Food Standards Update Introduction 2 Food Additives 3





































































































- Slides: 131

Food Standards Update

Introduction 2

Food Additives 3

What is an additive? n n n Food additives are substances deliberately added to a food to achieve a specific function i. e. preservative, raising agents, flavour improvers etc. Added in small quantities Usually consumed in product

Codex definition ‘. . Any substance not normally consumed as a food by itself and not normally used as a typical ingredient of the food…the intentional addition of which to food for a technological. . . purpose in the manufacture…of such foods, results…. in it or its by products becoming a component of or otherwise affecting the characteristics of such foods. ’

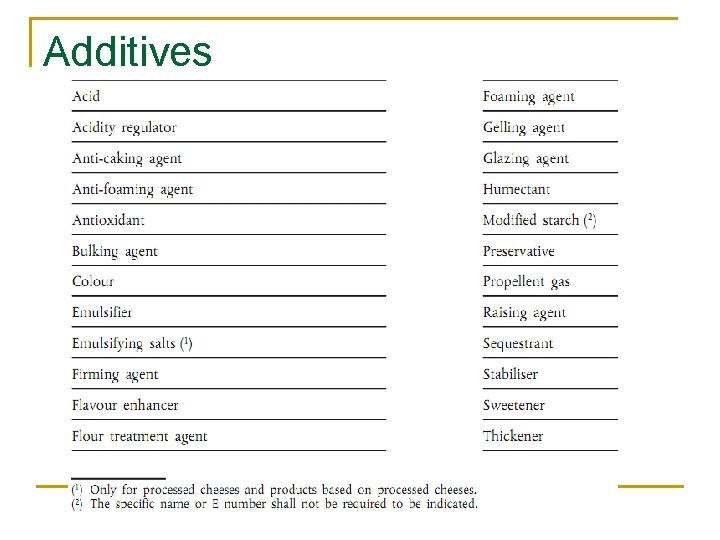
What are additives? n n n Colours Preservative Anti - oxidant Emulsifier Emulsifying salt Thickener Gelling agent Stabiliser Flavour enhancer Glazing agent Flour treatment agent Bulking agent n n n Acidity regulator Anti-caking agent Modified starch Sweetener Raising agent Antifoaming agent Firming agent Humectant Sequestrant Enzyme Propellant/packing gas

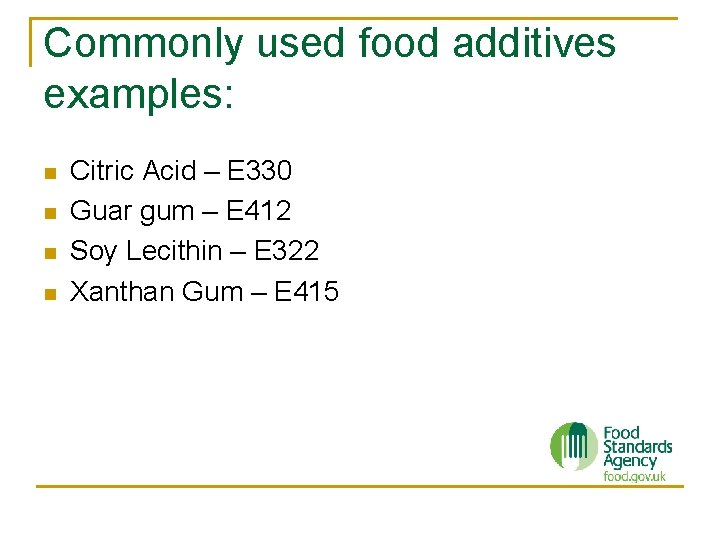
Commonly used food additives examples: n n Citric Acid – E 330 Guar gum – E 412 Soy Lecithin – E 322 Xanthan Gum – E 415

The nature of food law n European legislation q q Regulations Directives Recitals Enacting terms n n n Articles Annexes Domestic legislation

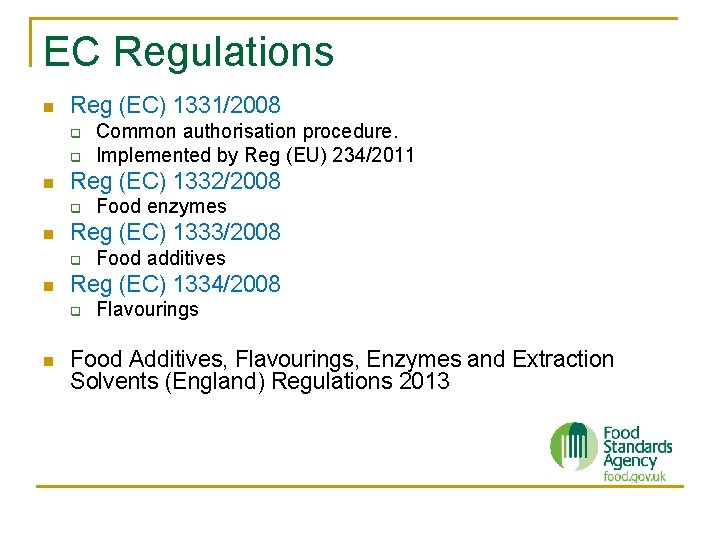
EC Regulations n Reg (EC) 1331/2008 q q n Reg (EC) 1332/2008 q n Food additives Reg (EC) 1334/2008 q n Food enzymes Reg (EC) 1333/2008 q n Common authorisation procedure. Implemented by Reg (EU) 234/2011 Flavourings Food Additives, Flavourings, Enzymes and Extraction Solvents (England) Regulations 2013

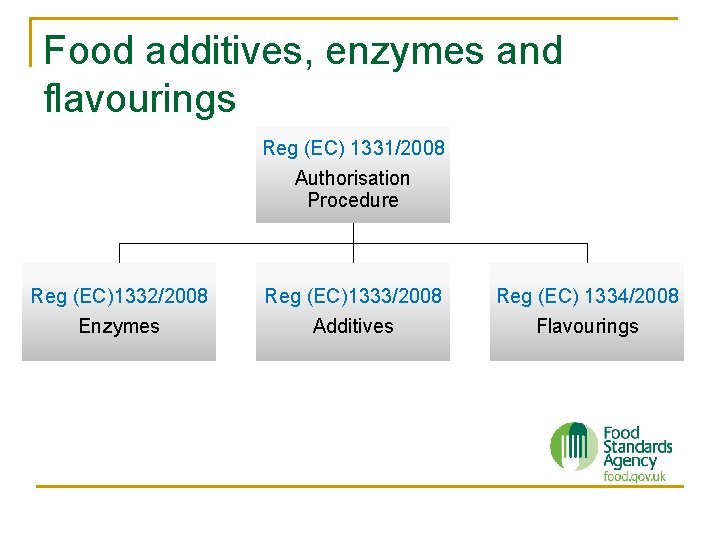
Food additives, enzymes and flavourings Reg (EC) 1331/2008 Authorisation Procedure Reg (EC)1332/2008 Enzymes Reg (EC)1333/2008 Additives Reg (EC) 1334/2008 Flavourings

Approval of additives Regulation (EC) No 1331/2008 11

Regulation (EC) 1331/2008 Common authorisation procedure. Applicant Commission EFSA Commission Normally seeks opinion from EFSA Provides an opinion within 9 months Regulation updating Community List Toxological/safety Risk management No requirement within 9 months of studies to consult for EFSA opinion removal/amendment

EFSA re-evaluation n Regulation (EU) No 257/2010 q Deadlines for the re-evaluation programme n n Food colours - 2015 Food additives q n Sweeteners – 2020 q n other than food colours & sweeteners - 2015– 2018 Exception: Aspartame 2013 Priority criteria – q Last evaluation, new scientific evidence, increased human exposure, EC request, emerging concern 13

Food Additives Regulation (EC) No 1333/2008 14

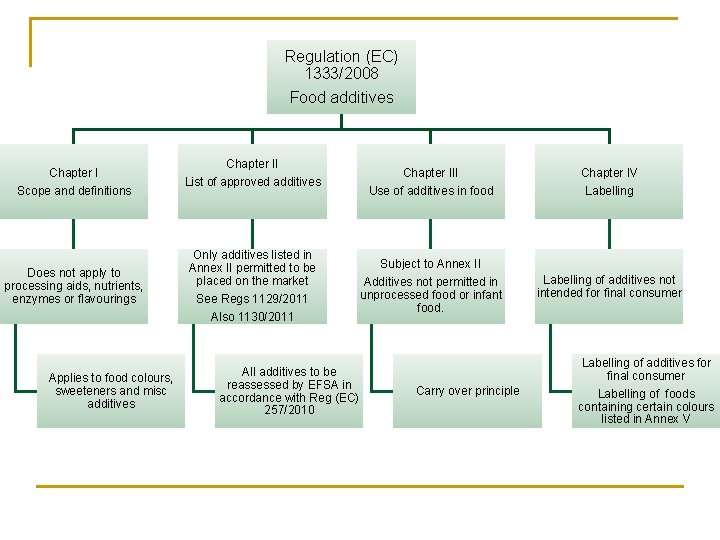
Regulation (EC) 1333/2008 Food additives Chapter I Scope and definitions Does not apply to processing aids, nutrients, enzymes or flavourings Applies to food colours, sweeteners and misc additives Chapter II List of approved additives Only additives listed in Annex II permitted to be placed on the market See Regs 1129/2011 Also 1130/2011 Chapter III Use of additives in food Chapter IV Labelling Subject to Annex II Additives not permitted in unprocessed food or infant food. Labelling of additives not intended for final consumer All additives to be reassessed by EFSA in accordance with Reg (EC) 257/2010 Carry over principle Labelling of additives for final consumer Labelling of foods containing certain colours listed in Annex V

Functional classes of additives n Annex 1 n For example q q Sweeteners Colours Preservatives Antioxidants etc 16

Regulation 1333/2008 Annex II n Annex II q q q Conditions of use Amended by Regulation (EU) 1129/2011 Food Categorisation System (FCS) Source: FSA Guidance - July 2014 17

Food Categorisation System n n n n n 0 All foodstuffs 1 Dairy 2 Fats and Oils 3 Edible Ice 4 Fruit and Vegetables 5 Confectionery 6 Cereals and Cereal Products 7 Bakery Wares 8 Meat and Meat Products 9 Fish and Fish Products n n n n n 10 Eggs and Egg Products 11 Sugars and TTS 12 Salt, Spices, Seasonings, Sauces etc. 13 PARNUTS 14 Beverages 15 Snacks 16 Desserts 17 Food Supplements 18 Processed foodstuffs not belonging to 1 - 17 18

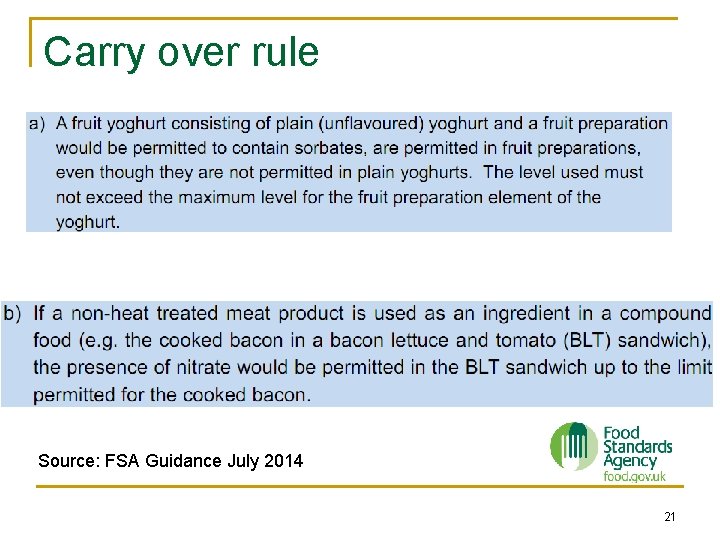
Regulation 1333/2008 Annex II n Part A q q Introduction to the Annex and general provisions on listed additives and conditions of use; Lists of colours that may not be sold directly to the consumer The requirement for nitrites to be sold in a mixture of salt or salt substitute, Lists of foods which are not permitted to contain additives or colours by way of the carry-over principle. 19

Carry over rules 20

Carry over rule Source: FSA Guidance July 2014 21

Reverse carry over Source: FSA Guidance July 2014 22

Exemptions to carry over rules n Article 18 q q Infant formulae Follow on formulae Processed cereal-based foods and baby foods Dietary foods for special medical purposes (Foods specially prepared for infants and young children) n Annex II – Part A (Table 1 & Table 2) 23

Regulation 1333/2008 Annex II n Part B q includes lists of all authorised additives n q colours, sweeteners and additives other than colours and sweeteners. The use of these additives in foods must comply with the provisions in Part E of Annex II. 24

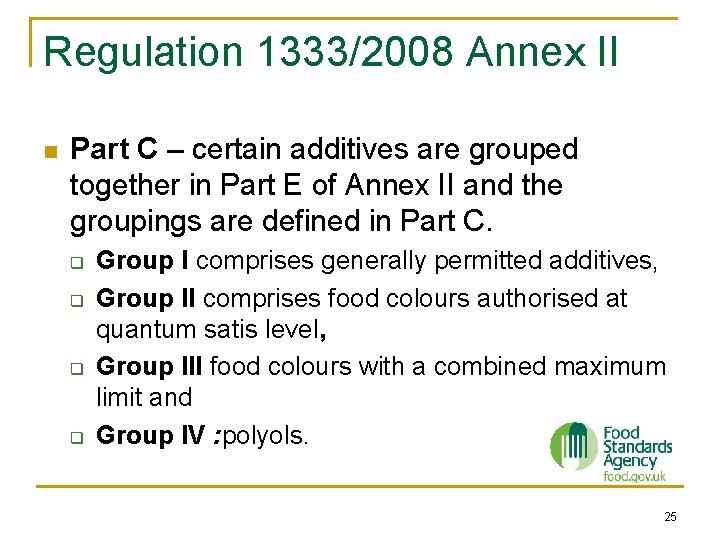
Regulation 1333/2008 Annex II n Part C – certain additives are grouped together in Part E of Annex II and the groupings are defined in Part C. q q Group I comprises generally permitted additives, Group II comprises food colours authorised at quantum satis level, Group III food colours with a combined maximum limit and Group IV : polyols. 25

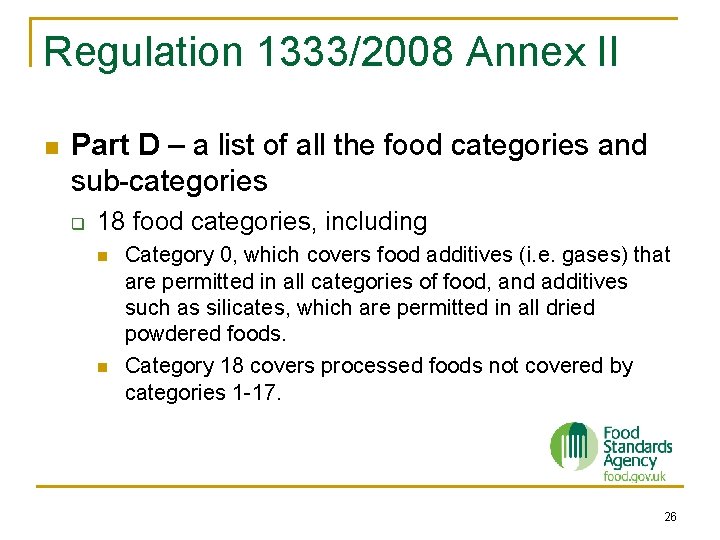
Regulation 1333/2008 Annex II n Part D – a list of all the food categories and sub-categories q 18 food categories, including n n Category 0, which covers food additives (i. e. gases) that are permitted in all categories of food, and additives such as silicates, which are permitted in all dried powdered foods. Category 18 covers processed foods not covered by categories 1 -17. 26

Regulation 1333/2008 Annex II n n Part E - comprises food categories and authorised additives. Additives are listed against the 153 subcategories by q n E number and name with conditions of use (including the maximum limit) indicated. Also indicated are: q q any restrictions or exceptions on the additive use in that sub-category and; footnotes are included where appropriate. 27

Purity specifications n For additives q Set out in Regulation 231/2012 28

Fresh meat, meat preparation, meat product 29

Imports of Soft Drinks from USA (FSA letter 16 th June 2014) n Consignments of soft drinks q q Excessive levels of the additive benzoic acid (E 210) and other non-permitted substances, including: - Brominated Vegetable Oil (BVO) n q non permitted food additive in the EU Calcium disodium EDTA (E 385) and Erythorbic acid (E 315) n permitted food additives for some foods but they are not permitted in drinks 30

Other points n Edible glitters and dusts 31

Labelling 32

Regulation 1333/2008 n When sold to the final consumer q q q Name and E-number Statement “for food” or “restricted use in food” Regulation 1169/2011 FICs still apply

Regulation 1169/2011 (FIC) Names of ingredients n Additives and enzymes: q q Other than carry over and processing aids must be designated by the name of that category, n n q followed by their specific name or, if appropriate, E number. If an ingredient belongs to more than one of the categories, n the category appropriate to the principal function in the case of the food in question shall be indicated.

Additives

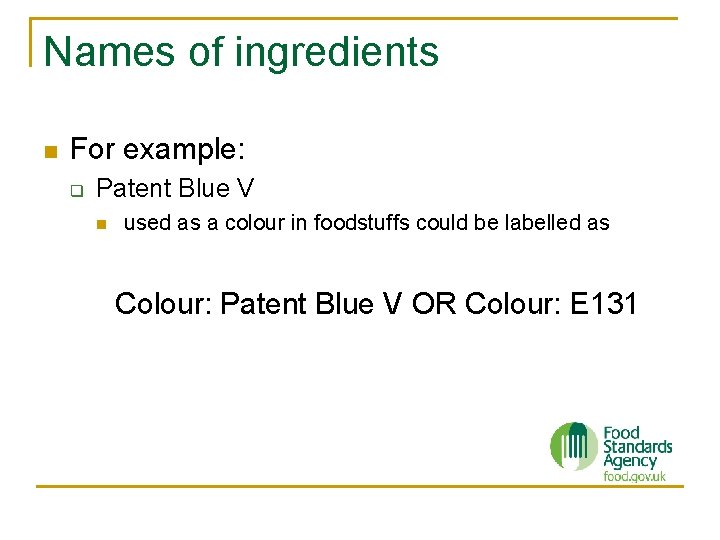
Names of ingredients n For example: q Patent Blue V n used as a colour in foodstuffs could be labelled as Colour: Patent Blue V OR Colour: E 131

Specific labelling requirements n Foods containing q q q n Tartrazine (E 102), Ponceau 4 R (E 124), Sunset yellow (E 110), Carmoisine (E 122), Quinoline yellow (E 104) and Allura Red (E 129) Required to be labelled with the following additional information; 37

The“Southampton” Study n Southampton University study q Link between consumption of six food colours n q and hyperactivity in 3 year old and 8/9 year old children in the general population. FSA have requested UK industry to voluntarily remove these six colours from food and drink. 38

Food Additives Database https: //webgate. ec. europa. eu/sanco_foods/main/? event=display 39

Food Additives Database n XXXXX

Food Additives Database n XXXXX

Food Additives Database n XXXXX

Quantum Satis n XXXXX

Food Additives Database n XXXXX

What would you do? n n n Unauthorised additive (for all foods) present in a food Unauthorised additive (in particular category) present in a food Excessive use of an additive in a food 45

Traceability 46

Traceability n Why is food traceability important? 47

Traceability “The traceability of food and food ingredients along the food chain is an essential element in ensuring food safety. ” Regulation (EC) 852/2004 48

Traceability Legal framework 49

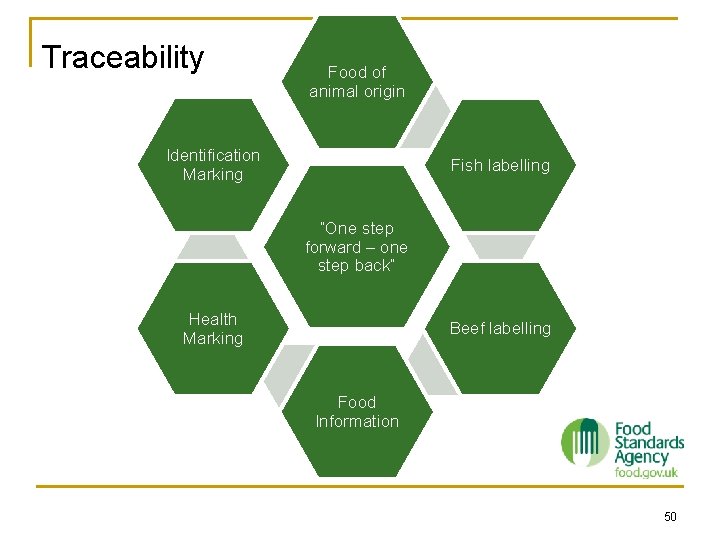
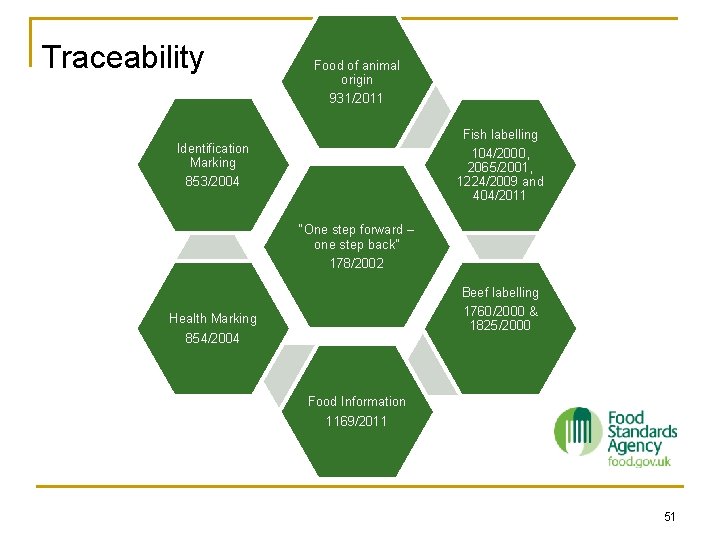
Traceability Food of animal origin Identification Marking Fish labelling “One step forward – one step back” Health Marking Beef labelling Food Information 50

Traceability Food of animal origin 931/2011 Fish labelling 104/2000, 2065/2001, 1224/2009 and 404/2011 Identification Marking 853/2004 “One step forward – one step back” 178/2002 Beef labelling 1760/2000 & 1825/2000 Health Marking 854/2004 Food Information 1169/2011 51

Regulation 178/2002 Article 18 “One step forward – one step back” 52

Article 18 178/2002 Food Safety and Hygiene (England) Regulations 2013 n Food businesses q q q n required to identify from whom and to whom a product has been supplied “one step forward”- “one step back” approach Does not include final consumer “Systems and procedures” q q in place that allow for this information to be made available to the competent Authorities Article 18 worded in terms of “goal and intended result” rather than prescribing how that result is to be achieved

Regulation (EU) 931/2011 Traceability of foods of animal origin 54

Regulation 931/2011 n Implements Article 18, 178/2002 q q q With respect to food of animal origin “Processed” and “Unprocessed” Does not apply to composite products 55

Art 3, Regulation (EU) 931/2011 n The following must be made available to FBO supplied: q q q Accurate description of the food; Volume or quantity of the food; Name and address of the n FBO from which the food has been dispatched; n Consignor (owner) q FBO to whom the food is dispatched; Consignee (owner), n n q q q if different from the FBO from which the food has been dispatched; if different from the FBO to whom the food is dispatched; Reference identifying the lot, batch or consignment & Date of dispatch.

Art 3, Regulation (EU) 931/2011 n Information must be: q q q Updated on a daily basis Kept until reasonably assumed food consumed Provided when requested by the competent authority n without undue delay. 57

Art 3, Regulation (EU) 931/2011 n Format in which the information made available q q Up to the choice of the supplier of the food as long as the information is n n clearly and unequivocally available to and retrievable by FBO to whom the food is supplied. 58

Health and Identification Marking 59

Health/Identification Marks n Health Marks: q Applied by inspectors to verify that the food itself has been subject to veterinary inspection. n Found on fresh meat carcasses, primal cuts q q q q cattle, including buffalo and bison sheep, goats and pigs horses camelids ratites farmed deer and wild boar large wild game, deer and wild boar

Health/Identification Marks n Identification marks q q q Applied by food business operators Indicate that the food has been handled in premises that have been approved but the food itself has not necessarily been subject to official controls. 61

Organic Foods 62

Legislation n EU Regulation 834/2007 q n EU Regulation 889/2008 q n detailed rules on the provisions of 834/2007 EU Regulation 1235/2008 q n organic production and labelling requirements detailed rules on provisions of 834/2007 with regards to imported organic products The Organic Products Regulations 2009 63

What is Organic Food? n Food that is produced q q according to Regulation 834/2007 Assessed and certified by a certification body n May not contain any genetically modified (GM) material or irradiated material n Only foods complying with these requirements may use the term “Organic” q or similar terms as used in EU such as “Eco” or “Bio” 64

Products Covered n Regulations apply to – q q q n Live or unprocessed agricultural products Processed agricultural products for use as food Feed Vegetative propagating material and seeds for cultivation Yeasts used as food or feed Products of hunting and fishing of wild animals shall not be considered as organic productions 65

Businesses covered? n Anyone who produces, prepares, stores, imports from a non-EU country, exports to a non-EU country or markets organic products n Businesses within scope of Regulations must register with a certification body – examples include q q q n Butcher who cuts organic meat Manufacturer selling organic products Retailer removing organic products from packaging to sell loose Regulations don’t apply to – q q Retailers selling prepacked products Mass catering operations 66

Certification Bodies n Currently 9 certification bodies in UK q q q q q Organic Farmers and Growers Ltd Organic Food Federation Soil Association Certification Ltd Bio-Dynamic Agricultural Association Irish Organic Farmers & Growers Association Organic Trust Limited Quality Welsh Food Certification Ltd Global Trust Certification Ltd Scottish Food Quality Certification Ltd – GB-ORG-02 – GB-ORG-04 – GB-ORG-05 – GB-ORG-06 – GB-ORG-07 – GB-ORG-09 – GB-ORG-13 – GB-ORG-16 – GB-ORG-17 67

When Can A Product Be Called Organic? n Processed foods q q Only be described as Organic where at least 95% of ingredients of agricultural origin are organic Where less than 95% products are organic, n q term “Organic” can only be applied to the relevant organic ingredients in the ingredients list Where ingredients indicated as “Organic” n list of ingredients shall state total percentage of organic ingredients. 68

Labelling n Products Labelled as Organic shall include – q Certification body code number (e. g. GB-ORG-05) q Community Logo q n Indication of the place where agricultural raw materials have been farmed – n EU Agriculture n Non-EU Agriculture n EU/Non-EU Agriculture n Or indication of Country of Origin of agricultural materials Requirements of Regulation 1169/2011 also apply 69

Organic Products Regulations 2009 Offences n Regulation 18 – offence to contravene provisions listed in Schedule, includes labelling n Summary conviction – fine not exceeding level 5 70

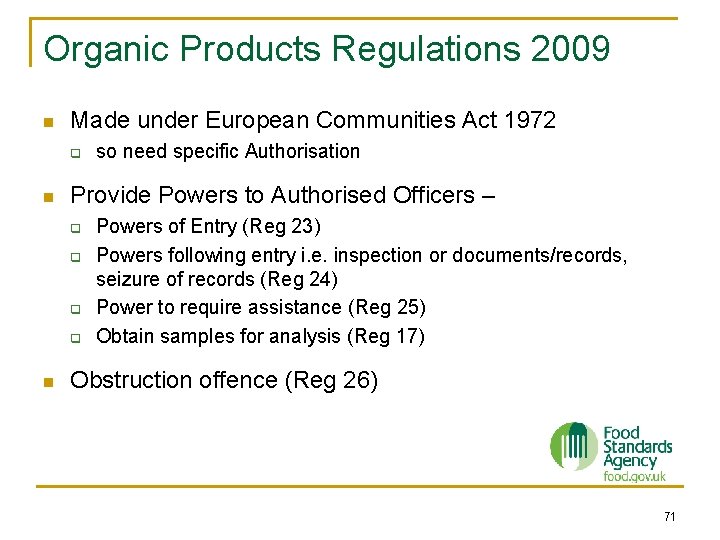
Organic Products Regulations 2009 n Made under European Communities Act 1972 q n Provide Powers to Authorised Officers – q q n so need specific Authorisation Powers of Entry (Reg 23) Powers following entry i. e. inspection or documents/records, seizure of records (Reg 24) Power to require assistance (Reg 25) Obtain samples for analysis (Reg 17) Obstruction offence (Reg 26) 71

What to Look for…. n Current Certification Body certificate covering products being produced/sold n Traceability – detailed traceability up the food chain to ensure organic certification maintained n Imported organic ingredients/products need a “Certificate of Inspection” which is kept at the first destination – check to see this is kept and certified by Ports n Sample – could sample products for irradiation/GM etc. 72

Food Crime Unit 73

Food Crime Unit n Information unavailable until September…………. 74

Food Law Prosecutions Outcomes Database (FLPO) 75

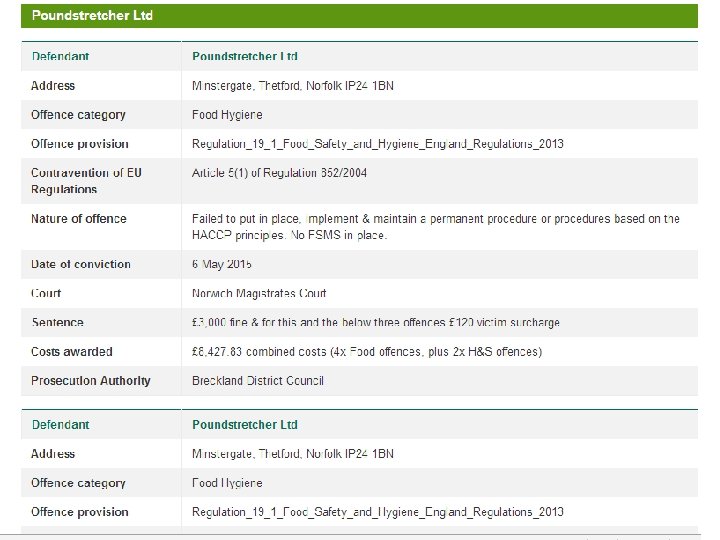
FLPO Database n Public record of all food standards, safety and hygiene prosecution cases n Information about action taken, allows consultation on prosecutions, sharing knowledge and good practice n Will include data from LA’s in England, Wales, Scotland Northern Ireland 76

FLPO Database Local Authorities asked to send details of successful prosecutions to – prosecutionsuccess@foodstandards. gsi. gov. uk n FLPO Database Weblink: http: //www. food. gov. uk/enforcement/monitoring/pro secutions n 77

78

79

Priorities for Food Standards 80

Priorities for Food Standards n Waiting for information from FSA…………. 81

Improvement & Compliance Notices 82

Notices n Food Standards generally moving away from criminal offences to civil notices n Not all notices are the same, there are: q improvement notices q compliance notices q warning notices q enforcement notices q penalty notices 83

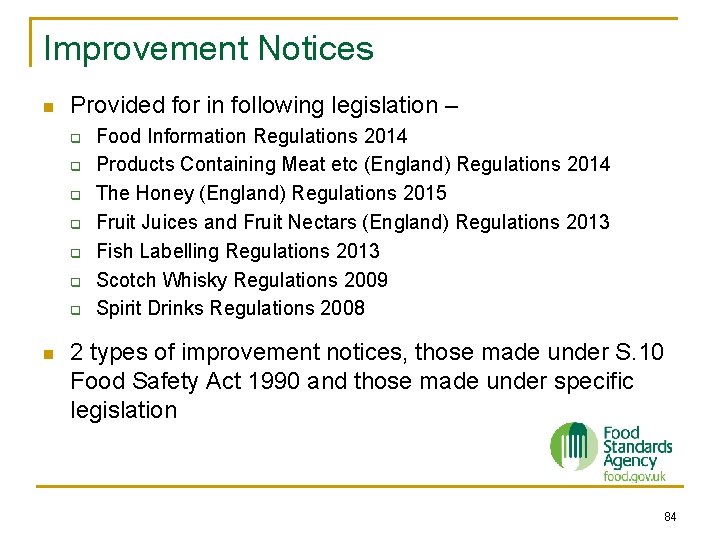
Improvement Notices n Provided for in following legislation – q q q q n Food Information Regulations 2014 Products Containing Meat etc (England) Regulations 2014 The Honey (England) Regulations 2015 Fruit Juices and Fruit Nectars (England) Regulations 2013 Fish Labelling Regulations 2013 Scotch Whisky Regulations 2009 Spirit Drinks Regulations 2008 2 types of improvement notices, those made under S. 10 Food Safety Act 1990 and those made under specific legislation 84

Improvement Notices n Improvement Notices must – q q q State Officers grounds for believing person failing to comply with legislation Specify matters which constitute persons failure to comply Specify measures, which in the Officers Opinion, the person must take to secure compliance Period of time which they have to comply Appeal Process 85

Grounds for officer believing that person has failed to comply with Regulations n Should include specific reference to: q q Food business Details of act or omission 86

Matters which constitute the person’s failure n Specific legal reference should be included 87

Measures to be taken to secure compliance n Officer should ensure: q Notice provides details of officer’s opinion n q n Should point out that alternatives may be acceptable. Notice does not require action beyond legal requirement Avoid q q Merely re-stating wording of legislation Using vague terms “adequate” “suitable” etc 88

Date of compliance n Varies between legislation q q n Some are instant notices, can state any date they need to comply with, i. e. Food Information Regulations Others have minimum 14 day time period, i. e. Honey (England) Regulations Needs to be appropriate in circumstances 89

Appeals n n General Regulatory Chamber of the First - tier Tribunal OR Magistrates Court Notice may be cancelled, affirmed or altered Time to appeal - 28 days Appeal may suspend notice period – check legislation 90

Non-compliance n Breach of notice: Criminal offence n Follow up action in accordance with enforcement policy (EP) q EP should be updated to include breach of Improvement Notices n Penalty = Fine n Publication Requirements – some legislation includes requirement to publish notices 91

Compliance Notices n Provided for in following legislation – q Food Additives, Flavourings, Enzymes and Extraction Solvents (England) Regulations 2013 92

Compliance Notices n A compliance notice must state — q q q the steps the person must take the date and, if appropriate, the time by which each step must be taken the reason for the service of the notice and for the steps required to be taken that a failure to comply with the notice is an offence the details of the right to appeal 93

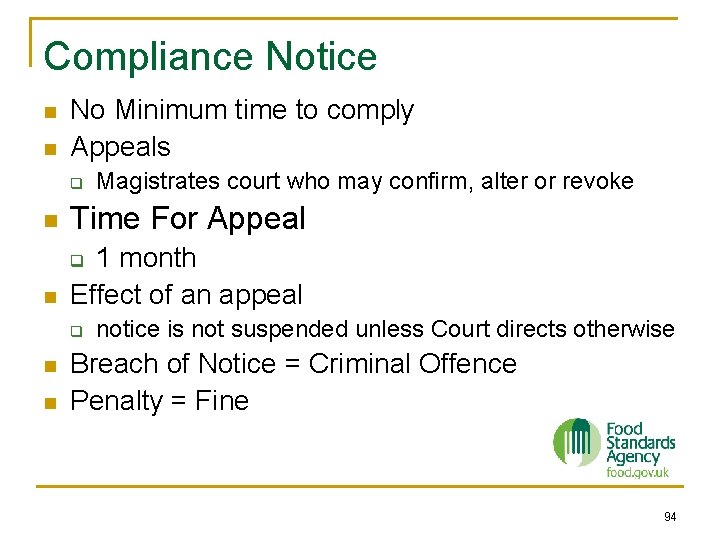
Compliance Notice n n No Minimum time to comply Appeals q Magistrates court who may confirm, alter or revoke n Time For Appeal n 1 month Effect of an appeal q q n n notice is not suspended unless Court directs otherwise Breach of Notice = Criminal Offence Penalty = Fine 94

Warning Notices n Provided for in following legislation – q n Wine Regulations 2011 A warning notice must state — q q q Provision of EU Regulations officer believes has been breached Notify person that any breach of that provision render person liable to prosecution the details of the right to appeal 95

Enforcement Notices n Provided for in following legislation – q n Wine Regulations 2011 An enforcement notice must — q q q State that the Officer is of the opinion that a Regulation has been contravened Specify matters constituting contravention or matter making it likely contravention will arise Specify steps must be taken to remedy contravention or remedy matters making it likely contravention arise Specify period of time which those steps must be taken Appeal Process 96

Prohibition Notice n Provided for in following legislation – q Wine Regulations 2011 n Prohibits movement, marketing or export of wine products n A prohibition notice must — q q State that the Officer is of the opinion that there has been a contravention of a provision of EU Regulation in relation to the product Appeal Process 97

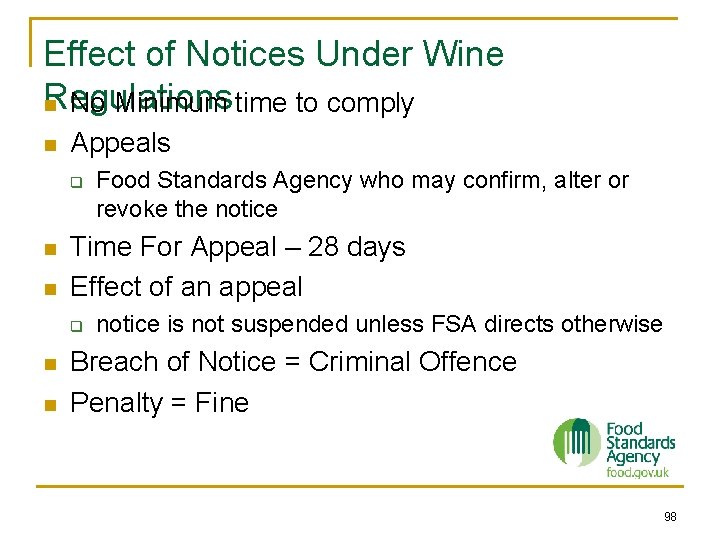
Effect of Notices Under Wine Regulations n No Minimum time to comply n Appeals q n n Time For Appeal – 28 days Effect of an appeal q n n Food Standards Agency who may confirm, alter or revoke the notice is not suspended unless FSA directs otherwise Breach of Notice = Criminal Offence Penalty = Fine 98

Penalty Notice n Provided for in following legislation – q q n Scotch Whisky Regulations 2011 Spirit Drinks Regulations 2008 A penalty notice must — q q q Give information on circumstances alleged to constitute the offence State amount of penalty State period of time which they have to pay State person and address penalty to be paid to and ways to pay State payment must not be paid in cash 99

Penalty Notice n Penalty amount between £ 1000 - £ 4000 n Aggravating and Mitigating factors to consider when setting amount in Schedule 4 n Money paid by Local Authority to Consolidated Fund 100

Top Tips For Notices n n n Ensure it is served on the correct person Check the type of notice Can the Trader identify the legislation from the notice and the exact breach? Check the specific requirements of the notice when drafting, e. g. time to comply Appeal process – time to appeal, who appeal is to, effect of appeal 101

Enforcement of Use by Dates 102

EU Regulation 1169/2011 Art 24 n In the case of foods which, from a microbiological point of view, are highly perishable and are therefore likely after a short period to constitute an immediate danger to human health, q the date of minimum durability shall be replaced by the ‘use by’ date. 103

EU Regulation 1169/2011 Art 24 n After the ‘use by’ date a food shall be deemed to be unsafe in accordance with Article 14(2) to (5) of Regulation (EC) No 178/2002. 104

Food Safety and Hygiene (England) Regulations 2013 Regulation 19 n “…any person who contravenes or fails to comply with any of the specified EU provisions commits an offence n Schedule 2 includes Art 14 of 178/2002 requirement that unsafe food must not be placed on the market n Penalties – q q n Summary – fine not exceeding statutory maximum Indictment – 2 years imprisonment and/or fine Time Limit – 3 years from offence/1 year from discovery 105

Enforcement Authority Regulation 5 n “Each food authority in its area or district shall execute and enforce the provisions of Regulation 178/2002 specified in Schedule 2 and these Regulations in so far as they relate to those provisions” n Food Authority includes – q q As respects each London Borough, district or non-metropolitan county, the council of that borough, district or country As respects the City of London, the Common Council 106

Authorisations and Powers n Regulations made under European Communities Act 1972 so Officers will need to be authorised specifically under the Food Safety and Hygiene (England) Regulations 2013 n Powers include – n n Powers of Entry (Reg 16) Procurement of Samples (Reg 14) Analysis etc of samples (Reg 15) Obstruction Offence (Reg 17) 107

S. 9 Food Safety Act 1990 n Unsafe food under 178/2002 fails to comply with food safety requirements (S. 8) n Therefore, Authorised Officer may either – a) give notice to the person in charge of the food that until the notice is withdrawn, the food or any specified portion of it – i. ii. b) is not to be used for human consumption; and Either is not to be removed or is not to be removed except to some place specified in the notice; OR Seize the food and remove it in order to have it dealt with by a Justice of the Peace 108

When do I take formal action? n When deciding whether to take formal action for selling food past use by date you should consider q Facts of case (e. g. compliance history, previous advice, etc. ) q Enforcement Policy q Regulators Code q Home Authority / Primary Authority Relationships 109

Best Before Dates n Best Before date is date manufacturer will guarantee the quality of their food until, after this date product may not be of a satisfactory quality n No specific offence for selling foods past before date n Could use other legislation for example q S. 14 Food Safety Act 1990 - food not of “nature, substance or quality” demanded 110

Food Supplements 111

Is it a food? n Medicinal Products n Medicines and Healthcare products Regulatory Agency (MHRA) n What is a Medicinal Product Guidance (https: //www. gov. uk/government/uploads/system/uploads/attachment_d ata/file/398998/A_guide_to_what_is_a_medicinal_product. pdf) 112

Is it novel? n n EC Regulation 258/97 (Novel Foods Regulation) A food that does not have a significant history of consumption within EU before 15 May 1997 Cannot legally be sold until the authorisation issued by EU EU Catalogue of Novel Foods - http: //ec. europa. eu/food/safety/novel_food/catalo gue/search/public/index. cfm# 113

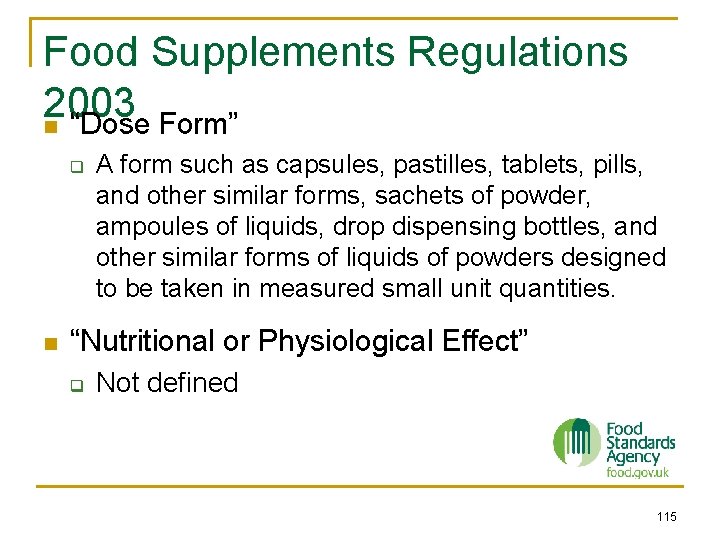
Food Supplements Regulations 2003 n Defined as - q Any food the purpose of which is to supplement the normal diet and which – a) Is a concentrated source of a vitamin or mineral or other substance with a nutritional or physiological effect, alone or in combination; AND b) Is sold in dose form 114

Food Supplements Regulations 2003 n “Dose Form” q n A form such as capsules, pastilles, tablets, pills, and other similar forms, sachets of powder, ampoules of liquids, drop dispensing bottles, and other similar forms of liquids of powders designed to be taken in measured small unit quantities. “Nutritional or Physiological Effect” q Not defined 115

Food Supplements Regulations 2003 n Food Supplements must be sold prepacked n Prepacked – q q Ready for sale to the ultimate consumer or a catering establishment, AND It is put into packaging before being offered for sale in such a way that the food supplement cannot be altered without opening or changing the packaging. 116

Labelling Requirements Regulation 6 n Must be marked with: q q q “Food Supplement” Name of Vitamin, Mineral or Other Substance characterising the product Recommended daily consumption/portion Warning not to exceed recommended daily dose State not used as a substitute for varied diet State store out of reach of young children 117

Labelling Requirements Regulation 6 n Amount of Vitamin, Mineral or Other Substance providing nutritional or physiological effect q q q In numerical form with relevant unit of measurement Average amount based on analysis Vitamins and Minerals listed in Annex 1 also state percentage of RDA 118

Regulation 1169/2011 n n Art 9 Mandatory Food Information must be: q n n Easily visible and Clearly Legible Exempt from nutritional labelling requirement Minimum font size – applies to mandatory requirements only 119

DNP/Dinosan/Nitrophen/Chemox. . . 120

Regulation (EC) 1924/2006 n Lays down q q q General principles for claims Conditions for the use of claims Requirement for scientific substantiation

Regulation (EC) 1924/2006 Article 3 n Nutrition and health claims made on food q Use of nutrition and health claims shall not: n n n Be false, ambiguous or misleading Give rise to doubt of safety of other foods Encourage or condone excessive consumption State/imply balanced diet insufficient Refer to bodily changes that q Give rise to/exploit fear in consumer

Use of nutrition and health claims n Can only be used if: q Presence, absence or reduced content of nutrient is beneficial q The nutrient is: n n Present in final food in significant quantity Absent or present at reduced level to provide nutritional effect In a form available to the body Available in reasonable amount of food n Claims only used q ‘If the average consumer can be expected to understand beneficial effects as expressed in the claim’ n Claims should refer to the food ‘ready for consumption’

Nutrition claims n Only permitted if listed in Annex q q q q for example: LOW ENERGY-REDUCED ENERGY-FREE LOW FAT-FREE LOW SATURATED FAT LOW SUGARS-FREE WITH NO ADDED SUGARS LOW SODIUM/SALT SODIUM-FREE or SALT-FREE SOURCE OF FIBRE LIGHT/LITE etc.

Health claims n Only permitted if: q Statement made: n n q n Importance of balanced diet Healthy lifestyle Quantity of food to provide effect given Where necessary: q q Statement of who should avoid food Health warning

Health claims register

Erucic acid in Foods 127

Erucic acid in foods n n n Rapeseed Mustard seed Wallflower seed Nasturtium seeds Marine animal oils 128

The Contaminants in Food Regulations 2013 Part 2 Erucic Acid in Food n Applies to: q q q Oils, fats and mixtures of the two Compound foodstuffs for infants and young children to which oils, fats and mixtures of the two have been added and the overall fat content exceeds 5% 129

The Contaminants in Food Regulations 2013 Part 2 Erucic Acid in Food n Offence = Regulation 4 n No person may place on the market to the final consumer a products to which this part applies with a level of Erucic acid above 5% 130

Questions? 131