Weak Acid Titration Calculations Paul Gilletti Ph D



![Strong acid: [H 3 O+] = concentration of acid so: p. H = -log Strong acid: [H 3 O+] = concentration of acid so: p. H = -log](https://slidetodoc.com/presentation_image/2b64565caf94119263c5b4e4a50362a9/image-13.jpg)
![Approximations: [H+] [A-] 4 2 p. OH=-Log[XS OH-] 3 1 [H+]=[A-] [OH-]=[HA] 15 Approximations: [H+] [A-] 4 2 p. OH=-Log[XS OH-] 3 1 [H+]=[A-] [OH-]=[HA] 15](https://slidetodoc.com/presentation_image/2b64565caf94119263c5b4e4a50362a9/image-15.jpg)


![Approximations: [H+] [A-] 2 4 STUDY FOR QUIZ p. OH=-Log[XS OH-] 3 1 [H+]=[A-] Approximations: [H+] [A-] 2 4 STUDY FOR QUIZ p. OH=-Log[XS OH-] 3 1 [H+]=[A-]](https://slidetodoc.com/presentation_image/2b64565caf94119263c5b4e4a50362a9/image-35.jpg)

- Slides: 41

Weak Acid Titration Calculations Paul Gilletti Ph. D. Mesa Community College Mesa, AZ 1

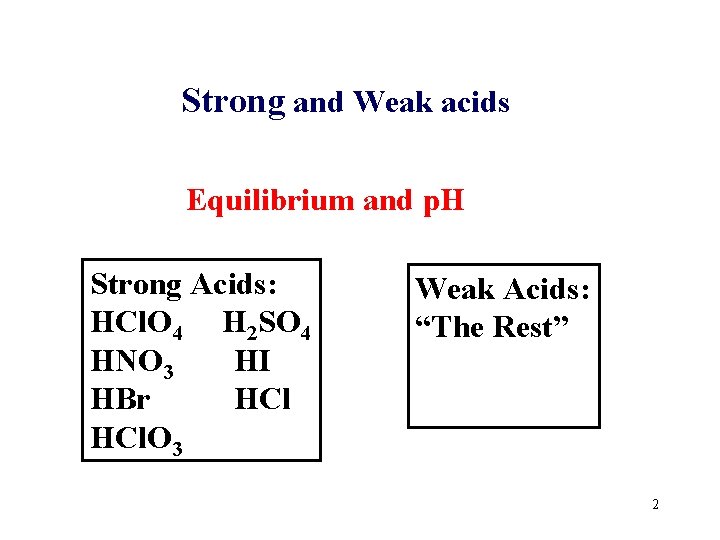
Strong and Weak acids Equilibrium and p. H Strong Acids: HCl. O 4 H 2 SO 4 HNO 3 HI HBr HCl. O 3 Weak Acids: “The Rest” 2

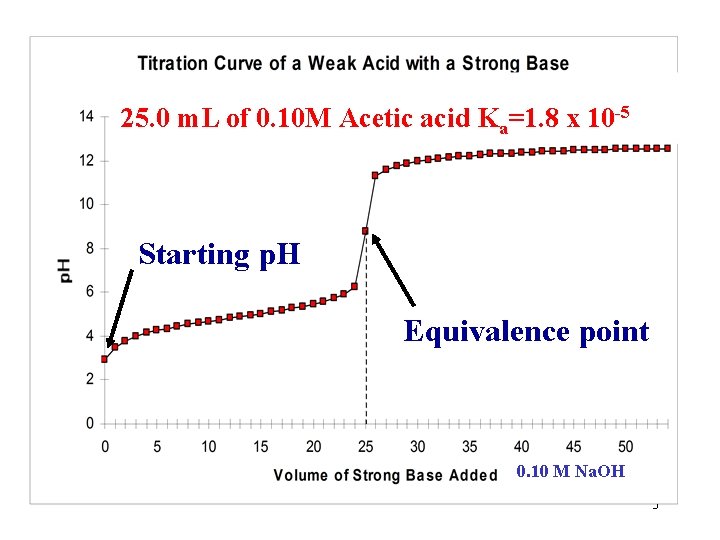
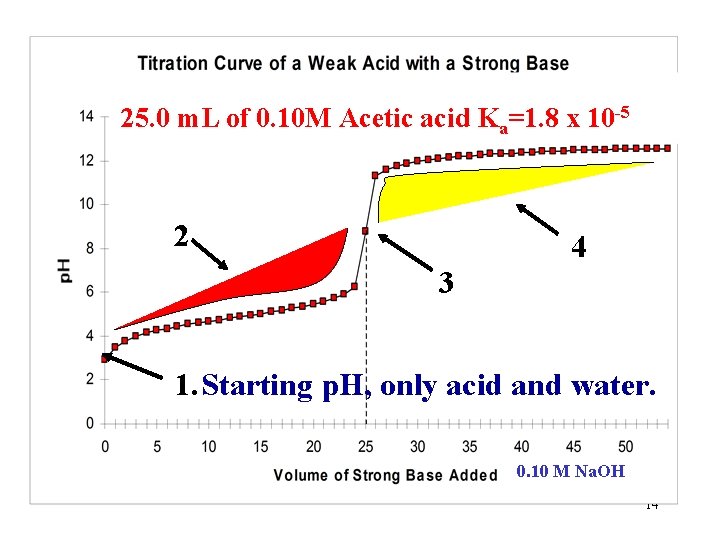
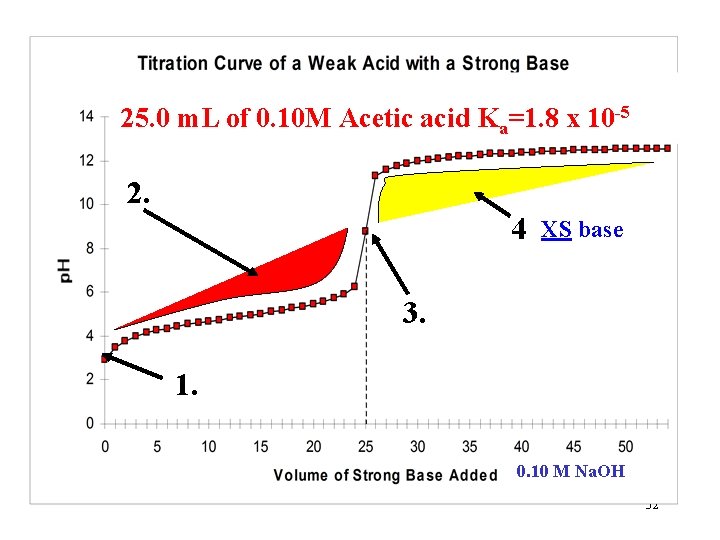
25. 0 m. L of 0. 10 M Acetic acid Ka=1. 8 x 10 -5 Starting p. H Equivalence point 0. 10 M Na. OH 3

Acetic acid is a weak acid. What would be the starting p. H of the solution if it were a strong acid? (25. 0 m. L of 0. 10 M Acetic acid) Do a quick calculation as if acetic acid were a strong acid and compare this value to that on the graph. How do the two values compare? Weak Acid: Graph starting p. H = 2. 87 Calculated as Strong acid: p. H = -log(0. 10) = 1. 0 How and Why are they different? “Let’s investigate!” 4

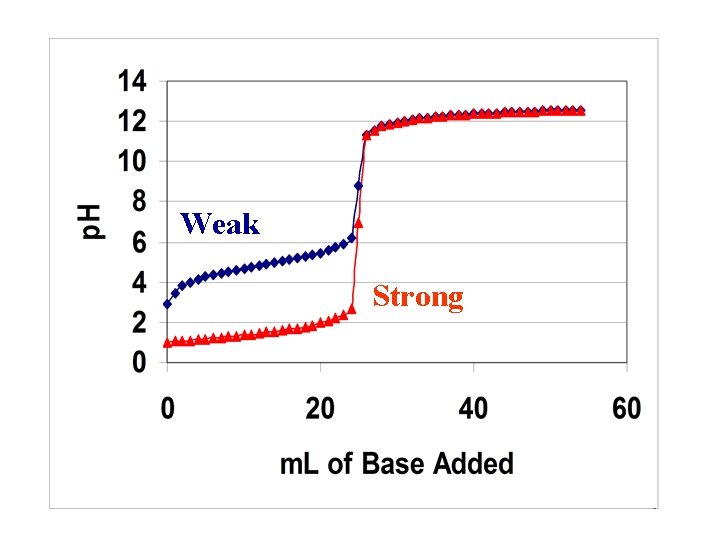
Weak Strong 5

How do the two values compare? Graph starting p. H = 2. 87 Strong acid calculation: p. H = -log(0. 10) = 1. 0 What is the significance of this difference? Since the two values differ by ~2 p. H units and p. H is a log scale, the concentration of H 3 O+ in the strong acid calculation is 1 x 102 or 100 times greater than that observed on the graph. Let’s see WHY they are different? 6

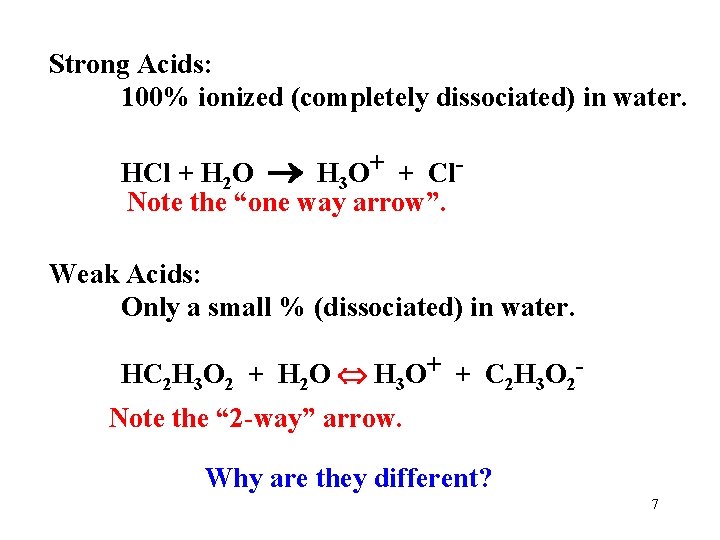
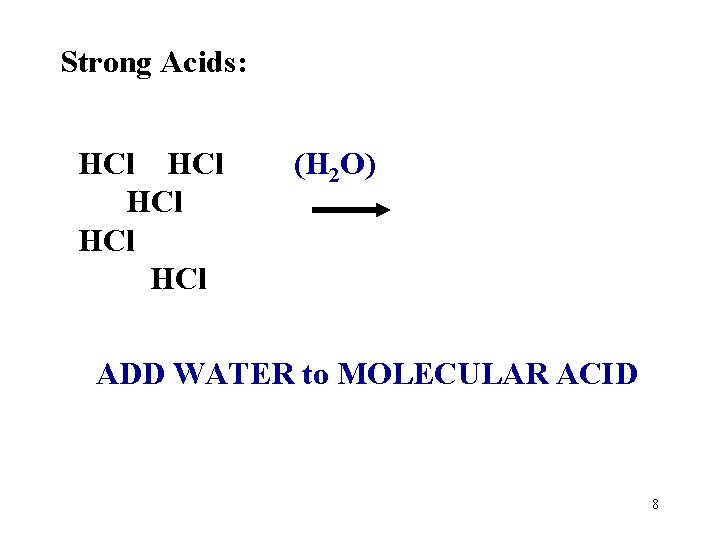
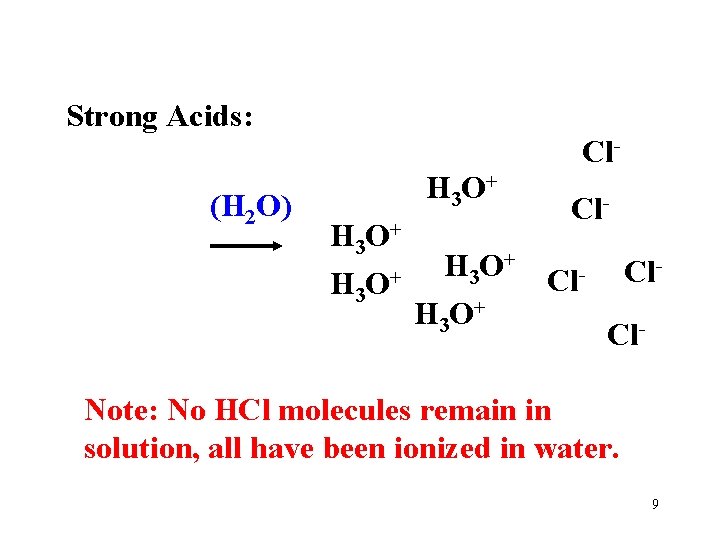
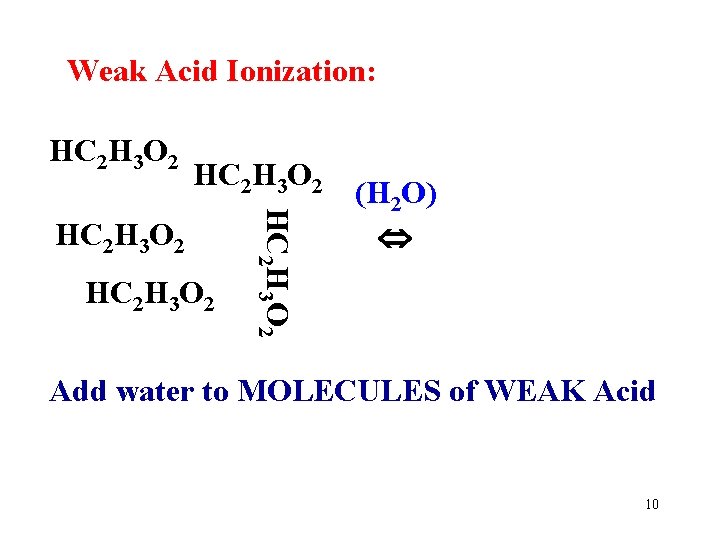
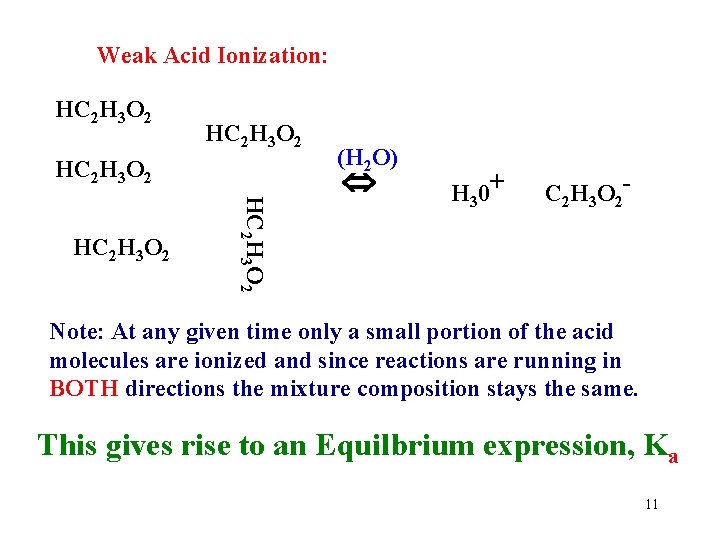
Strong Acids: 100% ionized (completely dissociated) in water. HCl + H 2 O H 3 O+ + Cl. Note the “one way arrow”. Weak Acids: Only a small % (dissociated) in water. HC 2 H 3 O 2 + H 2 O H 3 O+ + C 2 H 3 O 2 Note the “ 2 -way” arrow. Why are they different? 7

Strong Acids: HCl HCl HCl (H 2 O) ADD WATER to MOLECULAR ACID 8

Strong Acids: Cl(H 2 O) H 3 O+ Cl- H 3 O+ Cl. Cl- Note: No HCl molecules remain in solution, all have been ionized in water. 9

Weak Acid Ionization: HC 2 H 3 O 2 HC 2 H 3 O 2 (H 2 O) Add water to MOLECULES of WEAK Acid 10

Weak Acid Ionization: HC 2 H 3 O 2 HC 2 H 3 O 2 (H 2 O) H 30+ C 2 H 3 O 2 - Note: At any given time only a small portion of the acid molecules are ionized and since reactions are running in BOTH directions the mixture composition stays the same. This gives rise to an Equilbrium expression, Ka 11

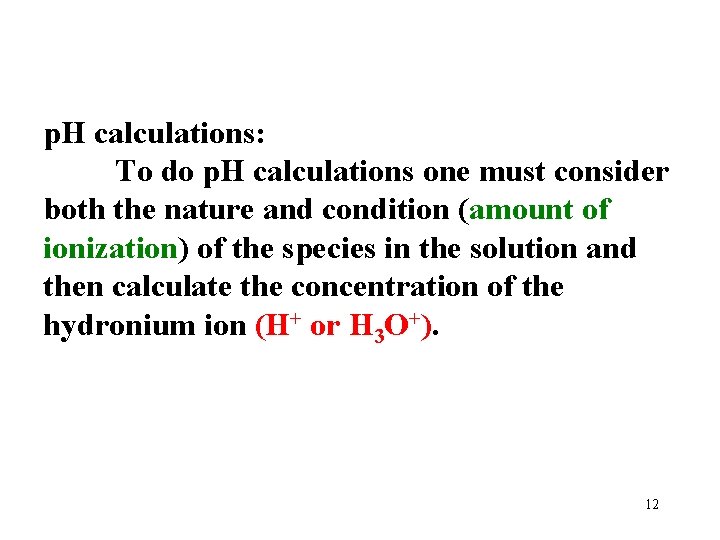
p. H calculations: To do p. H calculations one must consider both the nature and condition (amount of ionization) of the species in the solution and then calculate the concentration of the hydronium ion (H+ or H 3 O+). 12
![Strong acid H 3 O concentration of acid so p H log Strong acid: [H 3 O+] = concentration of acid so: p. H = -log](https://slidetodoc.com/presentation_image/2b64565caf94119263c5b4e4a50362a9/image-13.jpg)
Strong acid: [H 3 O+] = concentration of acid so: p. H = -log [H 3 O+] = -log[acid] Weak Acid: one must calculate the [H 3 O+] from an equilibrium ionization expression. HA + H 2 O H 3 O+ + AKa = _ [H 3 O+][A-] [HA] These are equilibrium concentrations. 13

25. 0 m. L of 0. 10 M Acetic acid Ka=1. 8 x 10 -5 2 4 3 1. Starting p. H, only acid and water. 0. 10 M Na. OH 14
![Approximations H A 4 2 p OHLogXS OH 3 1 HA OHHA 15 Approximations: [H+] [A-] 4 2 p. OH=-Log[XS OH-] 3 1 [H+]=[A-] [OH-]=[HA] 15](https://slidetodoc.com/presentation_image/2b64565caf94119263c5b4e4a50362a9/image-15.jpg)
Approximations: [H+] [A-] 4 2 p. OH=-Log[XS OH-] 3 1 [H+]=[A-] [OH-]=[HA] 15

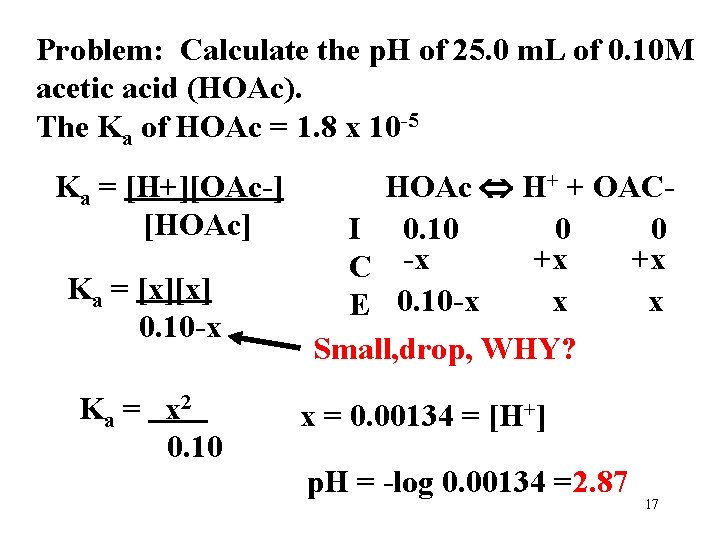
Problem: Calculate the p. H of 25. 0 m. L of 0. 10 M acetic acid (HOAc). The Ka of HOAc = 1. 8 x 10 -5 Which region of a titration curve would this be? 16

Problem: Calculate the p. H of 25. 0 m. L of 0. 10 M acetic acid (HOAc). The Ka of HOAc = 1. 8 x 10 -5 Ka = [H+][OAc-] [HOAc] Ka = [x][x] 0. 10 -x Ka = x 2 0. 10 HOAc H+ + OACI 0. 10 0 0 +x +x C -x x x E 0. 10 -x Small, drop, WHY? x = 0. 00134 = [H+] p. H = -log 0. 00134 =2. 87 17

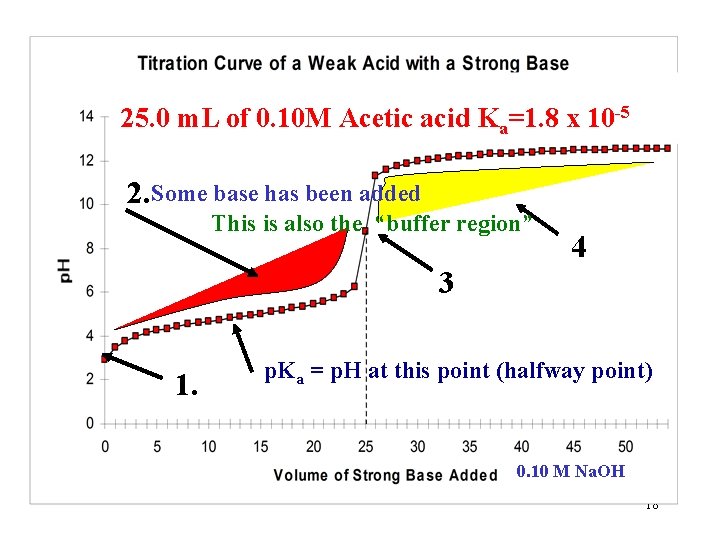
25. 0 m. L of 0. 10 M Acetic acid Ka=1. 8 x 10 -5 2. Some base has been added This is also the “buffer region” 4 3 1. p. Ka = p. H at this point (halfway point) 0. 10 M Na. OH 18

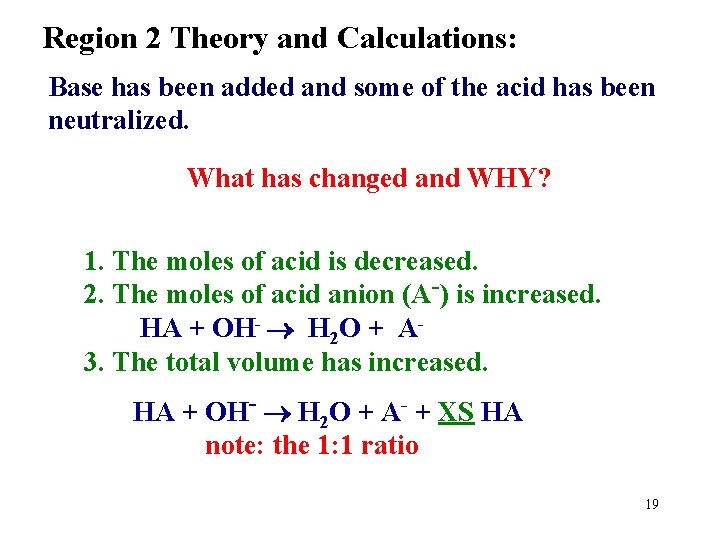
Region 2 Theory and Calculations: Base has been added and some of the acid has been neutralized. What has changed and WHY? 1. The moles of acid is decreased. 2. The moles of acid anion (A-) is increased. HA + OH- H 2 O + A 3. The total volume has increased. HA + OH- H 2 O + A- + XS HA note: the 1: 1 ratio 19

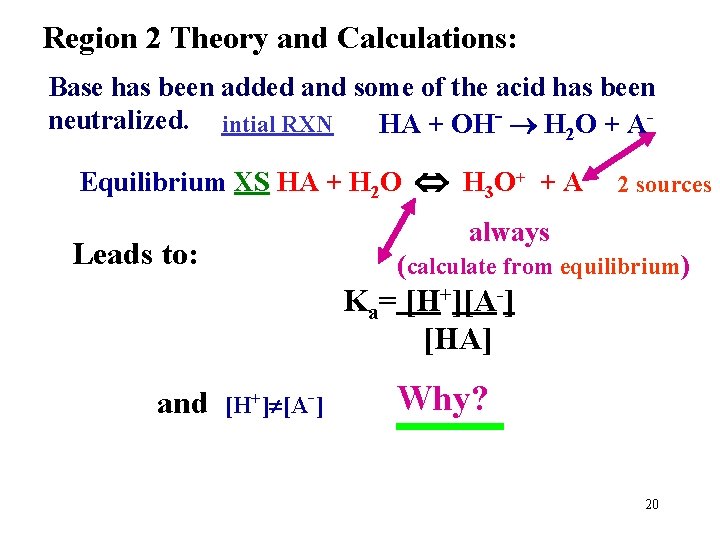
Region 2 Theory and Calculations: Base has been added and some of the acid has been neutralized. intial RXN HA + OH- H 2 O + AEquilibrium XS HA + H 2 O H 3 O+ + A- 2 sources always Leads to: (calculate from equilibrium) Ka= [H+][A-] [HA] and [H+] [A-] Why? 20

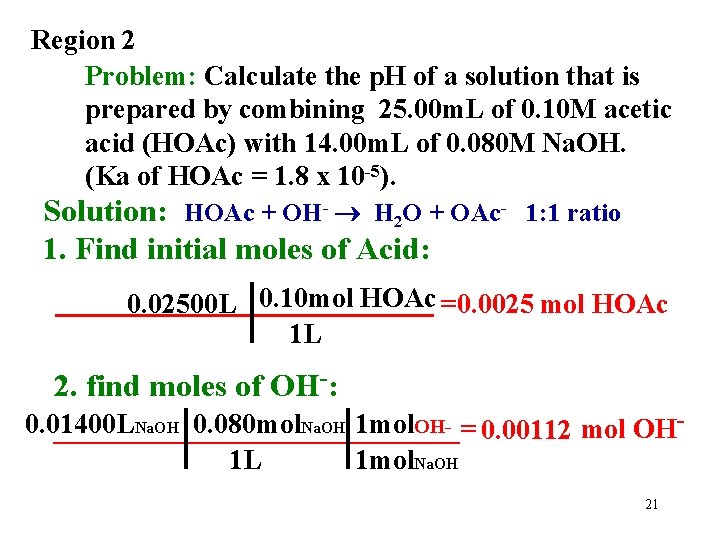
Region 2 Problem: Calculate the p. H of a solution that is prepared by combining 25. 00 m. L of 0. 10 M acetic acid (HOAc) with 14. 00 m. L of 0. 080 M Na. OH. (Ka of HOAc = 1. 8 x 10 -5). Solution: HOAc + OH- H 2 O + OAc- 1: 1 ratio 1. Find initial moles of Acid: 0. 02500 L 0. 10 mol HOAc = 0. 0025 mol HOAc 1 L 2. find moles of OH-: 0. 01400 L Na. OH 0. 080 mol. Na. OH 1 mol. OH_______________= 0. 00112 mol OH 1 L 1 mol. Na. OH 21

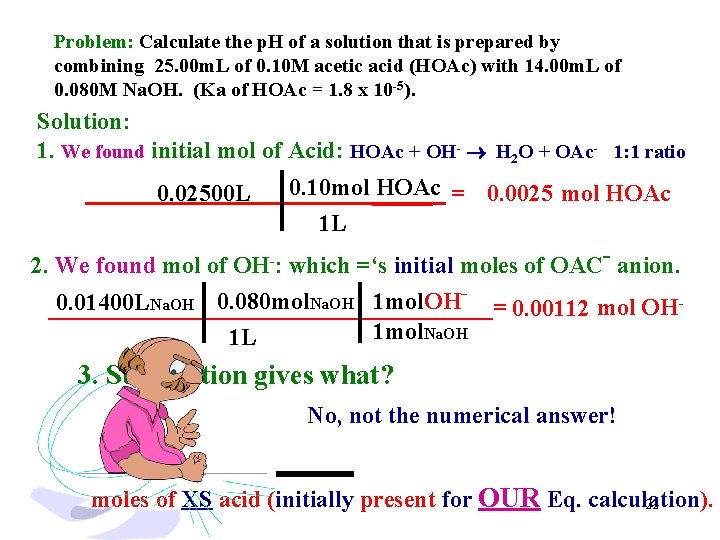
Problem: Calculate the p. H of a solution that is prepared by combining 25. 00 m. L of 0. 10 M acetic acid (HOAc) with 14. 00 m. L of 0. 080 M Na. OH. (Ka of HOAc = 1. 8 x 10 -5). Solution: 1. We found initial mol of Acid: HOAc + OH- H 2 O + OAc- 1: 1 ratio 0. 10 mol _____ HOAc = 0. 0025 mol HOAc 1 L 2. We found mol of OH-: which =‘s initial moles of OAC- anion. 0. 02500 L 0. 080 mol Na. OH 1 mol. OH 0. 01400 L Na. OH ___________________= 0. 00112 mol OH 1 mol. Na. OH 1 L 3. Subtraction gives what? No, not the numerical answer! moles of XS acid (initially present for OUR Eq. calculation). 22

Problem: Calculate the p. H of a solution that is prepared by combining 25. 00 m. L of 0. 10 M acetic acid (HOAc) with 14. 00 m. L of 0. 080 M Na. OH. (Ka of HOAc = 1. 8 x 10 -5). Solution: 1. Find initial moles of Acid: HOAc + OH- H 2 O + OAc- 1: 1 ratio 0. 10 mol _____ HOAc = 0. 0025 mol HOAc 1 L 2. find moles of OH-: which =‘s initial moles of OAC- anion. 0. 02500 L 0. 01400 LNa. OH 0. 080 mol. Na. OH 1 mol. OH___________________= 0. 00112 mol OH 1 L 1 mol. Na. OH 3. Subtraction gives moles of XS acid (initial for I. C. E. ). 0. 0025 - 0. 00112 = 0. 00138 mole acid INITIAL 4. and. . we have 0. 00112 mole OAc- INITIAL for I. C. E. 23

Problem: Calculate the p. H of a solution that is prepared by combining 25. 00 m. L of 0. 10 M acetic acid (HOAc) with 14. 00 m. L of 0. 080 M Na. OH. (Ka of HOAc = 1. 8 x 10 -5). Solution: FROM PREVIOUS SLIDE. 1. initial M of Acid: HOAc + OH- H 2 O + OAc- 1: 1 ratio 0. 00138 mole/0. 039 L = 0. 0354 M acid after RXN =INITIAL 2. M of OH-: which =‘s initial M of OAC- anion. 0. 00112 mole/0. 039 L = 0. 0287 M OAc- after RXN =INITIAL** [HOAc] [H+] + [OAc-] I** 0. 0354 0 0. 0287 -x +x +x C E 0. 0354 -x x 0. 0287+x Can we drop these? x = 0. 0000222 =[H+] p. H = - Log [0. 0000222] = 4. 65 24

Region 2 summary: 1. write balanced equation for initial weak/strong RXN. 2. find moles of acid 3. find moles of base 4. subtract to determine XS (and limiting reactant which equals initial moles of salt formed) 5. Change XS to M and limiting Reactant to M 6. Write balanced Chemical Equilibrium Equation: HA H+ + A 7. Use values from #5 a initial values in I. C. E. Chart 8. Work as Equilibrium problem. 25

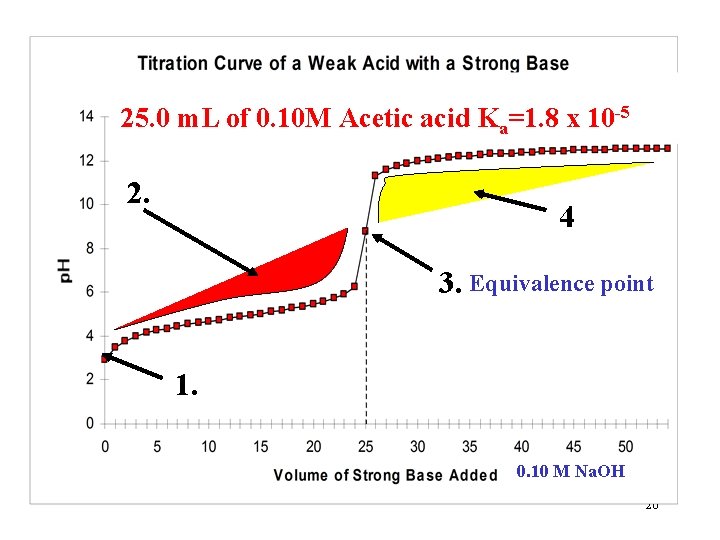
25. 0 m. L of 0. 10 M Acetic acid Ka=1. 8 x 10 -5 2. 4 3. Equivalence point 1. 0. 10 M Na. OH 26

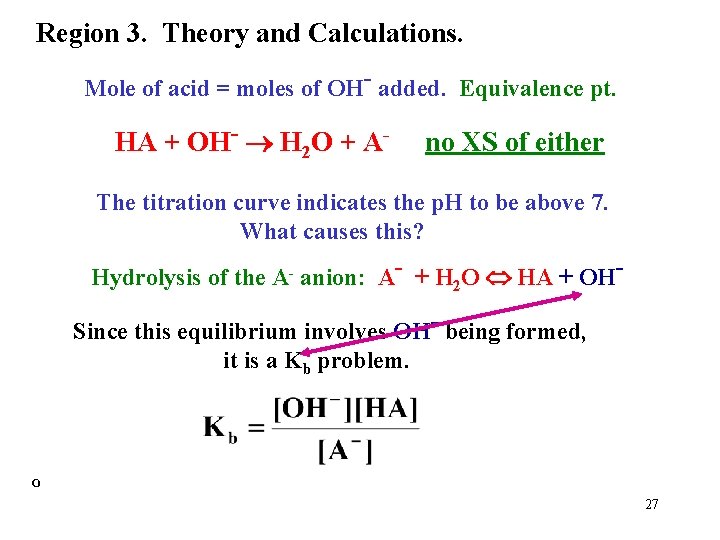
Region 3. Theory and Calculations. Mole of acid = moles of OH- added. Equivalence pt. HA + OH- H 2 O + A- no XS of either The titration curve indicates the p. H to be above 7. What causes this? Hydrolysis of the A- anion: A- + H O HA + OH 2 Since this equilibrium involves OH- being formed, it is a Kb problem. º 27

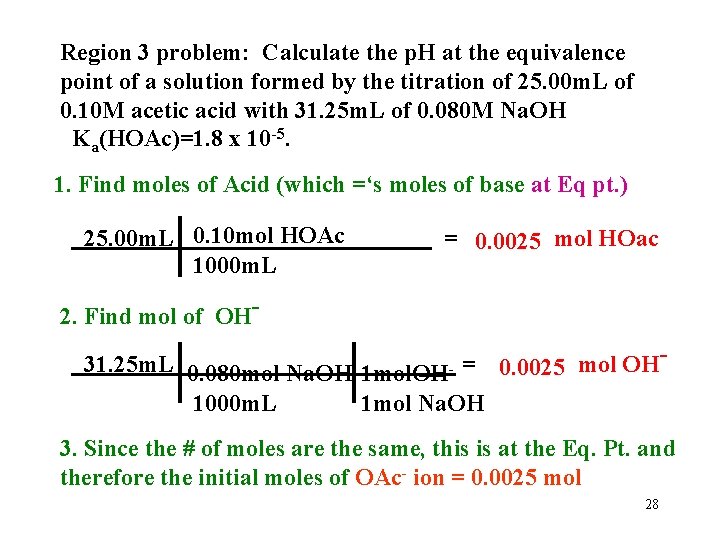
Region 3 problem: Calculate the p. H at the equivalence point of a solution formed by the titration of 25. 00 m. L of 0. 10 M acetic acid with 31. 25 m. L of 0. 080 M Na. OH Ka(HOAc)=1. 8 x 10 -5. 1. Find moles of Acid (which =‘s moles of base at Eq pt. ) 25. 00 m. L 0. 10 mol HOAc 1000 m. L = 0. 0025 mol HOac 2. Find mol of OH 31. 25 m. L 0. 080 mol Na. OH 1 mol. OH __ - = 0. 0025 mol OH 1000 m. L 1 mol Na. OH 3. Since the # of moles are the same, this is at the Eq. Pt. and therefore the initial moles of OAc- ion = 0. 0025 mol 28

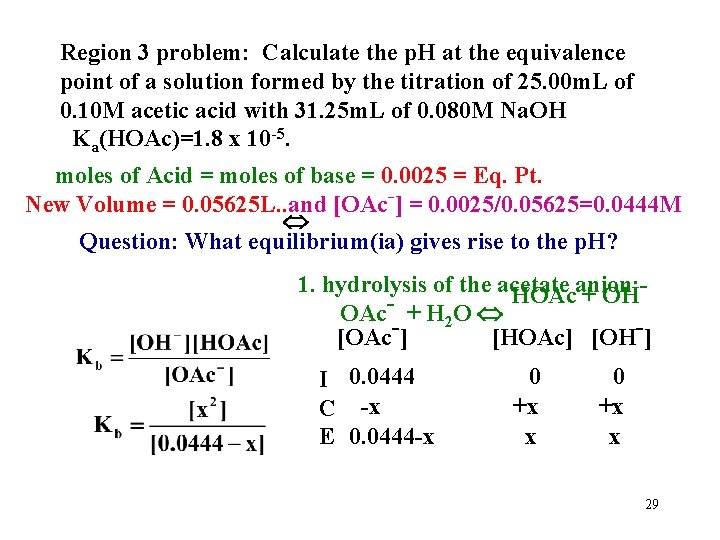
Region 3 problem: Calculate the p. H at the equivalence point of a solution formed by the titration of 25. 00 m. L of 0. 10 M acetic acid with 31. 25 m. L of 0. 080 M Na. OH Ka(HOAc)=1. 8 x 10 -5. moles of Acid = moles of base = 0. 0025 = Eq. Pt. New Volume = 0. 05625 L. . and [OAc-] = 0. 0025/0. 05625=0. 0444 M Question: What equilibrium(ia) gives rise to the p. H? 1. hydrolysis of the acetate anion: HOAc + OH OAc- + H 2 O [OAc-] [HOAc] [OH-] I 0. 0444 C -x E 0. 0444 -x 0 +x x 29

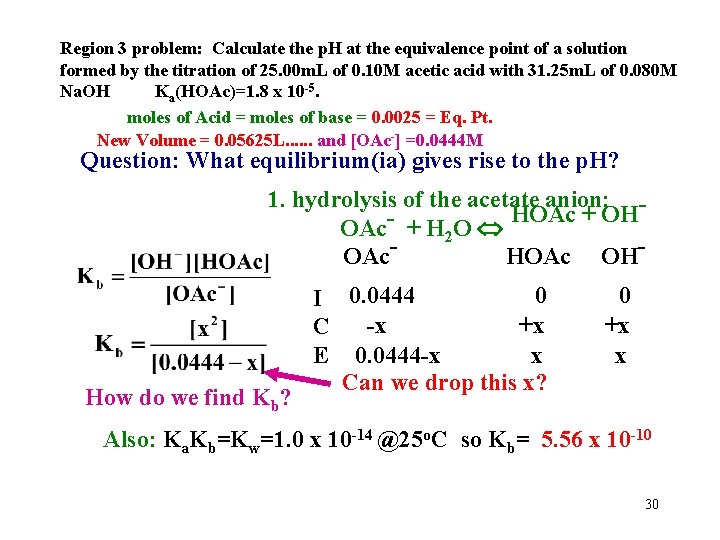
Region 3 problem: Calculate the p. H at the equivalence point of a solution formed by the titration of 25. 00 m. L of 0. 10 M acetic acid with 31. 25 m. L of 0. 080 M Na. OH Ka(HOAc)=1. 8 x 10 -5. moles of Acid = moles of base = 0. 0025 = Eq. Pt. New Volume = 0. 05625 L. . . and [OAc-] =0. 0444 M Question: What equilibrium(ia) gives rise to the p. H? 1. hydrolysis of the acetate anion: HOAc + OH OAc- + H 2 O OAc. HOAc OH- How do we find Kb? 0 I 0. 0444 -x +x C x E 0. 0444 -x Can we drop this x? 0 +x x Also: Ka. Kb=Kw=1. 0 x 10 -14 @25 o. C so Kb= 5. 56 x 10 -10 30

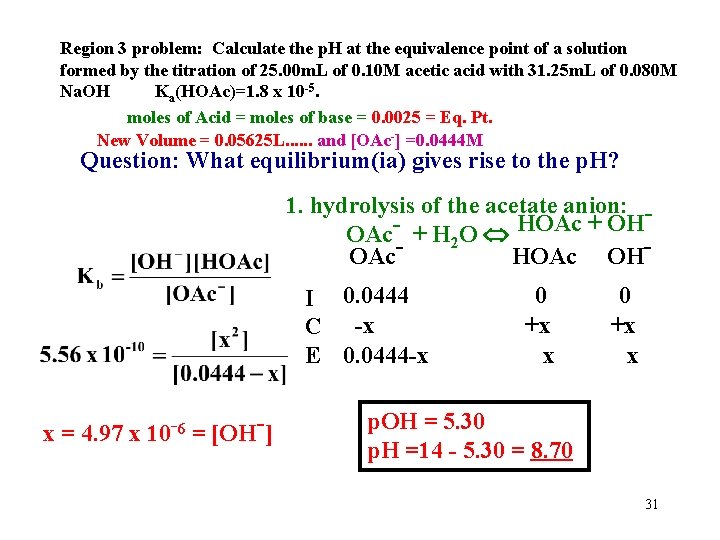
Region 3 problem: Calculate the p. H at the equivalence point of a solution formed by the titration of 25. 00 m. L of 0. 10 M acetic acid with 31. 25 m. L of 0. 080 M Na. OH Ka(HOAc)=1. 8 x 10 -5. moles of Acid = moles of base = 0. 0025 = Eq. Pt. New Volume = 0. 05625 L. . . and [OAc-] =0. 0444 M Question: What equilibrium(ia) gives rise to the p. H? 1. hydrolysis of the acetate anion: OAc-- + H 2 O HOAc + OHOAc OH I 0. 0444 C -x E 0. 0444 -x x = 4. 97 x 10 -6 = [OH-] 0 +x x p. OH = 5. 30 p. H =14 - 5. 30 = 8. 70 31

25. 0 m. L of 0. 10 M Acetic acid Ka=1. 8 x 10 -5 2. 4 XS base 3. 1. 0. 10 M Na. OH 32

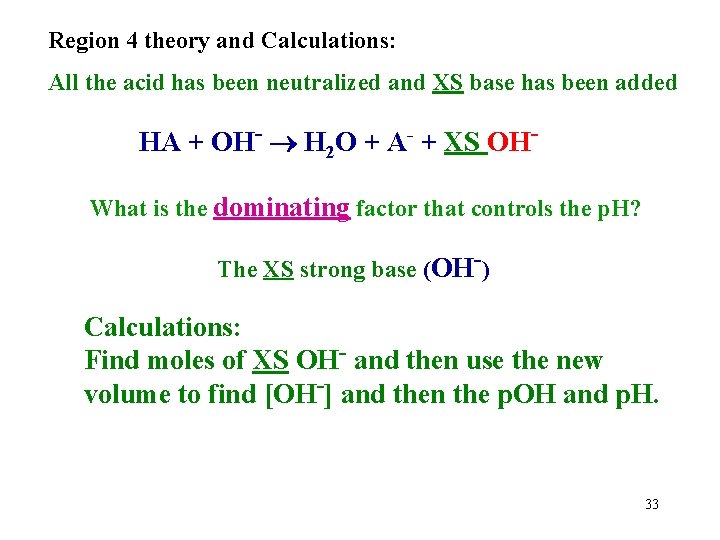
Region 4 theory and Calculations: All the acid has been neutralized and XS base has been added HA + OH- H 2 O + A- + XS OHWhat is the dominating factor that controls the p. H? The XS strong base (OH-) Calculations: Find moles of XS OH- and then use the new volume to find [OH-] and then the p. OH and p. H. 33

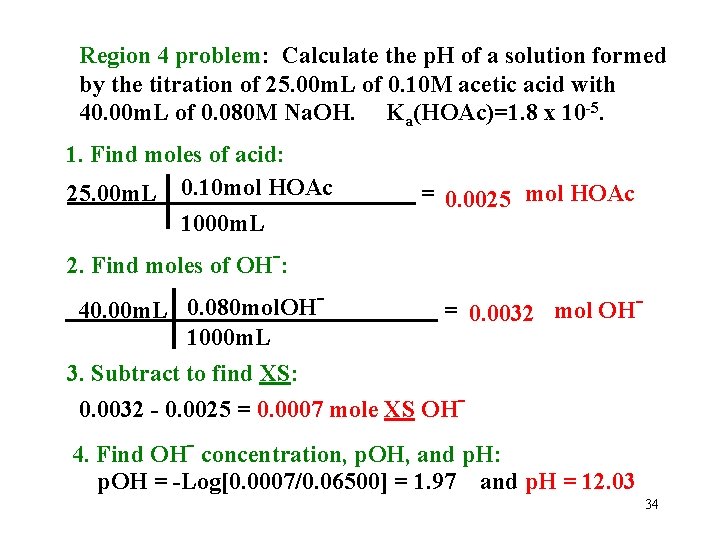
Region 4 problem: Calculate the p. H of a solution formed by the titration of 25. 00 m. L of 0. 10 M acetic acid with 40. 00 m. L of 0. 080 M Na. OH. Ka(HOAc)=1. 8 x 10 -5. 1. Find moles of acid: 25. 00 m. L 0. 10 mol HOAc 1000 m. L 2. Find moles of OH-: = 0. 0025 mol HOAc 0. 080 mol. OH 40. 00 m. L = 0. 0032 mol OH 1000 m. L 3. Subtract to find XS: 0. 0032 - 0. 0025 = 0. 0007 mole XS OH 4. Find OH- concentration, p. OH, and p. H: p. OH = -Log[0. 0007/0. 06500] = 1. 97 and p. H = 12. 03 34
![Approximations H A 2 4 STUDY FOR QUIZ p OHLogXS OH 3 1 HA Approximations: [H+] [A-] 2 4 STUDY FOR QUIZ p. OH=-Log[XS OH-] 3 1 [H+]=[A-]](https://slidetodoc.com/presentation_image/2b64565caf94119263c5b4e4a50362a9/image-35.jpg)
Approximations: [H+] [A-] 2 4 STUDY FOR QUIZ p. OH=-Log[XS OH-] 3 1 [H+]=[A-] [OH-]=[HA] 35

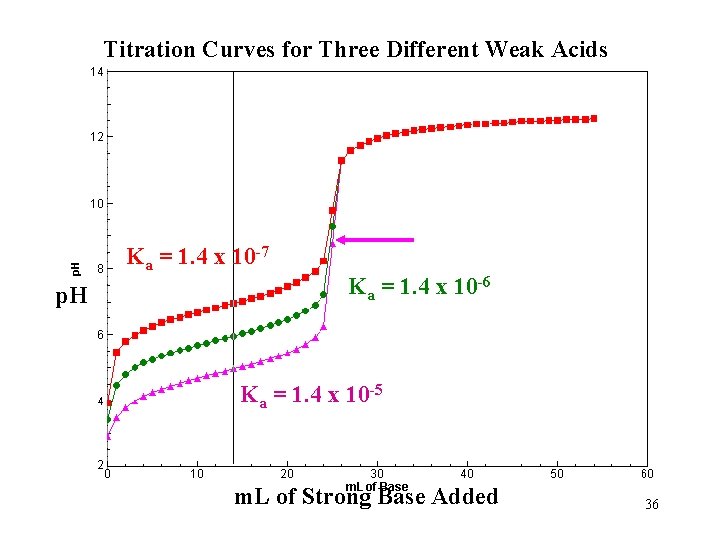
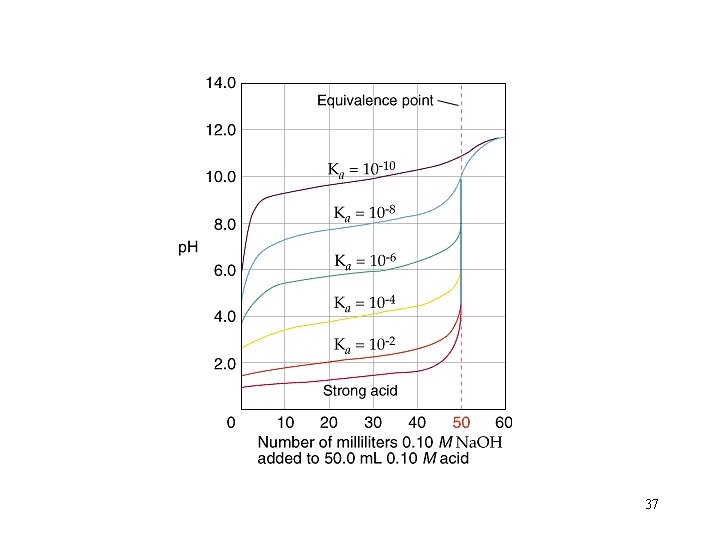
Titration Curves for Three Different Weak Acids Ka = 1. 4 x 10 -7 p. H Ka = 1. 4 x 10 -6 Ka = 1. 4 x 10 -5 m. L of Strong Base Added 36

37

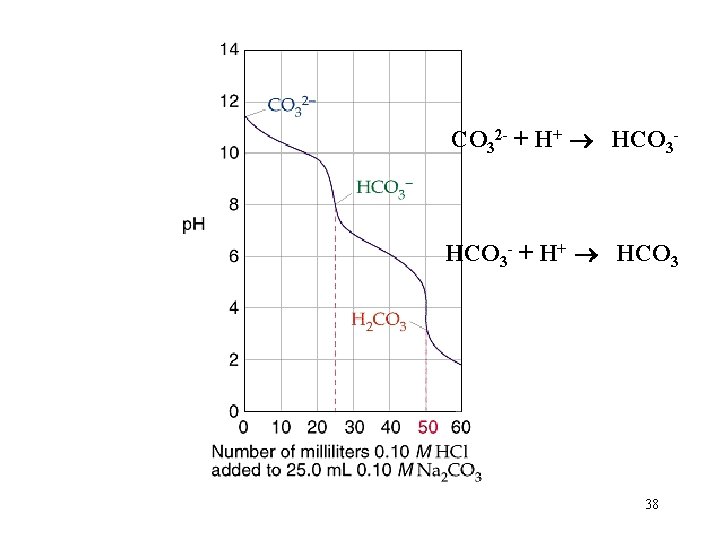
CO 32 - + H+ HCO 3 - + H+ HCO 3 38

39

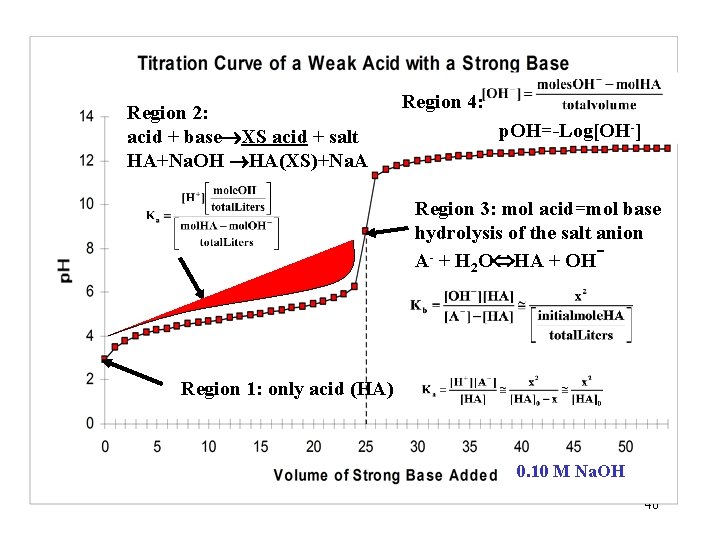
Region 2: acid + base XS acid + salt HA+Na. OH HA(XS)+Na. A Region 4: p. OH=-Log[OH-] Region 3: mol acid=mol base hydrolysis of the salt anion A- + H 2 O HA + OH Region 1: only acid (HA) 0. 10 M Na. OH 40

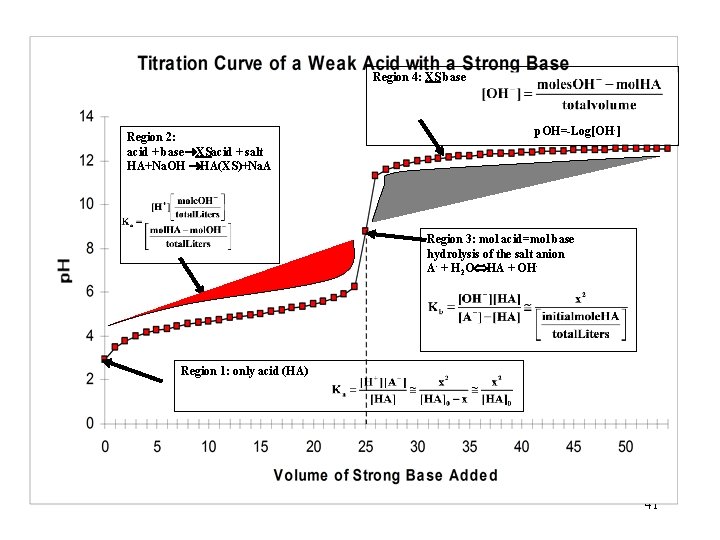
Region 4: XS base Region 2: acid + base XSacid + salt HA+Na. OH HA(XS)+Na. A p. OH=-Log[OH-] Region 3: mol acid=mol base hydrolysis of the salt anion A- + H 2 O HA + OH- Region 1: only acid (HA) 41