FIRST LAW OF THERMODYNAMICS PDT 152 Materials Chemistry


- Slides: 41

FIRST LAW OF THERMODYNAMICS PDT 152 Materials Chemistry Prepared by: Dr. Tan Soo Jin

AN INTRODUCTION TO (OR SOME REVISION ON) THERMODYNAMICS: SOME DEFINITIONS A system is some part of the universe that you want to study and understand. The surroundings are everything else in the universe that is not in our system. The system can be open or closed to (isolated from) the surroundings in terms of both matter and energy. All changes in a system are associated with the transfer of energy. Natural systems tend toward states of minimum energy

AN INTRODUCTION TO (OR SOME REVISION ON) THERMODYNAMICS: SOME DEFINITIONS System We call the fixed research object as system, also substance system. System is separated from the other parts, the boundary can be visual or imaginable. Surroundings The part which has reciprocity with system, or correlate with the system closely.

ISOLATED, OPEN AND CLOSED SYSTEM An isolated system cannot exchange mass or energy with its surroundings. The wall of an isolated system must be adiabatic. A closed system can exchange energy, but not mass, with its surroundings. The energy exchange may be mechanical (associated with a volume change) or thermal (associated with heat transfer through a diathermal wall). An open system can exchange both mass and energy with its surroundings.

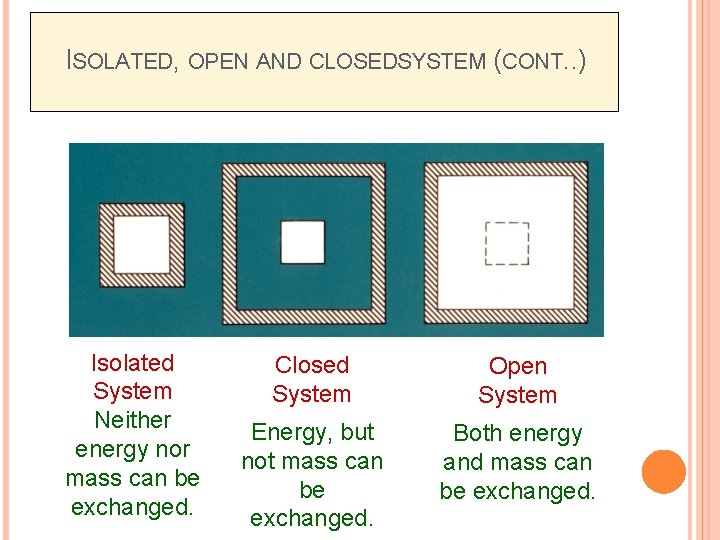
ISOLATED, OPEN AND CLOSEDSYSTEM (CONT. . ) Isolated System Neither energy nor mass can be exchanged. Closed System Open System Energy, but not mass can be exchanged. Both energy and mass can be exchanged.

AN INTRODUCTION Thermodynamics is derived from two words: ‘Thermo’ which means ‘Heat energy’ and ‘Dynamics’ which means ‘conversion’ or ‘transformation’. Concisely, thermodynamics is a division of science that deals with conversion of energy from one form to another. The main forms of energy of interest in engineering thermodynamics are heat and work.

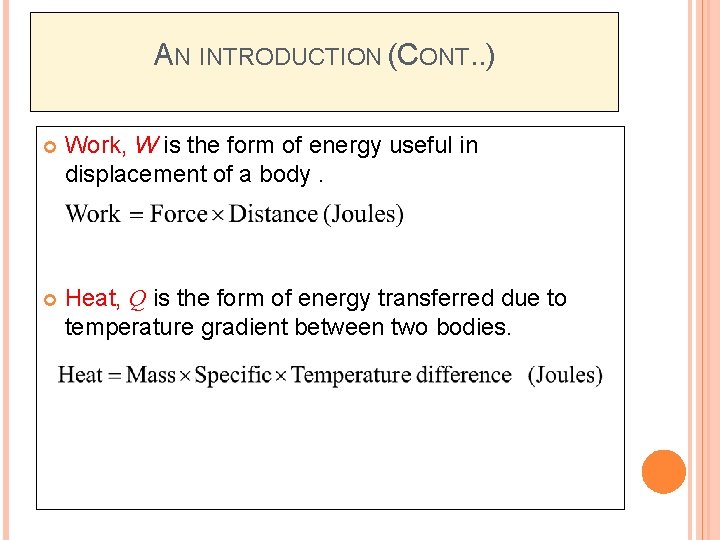
AN INTRODUCTION (CONT. . ) Work, W is the form of energy useful in displacement of a body. Heat, Q is the form of energy transferred due to temperature gradient between two bodies.

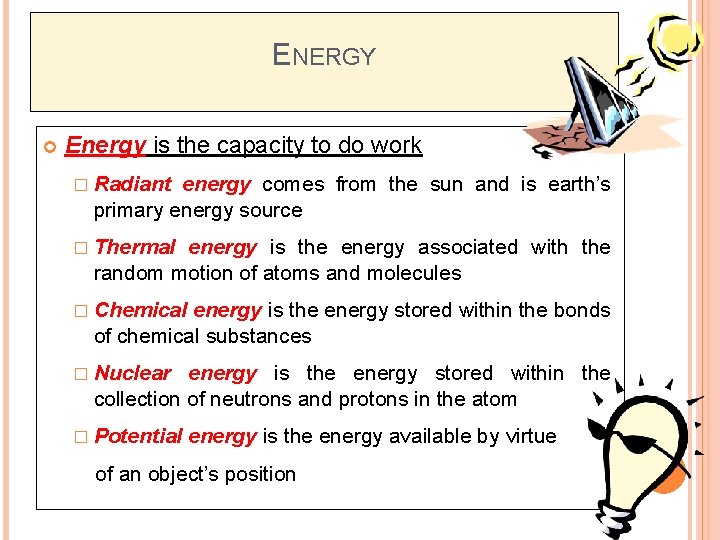
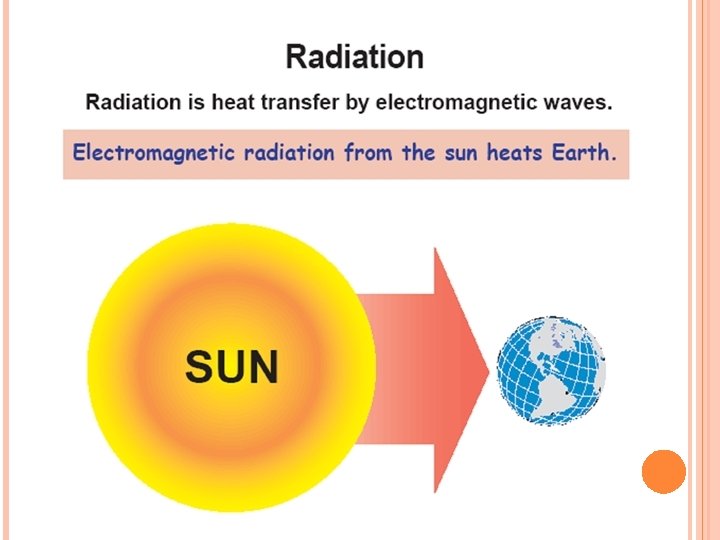
ENERGY Energy is the capacity to do work � Radiant energy comes from the sun and is earth’s primary energy source � Thermal energy is the energy associated with the random motion of atoms and molecules � Chemical energy is the energy stored within the bonds of chemical substances � Nuclear energy is the energy stored within the collection of neutrons and protons in the atom � Potential energy is the energy available by virtue of an object’s position

HEAT TRANSFER Heat is the transfer of thermal energy between two bodies that are at different temperatures. Temperature is a measure of thermal energy Temperature = Thermal Energy

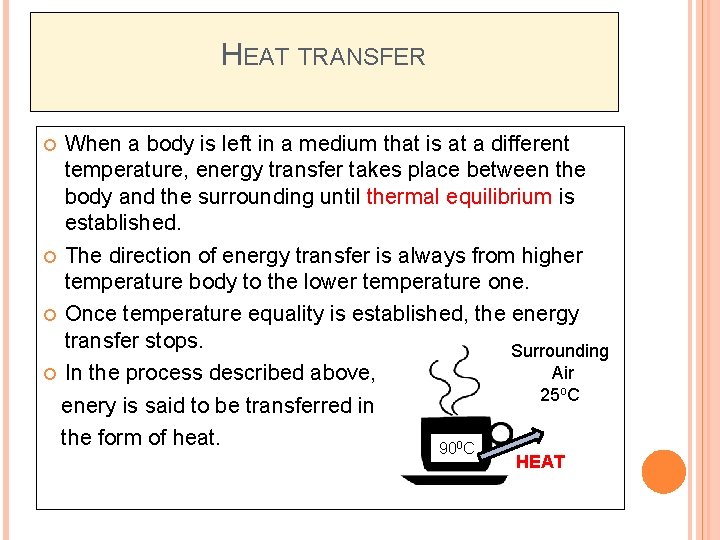
HEAT TRANSFER When a body is left in a medium that is at a different temperature, energy transfer takes place between the body and the surrounding until thermal equilibrium is established. The direction of energy transfer is always from higher temperature body to the lower temperature one. Once temperature equality is established, the energy transfer stops. Surrounding Air In the process described above, 25 o. C enery is said to be transferred in the form of heat. 900 C HEAT

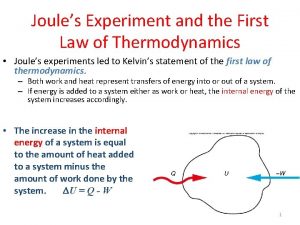
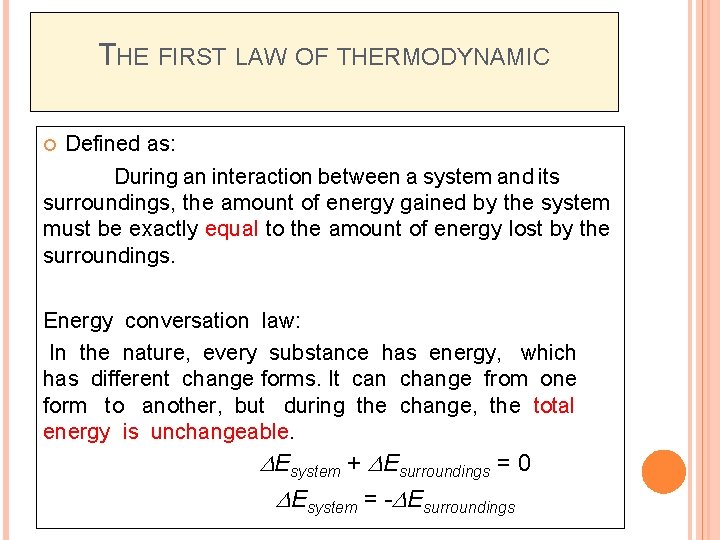
THE FIRST LAW OF THERMODYNAMIC Defined as: During an interaction between a system and its surroundings, the amount of energy gained by the system must be exactly equal to the amount of energy lost by the surroundings. Energy conversation law: In the nature, every substance has energy, which has different change forms. It can change from one form to another, but during the change, the total energy is unchangeable. DEsystem + DEsurroundings = 0 DEsystem = -DEsurroundings

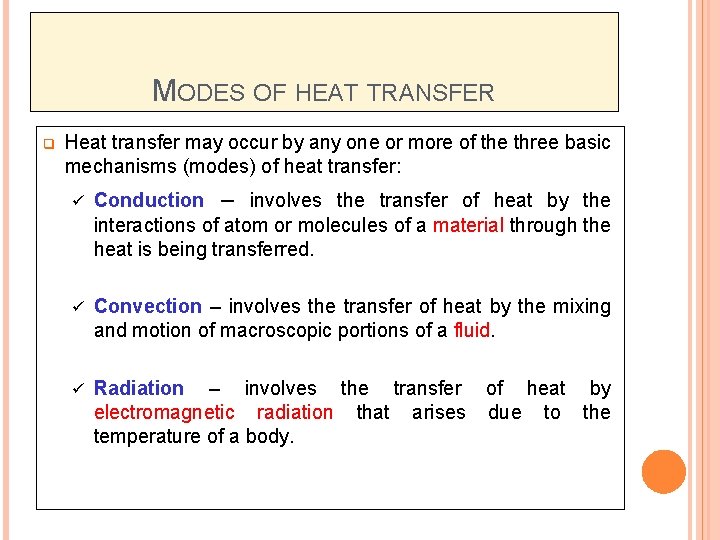
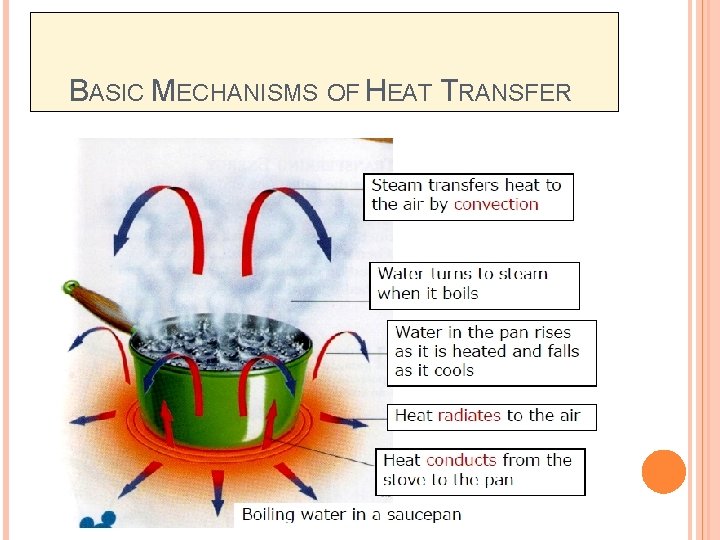
MODES OF HEAT TRANSFER q Heat transfer may occur by any one or more of the three basic mechanisms (modes) of heat transfer: ü Conduction – involves the transfer of heat by the interactions of atom or molecules of a material through the heat is being transferred. ü Convection – involves the transfer of heat by the mixing and motion of macroscopic portions of a fluid. ü Radiation – involves the transfer of heat by electromagnetic radiation that arises due to the temperature of a body.

BASIC MECHANISMS OF HEAT TRANSFER

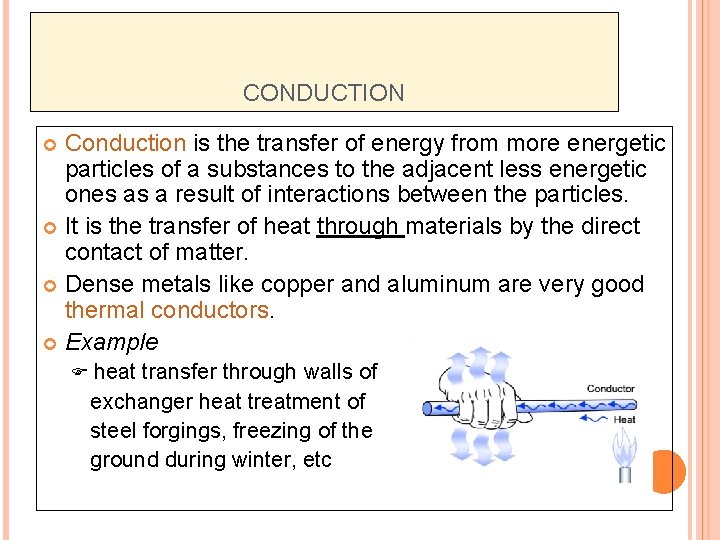
CONDUCTION Conduction is the transfer of energy from more energetic particles of a substances to the adjacent less energetic ones as a result of interactions between the particles. It is the transfer of heat through materials by the direct contact of matter. Dense metals like copper and aluminum are very good thermal conductors. Example F heat transfer through walls of exchanger heat treatment of steel forgings, freezing of the ground during winter, etc

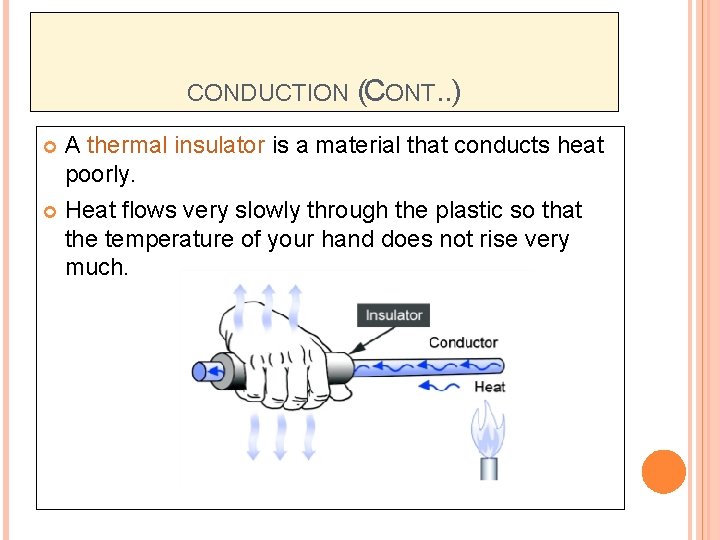
CONDUCTION (CONT. . ) A thermal insulator is a material that conducts heat poorly. Heat flows very slowly through the plastic so that the temperature of your hand does not rise very much.

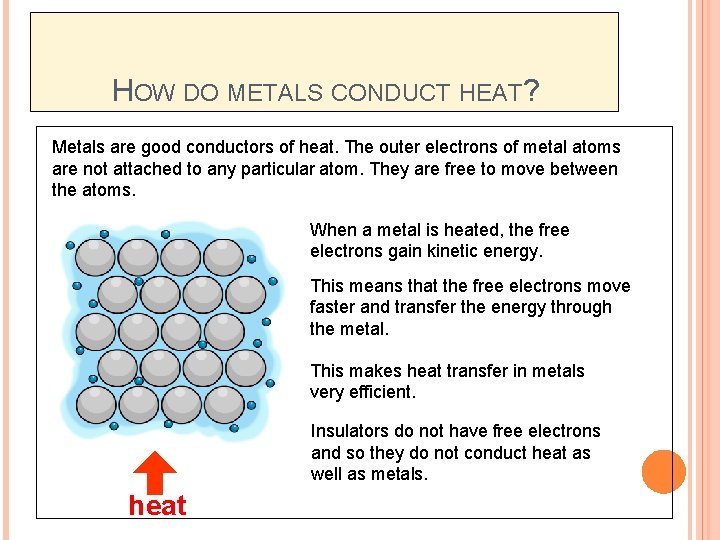
HOW DO METALS CONDUCT HEAT? Metals are good conductors of heat. The outer electrons of metal atoms are not attached to any particular atom. They are free to move between the atoms. When a metal is heated, the free electrons gain kinetic energy. This means that the free electrons move faster and transfer the energy through the metal. This makes heat transfer in metals very efficient. Insulators do not have free electrons and so they do not conduct heat as well as metals. heat

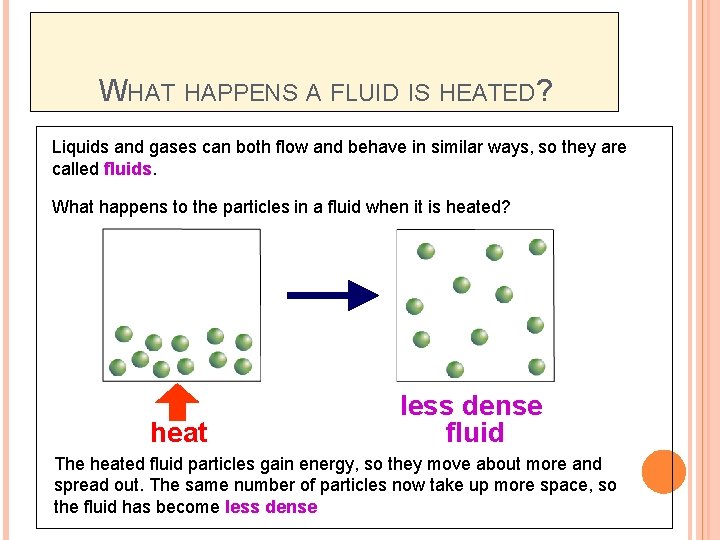
WHAT HAPPENS A FLUID IS HEATED? Liquids and gases can both flow and behave in similar ways, so they are called fluids. What happens to the particles in a fluid when it is heated? heat less dense fluid The heated fluid particles gain energy, so they move about more and spread out. The same number of particles now take up more space, so the fluid has become less dense

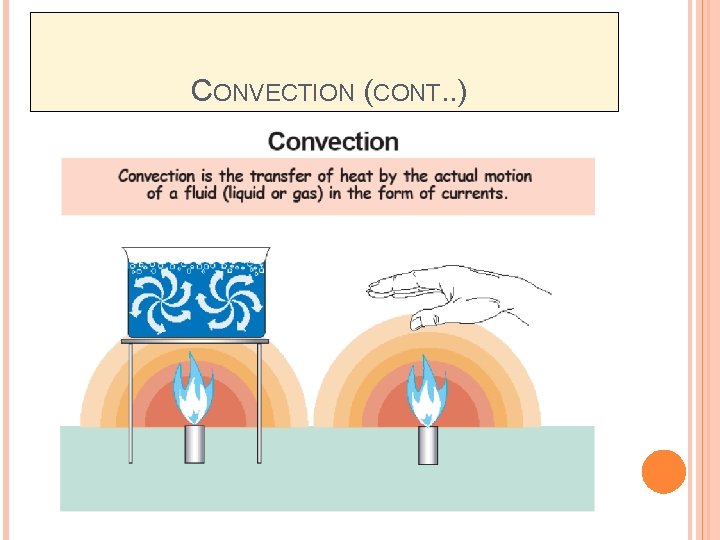
CONVECTION Convection is the mode of energy transfer between a solid surface and the adjacent liquid or gas which is in motion, and it involves the combined effects of conduction and fluid motion. The faster the fluid motion, the greater the convection heat transfer. It also refers to the energy exchange between a solid surface and a fluid.

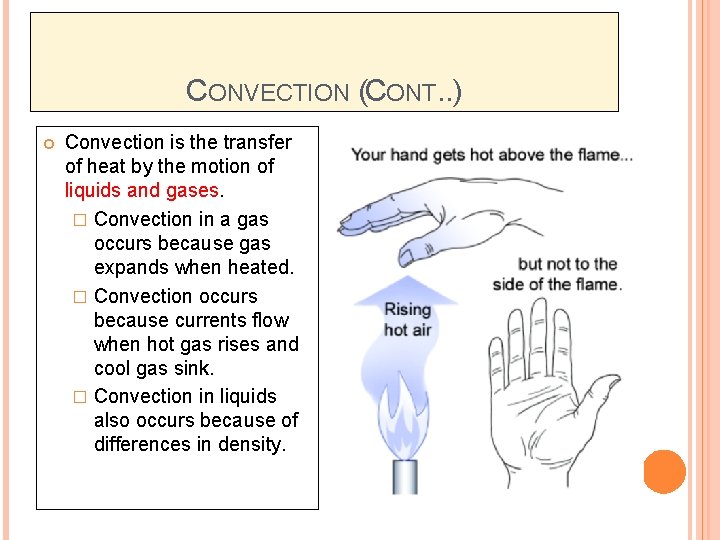
CONVECTION (CONT. . ) Convection is the transfer of heat by the motion of liquids and gases. � Convection in a gas occurs because gas expands when heated. � Convection occurs because currents flow when hot gas rises and cool gas sink. � Convection in liquids also occurs because of differences in density.

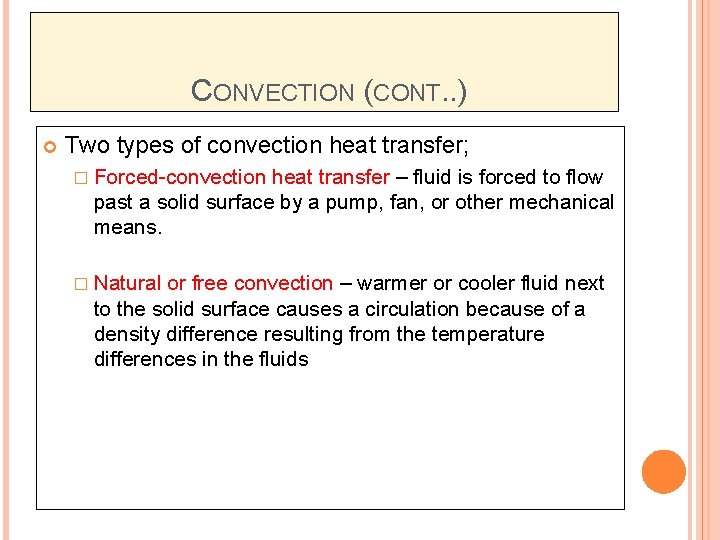
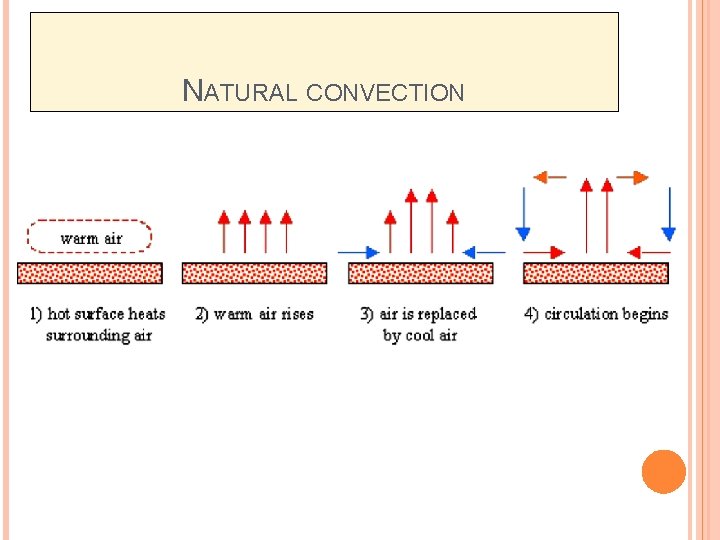
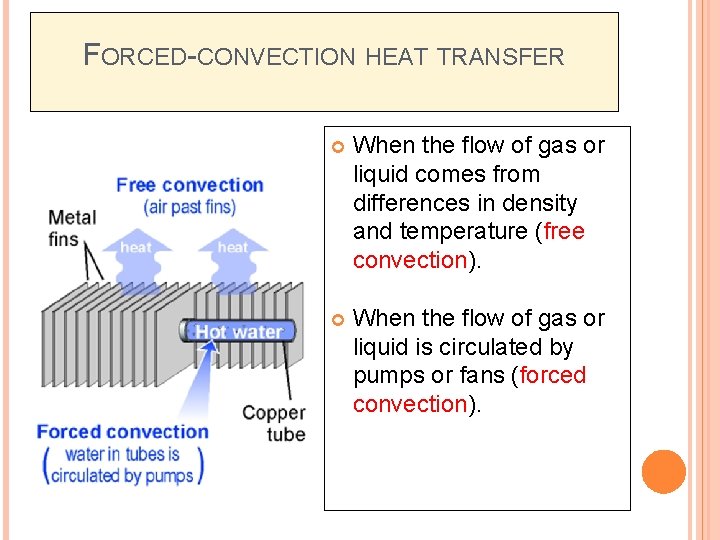
CONVECTION (CONT. . ) Two types of convection heat transfer; � Forced-convection heat transfer – fluid is forced to flow past a solid surface by a pump, fan, or other mechanical means. � Natural or free convection – warmer or cooler fluid next to the solid surface causes a circulation because of a density difference resulting from the temperature differences in the fluids

CONVECTION (CONT. . )

NATURAL CONVECTION

FORCED-CONVECTION HEAT TRANSFER When the flow of gas or liquid comes from differences in density and temperature (free convection). When the flow of gas or liquid is circulated by pumps or fans (forced convection).

RADIATION Key Question: How does heat from the sun get to Earth?


RADIATION (CONT. . ) Radiation – the transfer of energy through space by means of electromagnetic waves. No physical medium is needed for its propagation. The same laws the govern the transfer of light govern the radiant transfer of heat. Solids and liquids tend to absorb the radiation being transferred through them, so that radiation is important primarily in transfer through space or gases. example: transport of heat to the earth from the sun, heating fluids in coils of tubing inside a combustion furnace

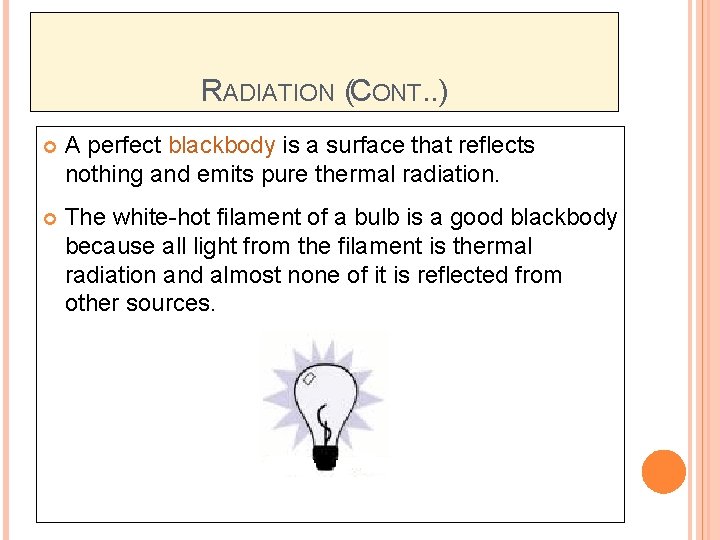
RADIATION (CONT. . ) A perfect blackbody is a surface that reflects nothing and emits pure thermal radiation. The white-hot filament of a bulb is a good blackbody because all light from the filament is thermal radiation and almost none of it is reflected from other sources.

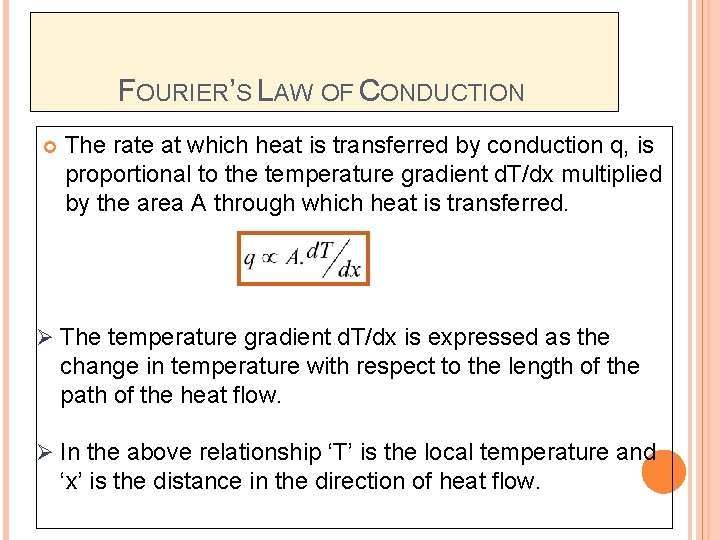
FOURIER’S LAW OF CONDUCTION The rate at which heat is transferred by conduction q, is proportional to the temperature gradient d. T/dx multiplied by the area A through which heat is transferred. Ø The temperature gradient d. T/dx is expressed as the change in temperature with respect to the length of the path of the heat flow. Ø In the above relationship ‘T’ is the local temperature and ‘x’ is the distance in the direction of heat flow.

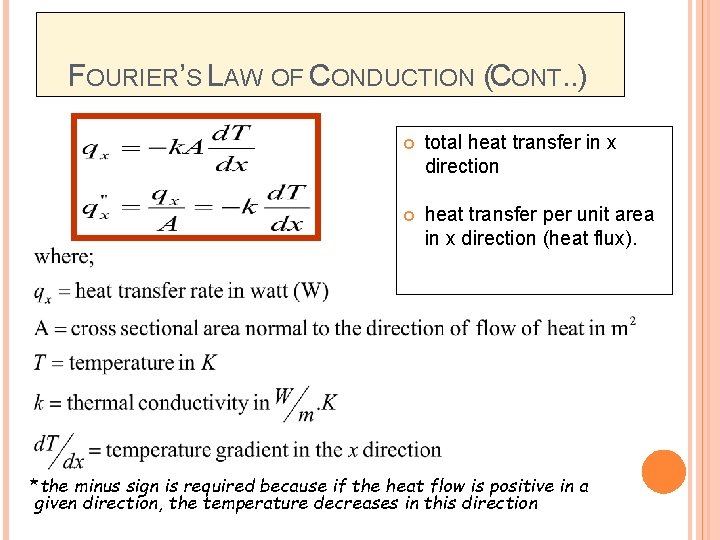
FOURIER’S LAW OF CONDUCTION (CONT. . ) total heat transfer in x direction heat transfer per unit area in x direction (heat flux). *the minus sign is required because if the heat flow is positive in a given direction, the temperature decreases in this direction

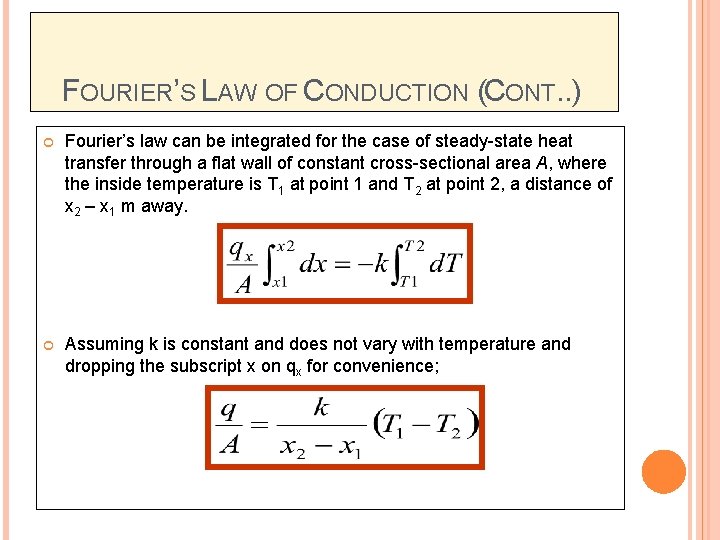
FOURIER’S LAW OF CONDUCTION (CONT. . ) Fourier’s law can be integrated for the case of steady-state heat transfer through a flat wall of constant cross-sectional area A, where the inside temperature is T 1 at point 1 and T 2 at point 2, a distance of x 2 – x 1 m away. Assuming k is constant and does not vary with temperature and dropping the subscript x on qx for convenience;

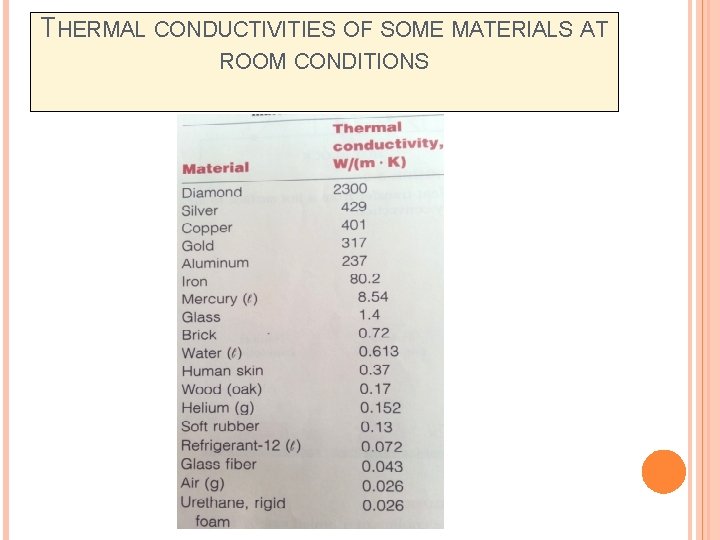
THERMAL CONDUCTIVITIES OF SOME MATERIALS AT ROOM CONDITIONS


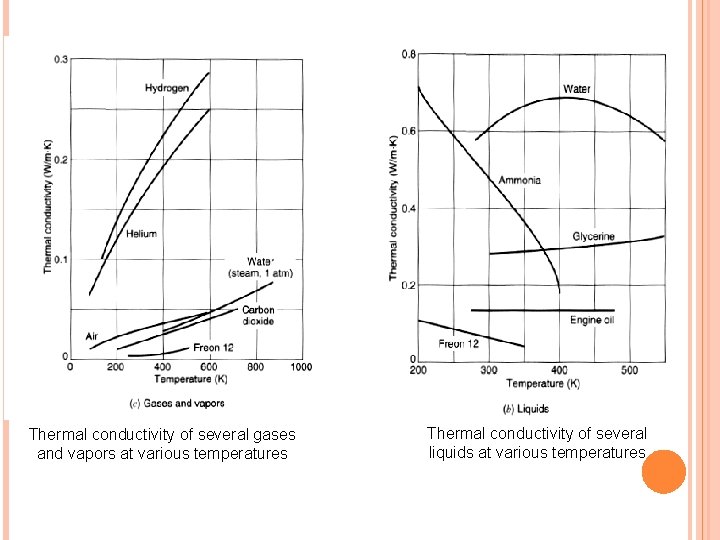
Thermal conductivity of several gases and vapors at various temperatures Thermal conductivity of several liquids at various temperatures

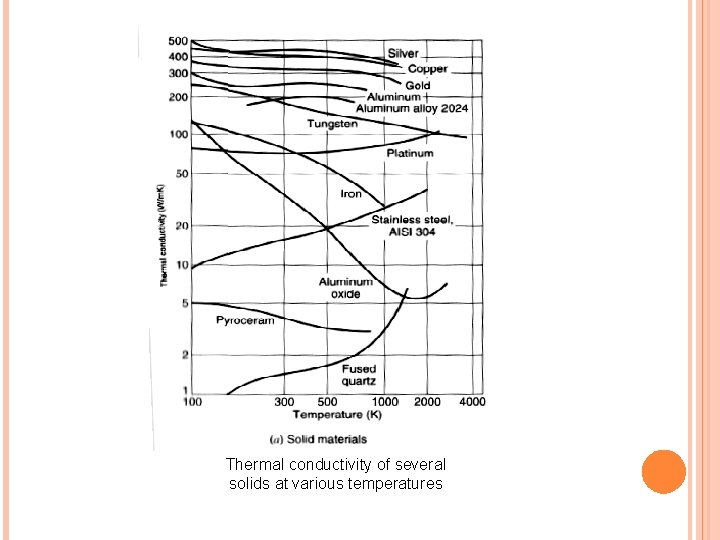
Thermal conductivity of several solids at various temperatures

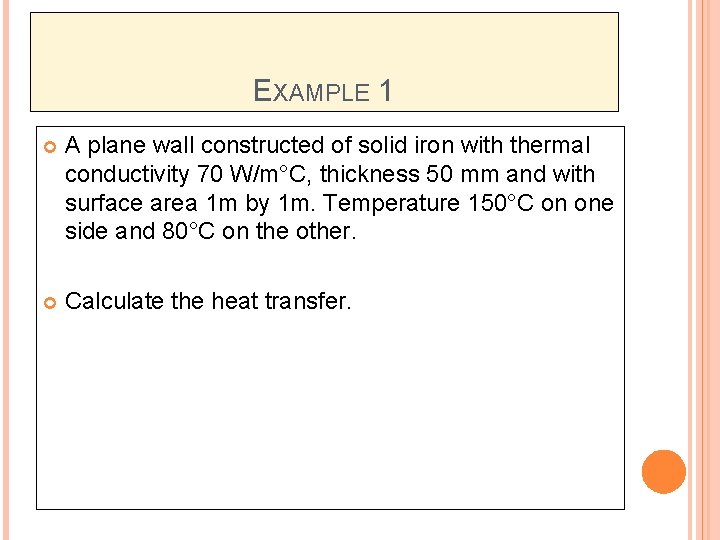
EXAMPLE 1 A plane wall constructed of solid iron with thermal conductivity 70 W/m°C, thickness 50 mm and with surface area 1 m by 1 m. Temperature 150°C on one side and 80°C on the other. Calculate the heat transfer.

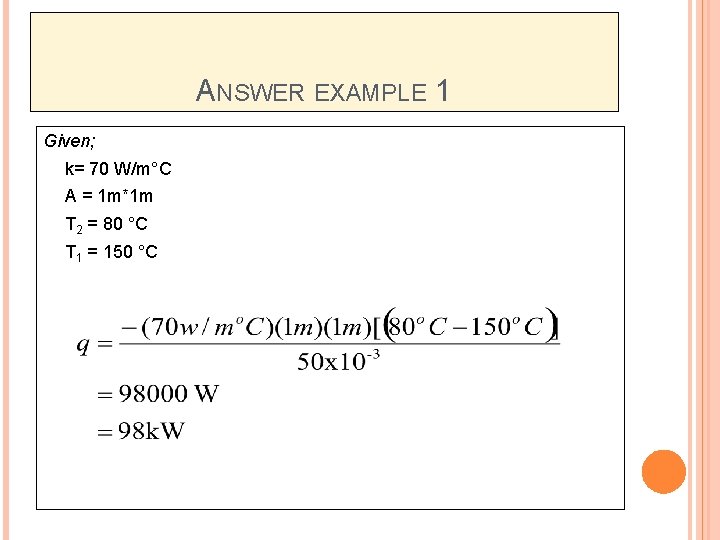
ANSWER EXAMPLE 1 Given; k= 70 W/m°C A = 1 m*1 m T 2 = 80 °C T 1 = 150 °C

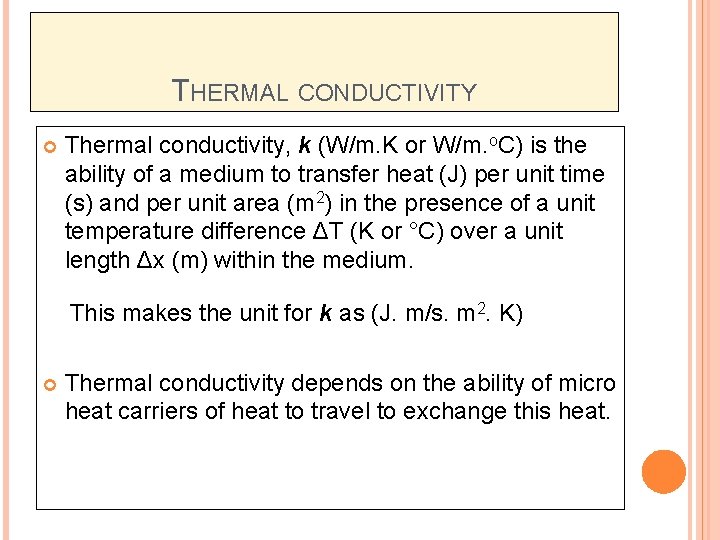
THERMAL CONDUCTIVITY Thermal conductivity, k (W/m. K or W/m. o. C) is the ability of a medium to transfer heat (J) per unit time (s) and per unit area (m 2) in the presence of a unit temperature difference ΔT (K or °C) over a unit length Δx (m) within the medium. This makes the unit for k as (J. m/s. m 2. K) Thermal conductivity depends on the ability of micro heat carriers of heat to travel to exchange this heat.

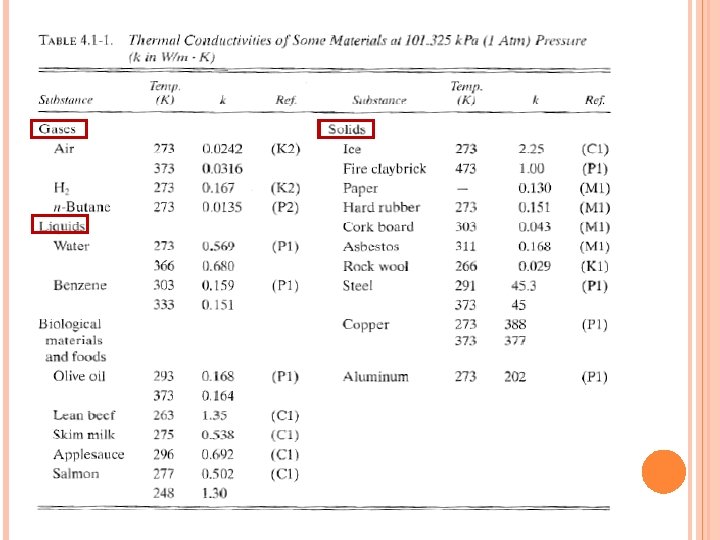
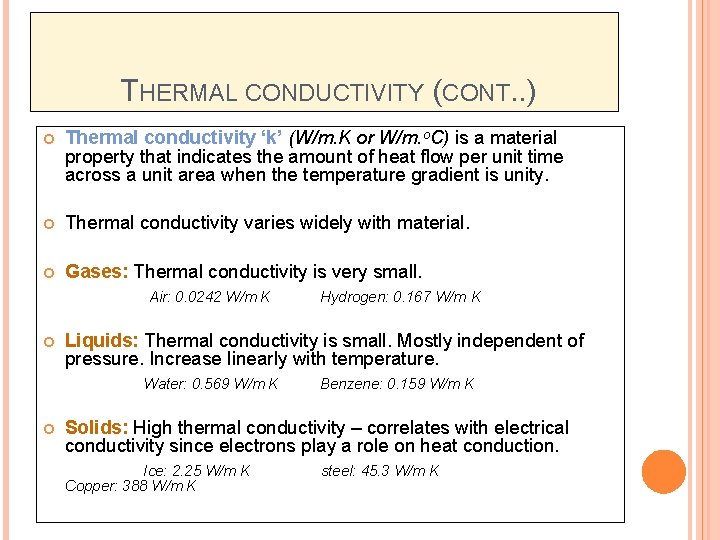
THERMAL CONDUCTIVITY (CONT. . ) Thermal conductivity ‘k’ (W/m. K or W/m. o. C) is a material property that indicates the amount of heat flow per unit time across a unit area when the temperature gradient is unity. Thermal conductivity varies widely with material. Gases: Thermal conductivity is very small. Air: 0. 0242 W/m K Liquids: Thermal conductivity is small. Mostly independent of pressure. Increase linearly with temperature. Water: 0. 569 W/m K Hydrogen: 0. 167 W/m K Benzene: 0. 159 W/m K Solids: High thermal conductivity – correlates with electrical conductivity since electrons play a role on heat conduction. Ice: 2. 25 W/m K Copper: 388 W/m K steel: 45. 3 W/m K

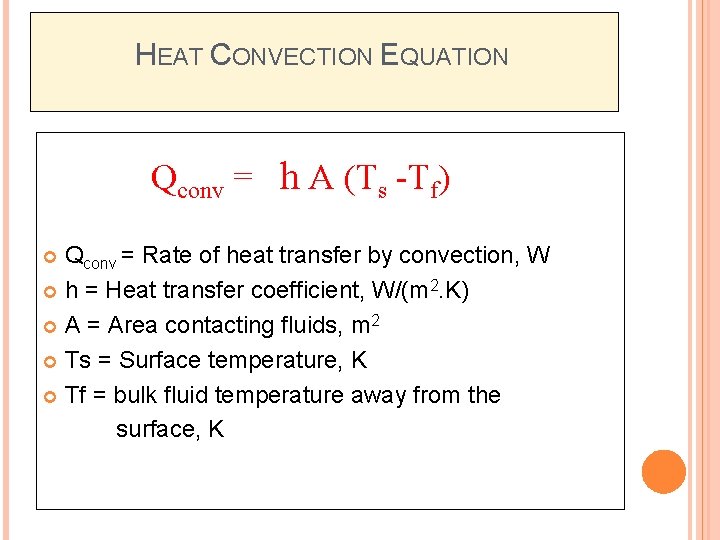
HEAT CONVECTION EQUATION Qconv = h A (Ts -Tf) Qconv = Rate of heat transfer by convection, W h = Heat transfer coefficient, W/(m 2. K) A = Area contacting fluids, m 2 Ts = Surface temperature, K Tf = bulk fluid temperature away from the surface, K

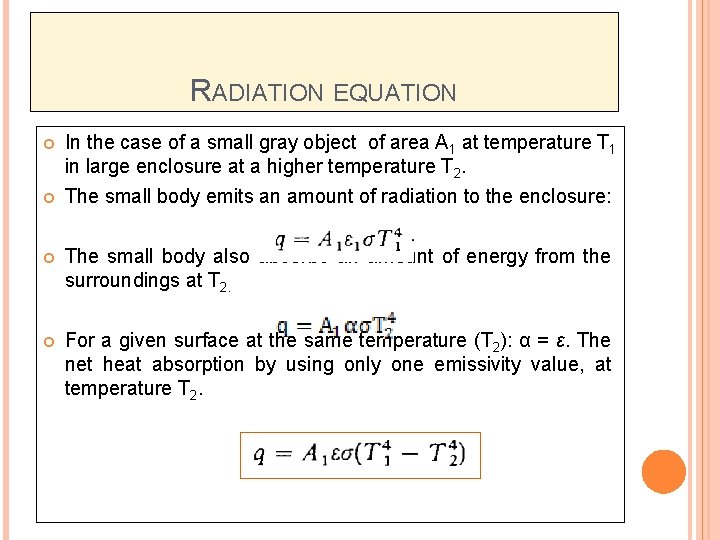
RADIATION EQUATION In the case of a small gray object of area A 1 at temperature T 1 in large enclosure at a higher temperature T 2. The small body emits an amount of radiation to the enclosure: The small body also absorbs an amount of energy from the surroundings at T 2. For a given surface at the same temperature (T 2): α = ε. The net heat absorption by using only one emissivity value, at temperature T 2.

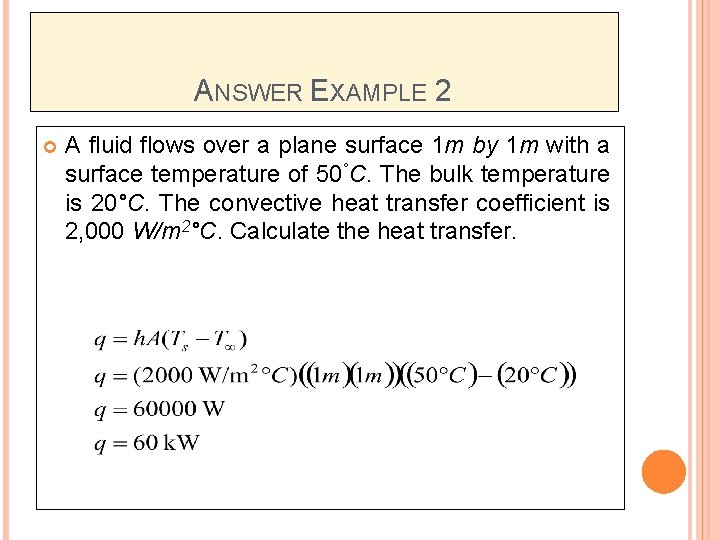
ANSWER EXAMPLE 2 A fluid flows over a plane surface 1 m by 1 m with a surface temperature of 50°C. The bulk temperature is 20°C. The convective heat transfer coefficient is 2, 000 W/m 2°C. Calculate the heat transfer.
 Third law of thermodynamics derivation
Third law of thermodynamics derivation First law of thermodynamics for open system
First law of thermodynamics for open system First law of thermodynamics
First law of thermodynamics 1th law of thermodynamics
1th law of thermodynamics Joule experiment for first law of thermodynamics
Joule experiment for first law of thermodynamics First law of thermodynamics
First law of thermodynamics First law of thermodynamics compressor
First law of thermodynamics compressor First law of thermodynamics sign convention
First law of thermodynamics sign convention First law of thermodynamics control mass
First law of thermodynamics control mass Gibbs free energy
Gibbs free energy First law of thermodynamics
First law of thermodynamics Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law of motion
Newton's first law of motion Khotbah gerry takaria
Khotbah gerry takaria Prorphyrin
Prorphyrin Pdt rokotus
Pdt rokotus En 14351-1
En 14351-1 Pdt anwar tjen
Pdt anwar tjen Pdt product development
Pdt product development Venturi p&id symbol
Venturi p&id symbol Aga pdt
Aga pdt Pdt manufacturing
Pdt manufacturing 2 pdt
2 pdt Pdt anwar tjen
Pdt anwar tjen 2 pdt
2 pdt Pdt manufacturing
Pdt manufacturing Pdt alex
Pdt alex Dos cosas te he demandado no me las niegues
Dos cosas te he demandado no me las niegues Rumah sungai lenggong
Rumah sungai lenggong Statistical thermodynamics in chemistry
Statistical thermodynamics in chemistry 11th chemistry thermodynamics lec 13
11th chemistry thermodynamics lec 13 Ap chemistry thermodynamics
Ap chemistry thermodynamics Thermodynamics ap chemistry
Thermodynamics ap chemistry 11th chemistry thermodynamics lec 10
11th chemistry thermodynamics lec 10 Dr tatiana erukhimova
Dr tatiana erukhimova 0th law of thermodynamics definition
0th law of thermodynamics definition Zeroth law of thermodynamics examples
Zeroth law of thermodynamics examples Entropy change formula
Entropy change formula State second law of thermodynamics
State second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Thermodynamics rules
Thermodynamics rules Second law of thermodynamics
Second law of thermodynamics