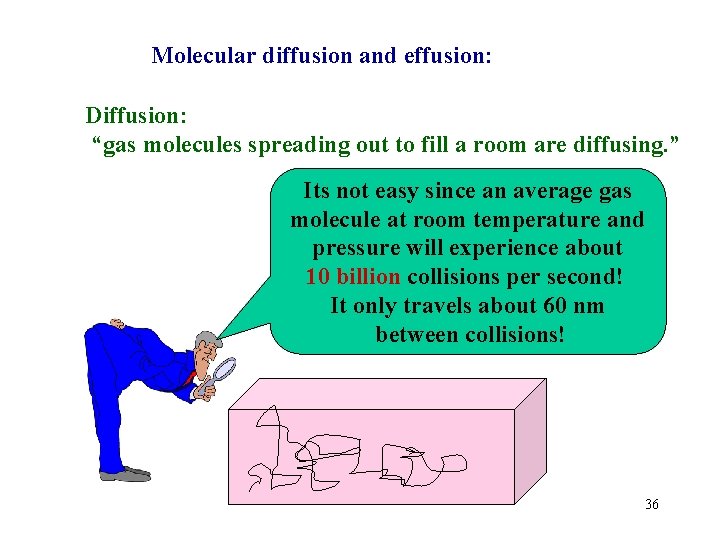
Molecular diffusion and effusion: Diffusion: “gas molecules spreading out to fill a room are diffusing. ” Its not easy since an average gas molecule at room temperature and pressure will experience about 10 billion collisions per second! It only travels about 60 nm between collisions! 36

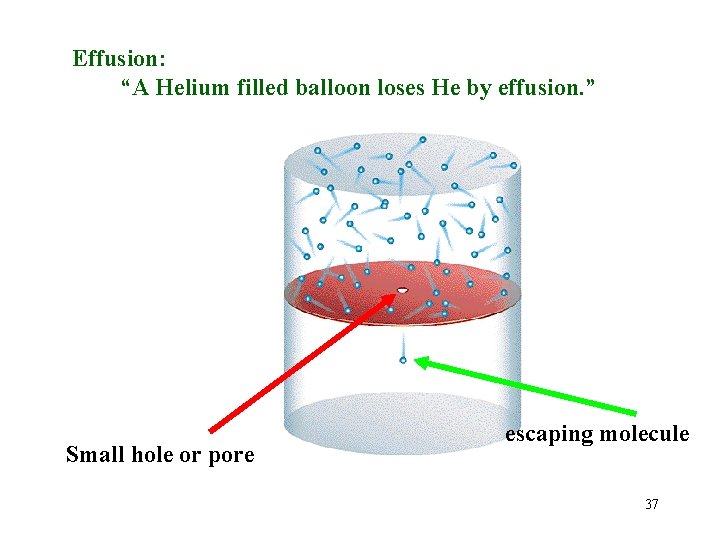
Effusion: “A Helium filled balloon loses He by effusion. ” Small hole or pore escaping molecule 37

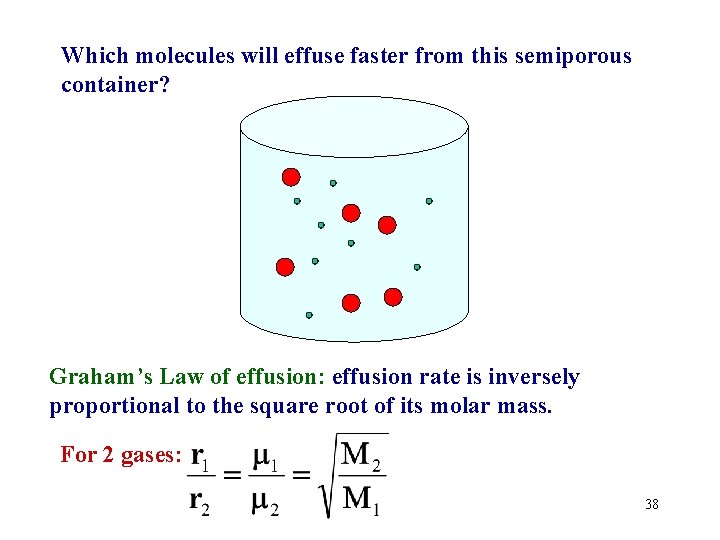
Which molecules will effuse faster from this semiporous container? Graham’s Law of effusion: effusion rate is inversely proportional to the square root of its molar mass. For 2 gases: 38

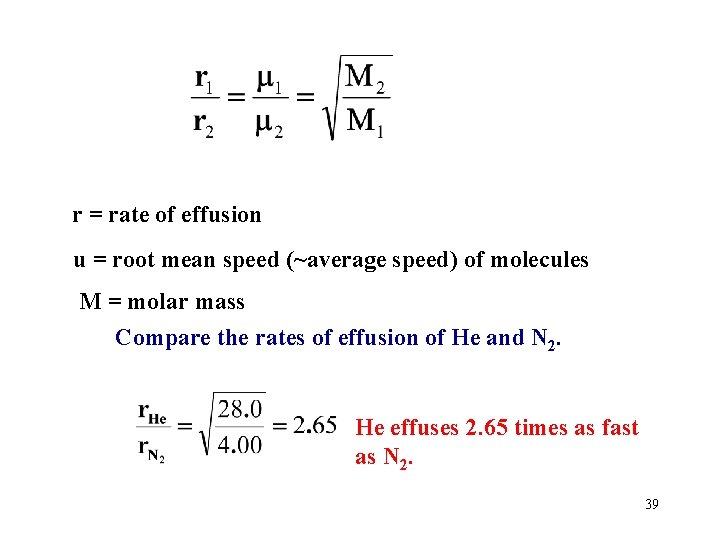
r = rate of effusion u = root mean speed (~average speed) of molecules M = molar mass Compare the rates of effusion of He and N 2. He effuses 2. 65 times as fast as N 2. 39

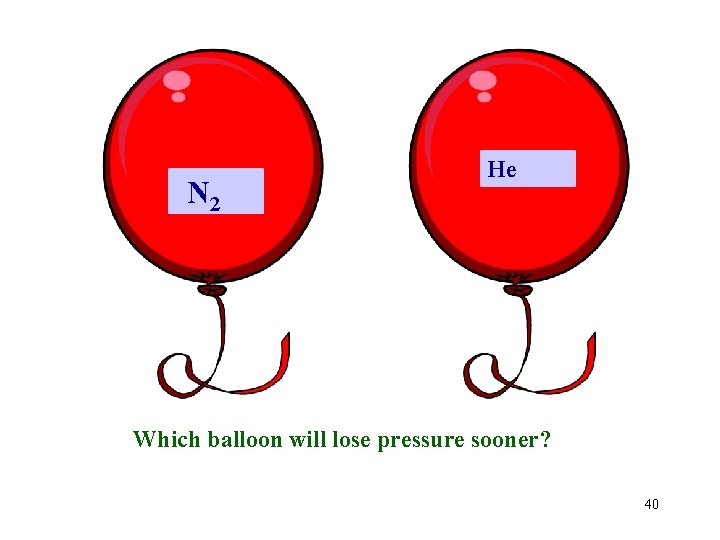
N 2 He Which balloon will lose pressure sooner? 40

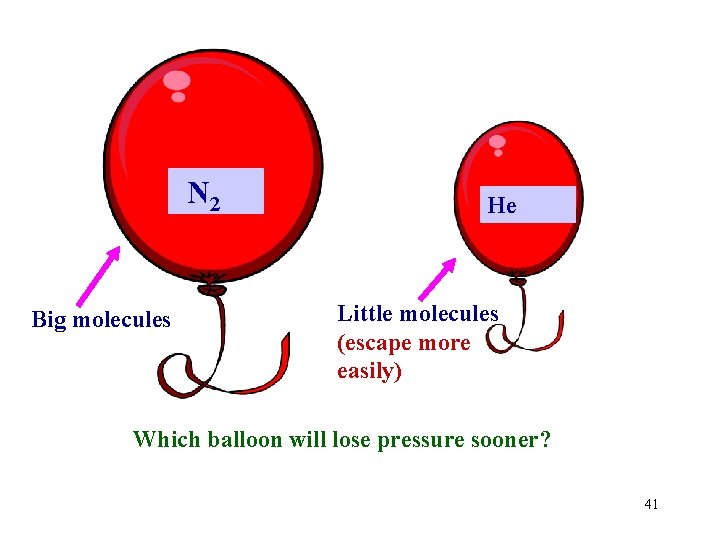
N 2 Big molecules He Little molecules (escape more easily) Which balloon will lose pressure sooner? 41

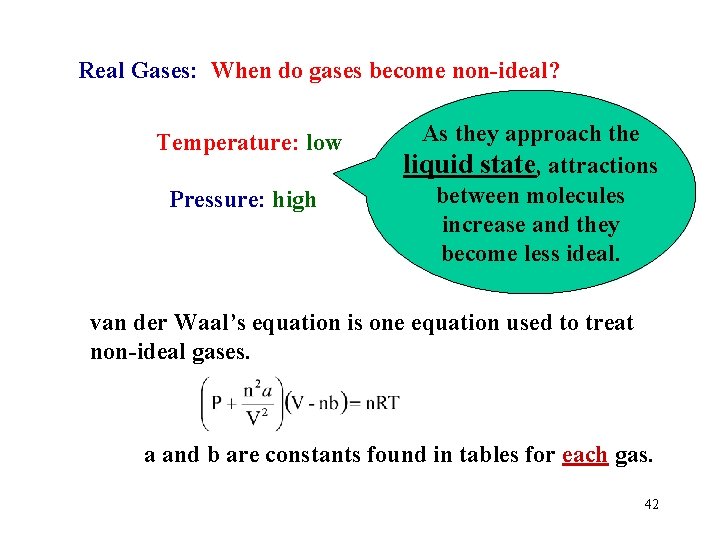
Real Gases: When do gases become non-ideal? Temperature: low Pressure: high As they approach the liquid state, attractions between molecules increase and they become less ideal. van der Waal’s equation is one equation used to treat non-ideal gases. a and b are constants found in tables for each gas. 42

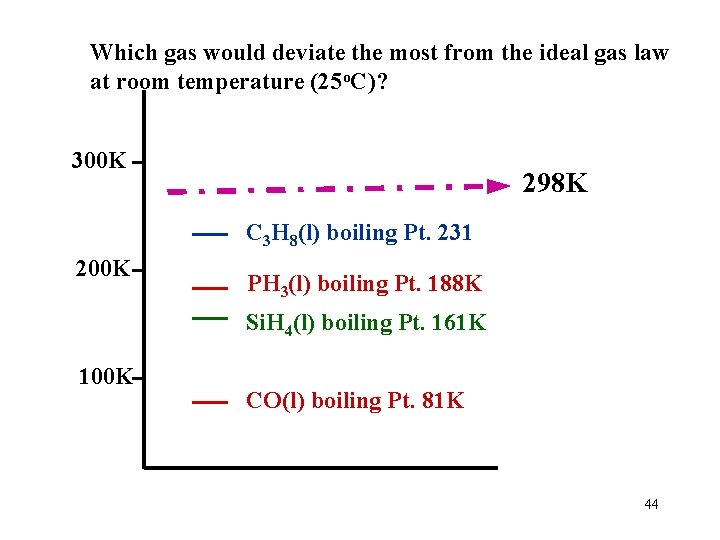
Which gas would deviate the most from the ideal gas law at room temperature (25 o. C)? C 3 H 8 boiling Pt. 231 K PH 3 boiling Pt. 188 K Si. H 4 boiling Pt. 161 K CO boiling Pt. 81 K 43

Which gas would deviate the most from the ideal gas law at room temperature (25 o. C)? 300 K 298 K C 3 H 8(l) boiling Pt. 231 200 K PH 3(l) boiling Pt. 188 K Si. H 4(l) boiling Pt. 161 K 100 K CO(l) boiling Pt. 81 K 44
 Ron gilletti
Ron gilletti H mol
H mol Paul gilletti
Paul gilletti Mesa de dios y mesa de los demonios
Mesa de dios y mesa de los demonios Collège paul eluard vermelles
Collège paul eluard vermelles Pronote college paul fort
Pronote college paul fort Community action cycle for community mobilization
Community action cycle for community mobilization Woodland community college counseling
Woodland community college counseling Chesterton community college sixth form
Chesterton community college sixth form Willow community college
Willow community college Willow international address
Willow international address Southwestern community college
Southwestern community college Shoreline community college nursing
Shoreline community college nursing Moraine valley testing center
Moraine valley testing center Pnc intellilink
Pnc intellilink Lahc verify my fafsa
Lahc verify my fafsa Johnson county community college nursing program
Johnson county community college nursing program Glendale community college library hours
Glendale community college library hours Glendale community college nursing prerequisites
Glendale community college nursing prerequisites Nursing program glendale
Nursing program glendale Glendale community college drop deadline
Glendale community college drop deadline National community college benchmark project
National community college benchmark project Tarrant county college - northeast campus
Tarrant county college - northeast campus Aims community college cpat
Aims community college cpat Wallace community college sparks campus
Wallace community college sparks campus Uwt fafsa
Uwt fafsa South seattle community college
South seattle community college Mathmaticious
Mathmaticious Ccd radiology program
Ccd radiology program Hotels near paradise valley community college
Hotels near paradise valley community college Norco college web advisor
Norco college web advisor Glendale community college transcript
Glendale community college transcript Rogue community college map
Rogue community college map Bergen community college academic advising
Bergen community college academic advising Pima community college advisors
Pima community college advisors George c wallace community college
George c wallace community college Wayne community college library
Wayne community college library River valley community college
River valley community college Joshua fiske
Joshua fiske Queensborough community college library
Queensborough community college library Capital community college grammar
Capital community college grammar Moodle carteret community college
Moodle carteret community college Clyst vale community college
Clyst vale community college Coastline pictures
Coastline pictures Chief instructional officer
Chief instructional officer