Lecture one Thermodynamics The First Law of Thermodynamics








- Slides: 33

Lecture one

Thermodynamics










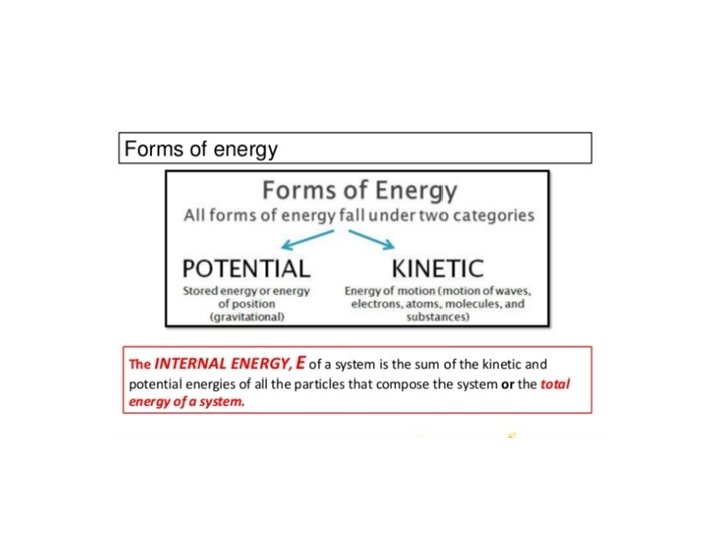
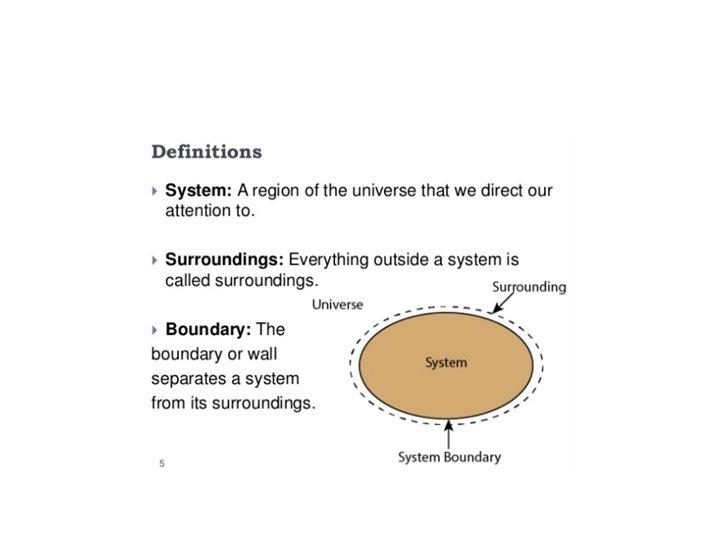
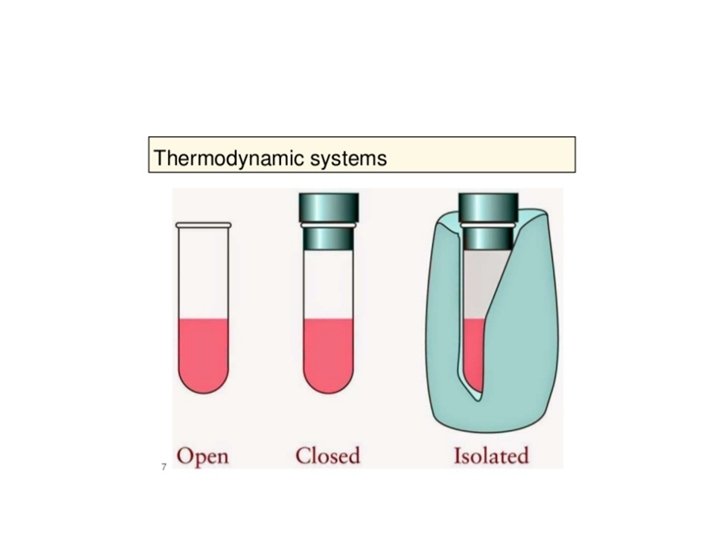
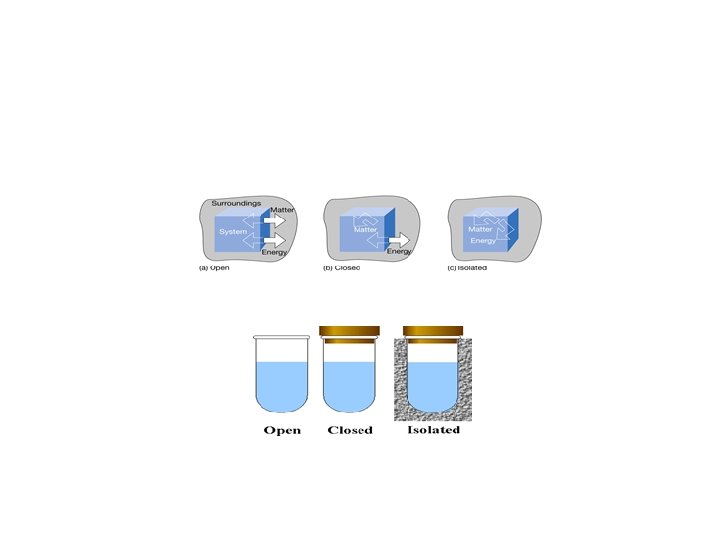
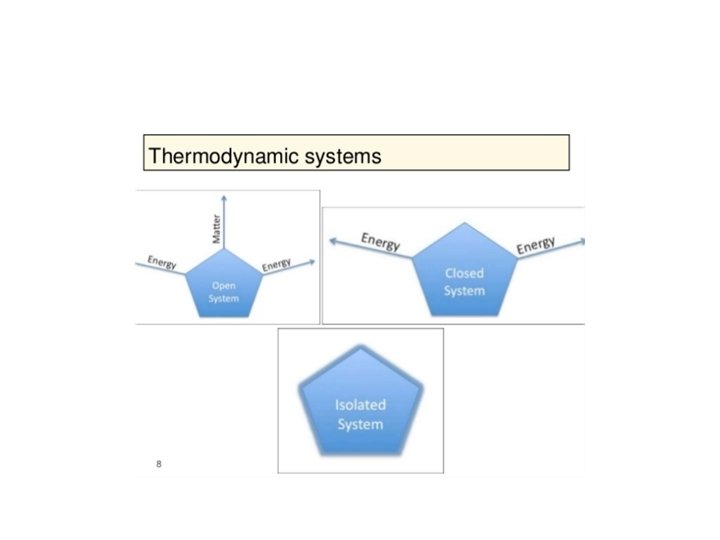
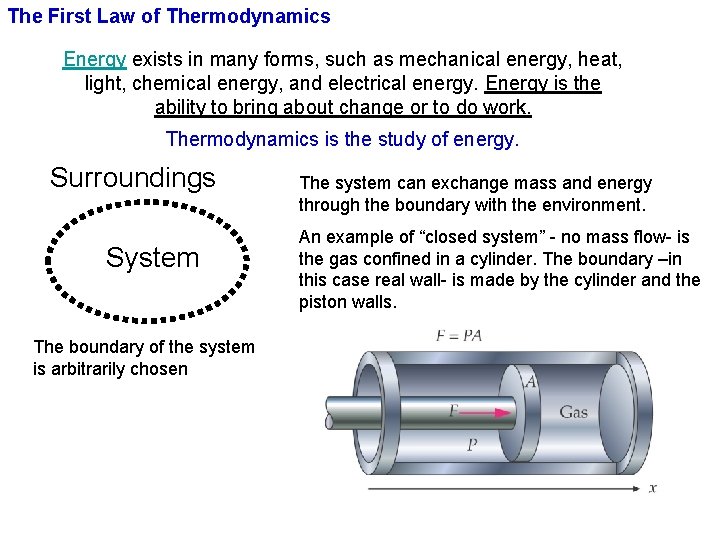
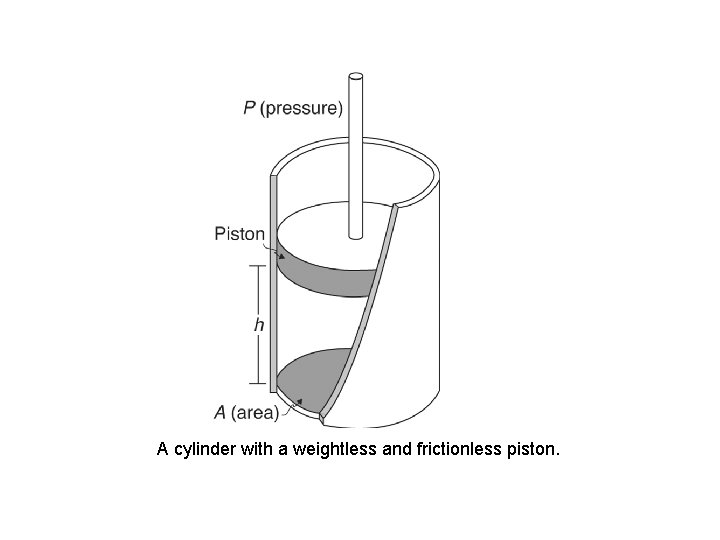
The First Law of Thermodynamics Energy exists in many forms, such as mechanical energy, heat, light, chemical energy, and electrical energy. Energy is the ability to bring about change or to do work. Thermodynamics is the study of energy. Surroundings System The boundary of the system is arbitrarily chosen The system can exchange mass and energy through the boundary with the environment. An example of “closed system” - no mass flow- is the gas confined in a cylinder. The boundary –in this case real wall- is made by the cylinder and the piston walls.

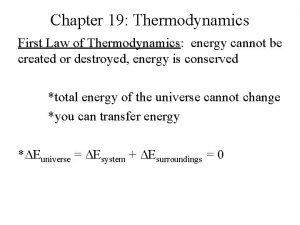
The First Law of Thermodynamics → Conservation of Energy: Energy can be changed from one form to another, but it cannot be created or destroyed. The total amount of energy and matter in the Universe remains constant, merely changing from one form to another. The First Law of Thermodynamics (Conservation) states that energy is always conserved, it cannot be created or destroyed. In essence, energy can be converted from one form into another. We need to define the concept of internal energy of the system, Eint as an energy stored in the system.

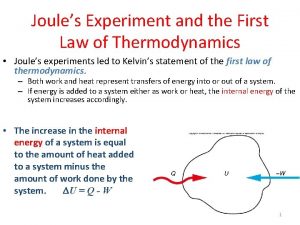
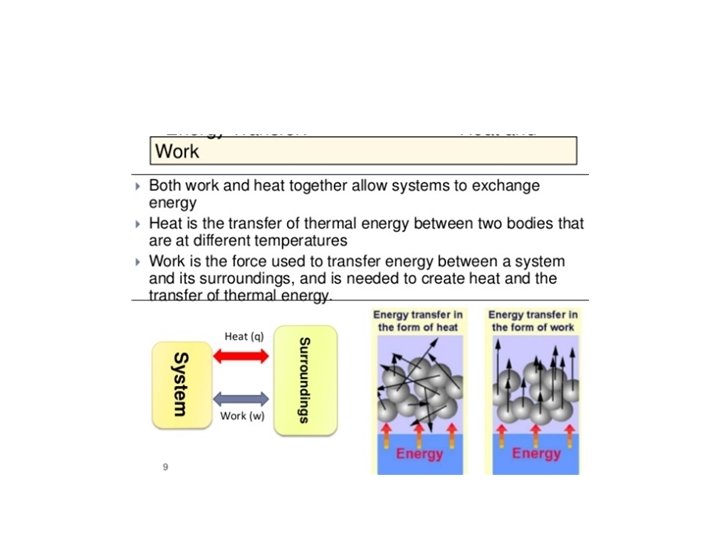
The First Law of Thermodynamics. Heat, Work and Internal Energy Joule’s Experiment and the First Law of Thermodynamics. Equivalence between work and heat 1 calorie = 4. 184 Joules Work is done on water. The energy is transferred to the water – i. e. the system-. The energy transferred appears as an increase in temperature. We can replace the insulating walls by conducting walls. We can transfer heat through the walls to the system to produce the same increase in temperature. The increase in temperature of the system is a consequence of an increase in Internal Energy. Internal energy is a state function of the system The sum of the heat transferred into the system and the work done on the system equals the change in the internal energy of the system

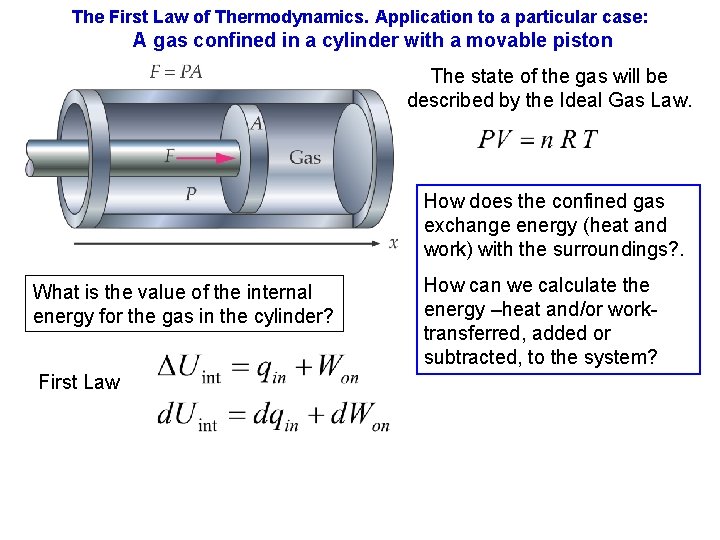
The First Law of Thermodynamics. Application to a particular case: A gas confined in a cylinder with a movable piston The state of the gas will be described by the Ideal Gas Law. How does the confined gas exchange energy (heat and work) with the surroundings? . What is the value of the internal energy for the gas in the cylinder? First Law How can we calculate the energy –heat and/or worktransferred, added or subtracted, to the system?

A cylinder with a weightless and frictionless piston.

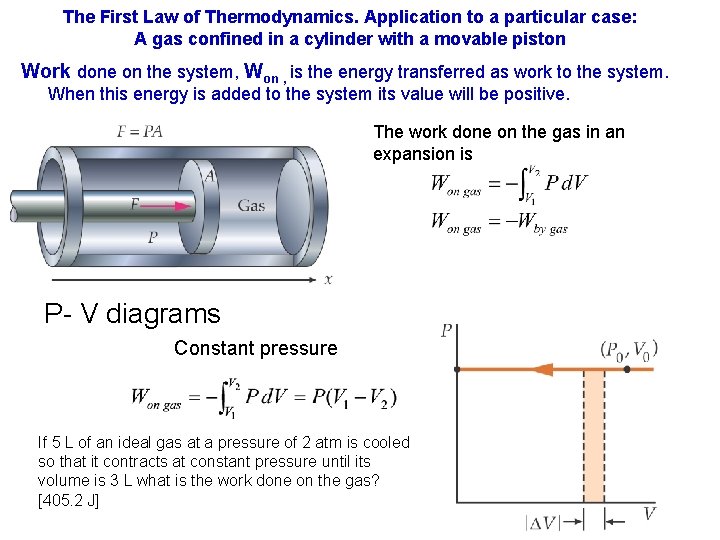
The First Law of Thermodynamics. Application to a particular case: A gas confined in a cylinder with a movable piston Work done on the system, Won , is the energy transferred as work to the system. When this energy is added to the system its value will be positive. The work done on the gas in an expansion is P- V diagrams Constant pressure If 5 L of an ideal gas at a pressure of 2 atm is cooled so that it contracts at constant pressure until its volume is 3 L what is the work done on the gas? [405. 2 J]

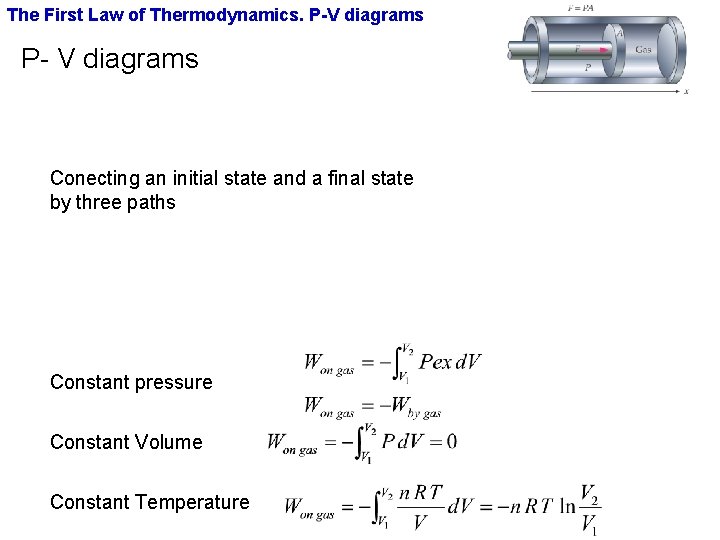
The First Law of Thermodynamics. P-V diagrams P- V diagrams Conecting an initial state and a final state by three paths Constant pressure Constant Volume Constant Temperature


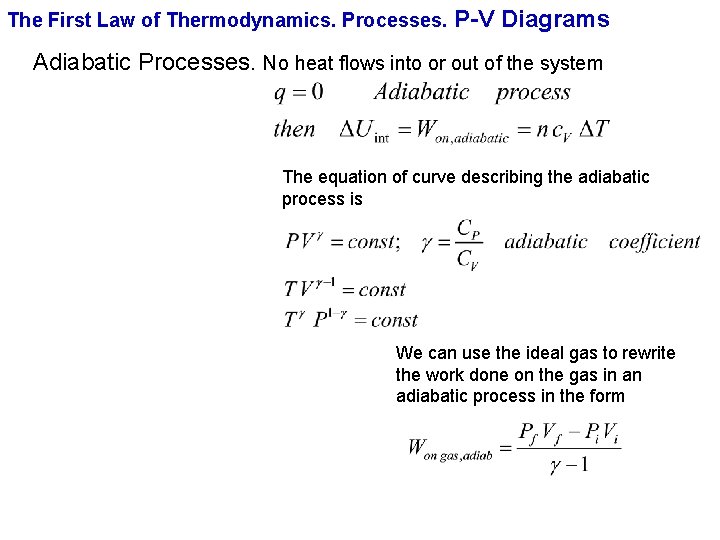
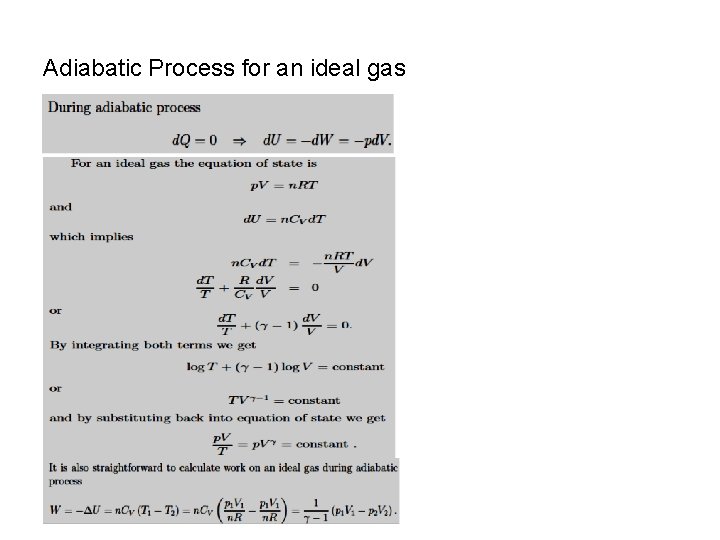
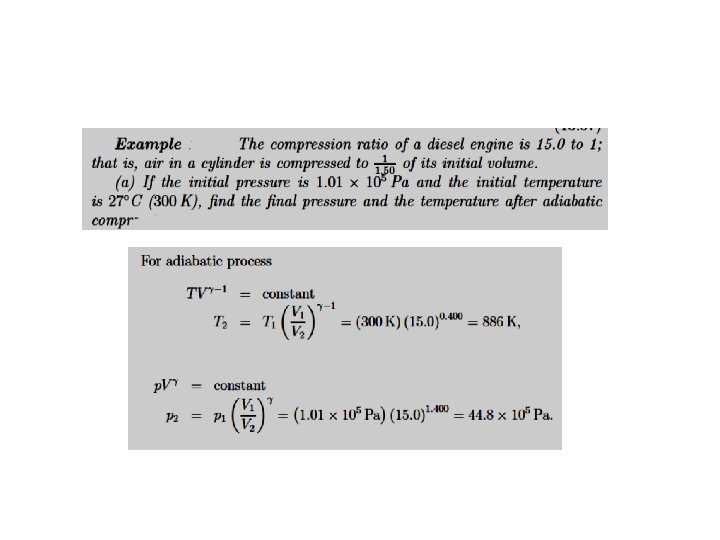
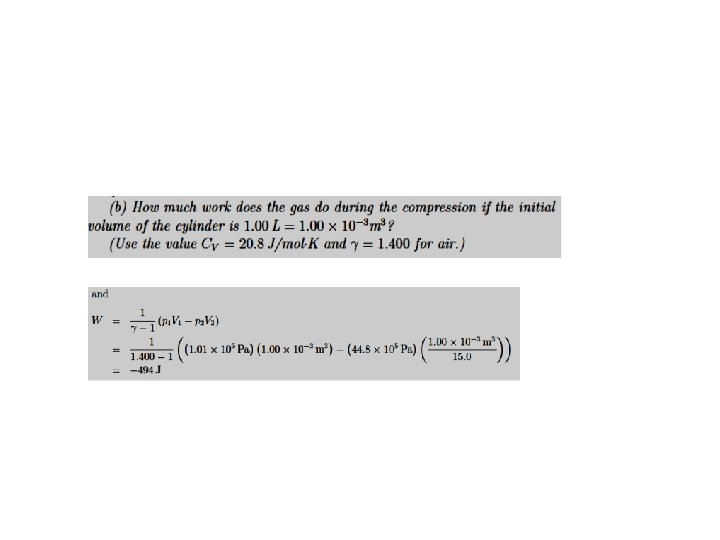
The First Law of Thermodynamics. Processes. P-V Diagrams Adiabatic Processes. No heat flows into or out of the system The equation of curve describing the adiabatic process is We can use the ideal gas to rewrite the work done on the gas in an adiabatic process in the form

Lecture 2

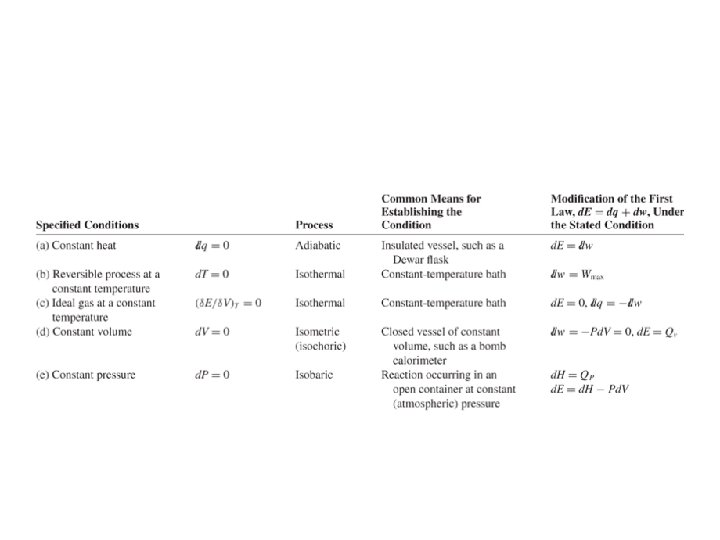
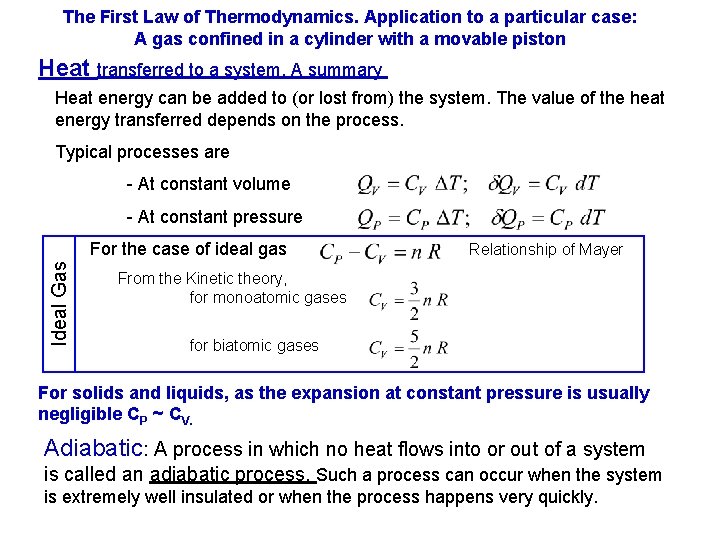
The First Law of Thermodynamics. Application to a particular case: A gas confined in a cylinder with a movable piston Heat transferred to a system. A summary Heat energy can be added to (or lost from) the system. The value of the heat energy transferred depends on the process. Typical processes are - At constant volume - At constant pressure Ideal Gas For the case of ideal gas Relationship of Mayer From the Kinetic theory, for monoatomic gases for biatomic gases For solids and liquids, as the expansion at constant pressure is usually negligible CP ~ CV. Adiabatic: A process in which no heat flows into or out of a system is called an adiabatic process. Such a process can occur when the system is extremely well insulated or when the process happens very quickly.

• Q 1 - The pressure exerted on an ideal gas at 2. 00 atm and 300 K is reduced suddenly to 1. 00 atm while heat is transferred to maintain the initial temperature of 300 K. Calculate q, w, and ΔU in joules for this process.


• • Suppose the pressure change in previous question carried out reversibly as well as isothermally. Calculate q, w, and ΔU in joules for this process and compare the answers to those in the irreversible expansion in one step. Solution:

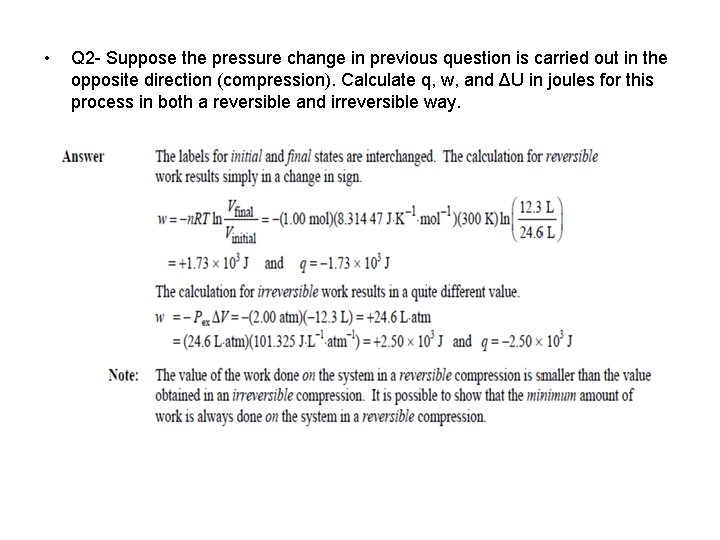
• Q 2 - Suppose the pressure change in previous question is carried out in the opposite direction (compression). Calculate q, w, and ΔU in joules for this process in both a reversible and irreversible way.

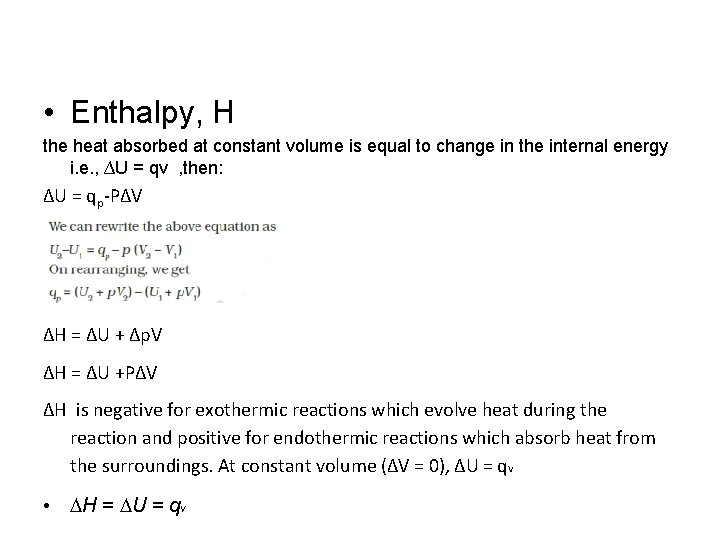
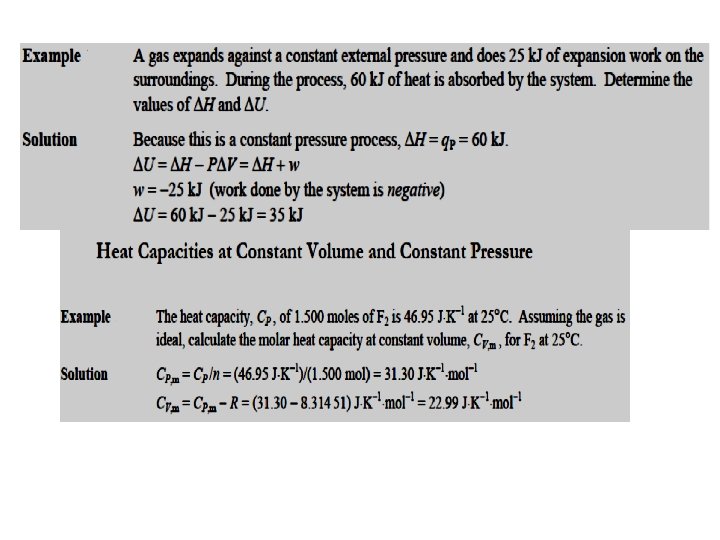
• Enthalpy, H the heat absorbed at constant volume is equal to change in the internal energy i. e. , ∆U = qv , then: ∆U = qp-P∆V ∆H = ∆U + ∆p. V ∆H = ∆U +P∆V ∆H is negative for exothermic reactions which evolve heat during the reaction and positive for endothermic reactions which absorb heat from the surroundings. At constant volume (∆V = 0), ∆U = q. V • DH = DU = q V



Adiabatic Process for an ideal gas



 Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law
Newton's first law One king one law one faith
One king one law one faith Energy balance equation thermodynamics open system
Energy balance equation thermodynamics open system Isobaric process formula
Isobaric process formula 1th law of thermodynamics
1th law of thermodynamics First law of thermodynamics joule's experiment
First law of thermodynamics joule's experiment Thermodynamics first law
Thermodynamics first law First law applied to flow process
First law applied to flow process First law of thermodynamics sign convention
First law of thermodynamics sign convention First law of thermodynamics control mass
First law of thermodynamics control mass Free energy
Free energy First law of thermodynamics
First law of thermodynamics 01:640:244 lecture notes - lecture 15: plat, idah, farad
01:640:244 lecture notes - lecture 15: plat, idah, farad V=k/p
V=k/p Charles law constant
Charles law constant One empire one god one emperor
One empire one god one emperor One one little dog run
One one little dog run One empire one god one emperor
One empire one god one emperor One team one plan one goal
One team one plan one goal See one do one teach one
See one do one teach one One price policy
One price policy Willow cabin speech
Willow cabin speech See one do one teach one
See one do one teach one Asean tourism strategic plan
Asean tourism strategic plan Graphic organizer with the aims of la liga filipina
Graphic organizer with the aims of la liga filipina A first course in atmospheric thermodynamics solutions
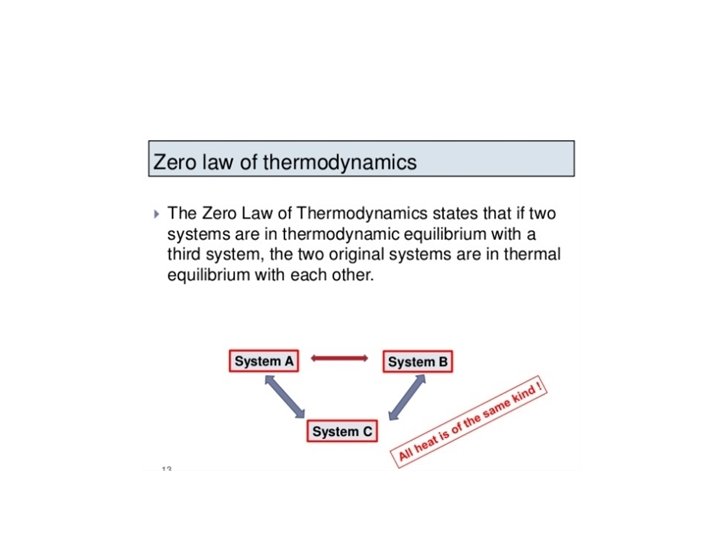
A first course in atmospheric thermodynamics solutions 0th law of thermodynamics definition
0th law of thermodynamics definition Third law of thermodynamics derivation
Third law of thermodynamics derivation Third law of thermodynamics
Third law of thermodynamics Isentropic compressor
Isentropic compressor State second law of thermodynamics
State second law of thermodynamics What is second law of thermodynamics
What is second law of thermodynamics Thermodynamics rules
Thermodynamics rules