Chapter 19 THE FIRST LAW OF THERMODYNAMICS THERMODYNAMICS







- Slides: 35

Chapter 19 THE FIRST LAW OF THERMODYNAMICS

THERMODYNAMICS “A theory is the more impressive the greater the simplicity of its premises, the more different kinds of things it relates, and the more extended its area of applicability. Therefore the deep impression that classical thermodynamics made upon me. It is the only physical theory of universal content which I am convinced will never be overthrown, within the framework of applicability of its basic concepts. ” A. Einstein

(INTERNAL) ENERGY OF A GAS

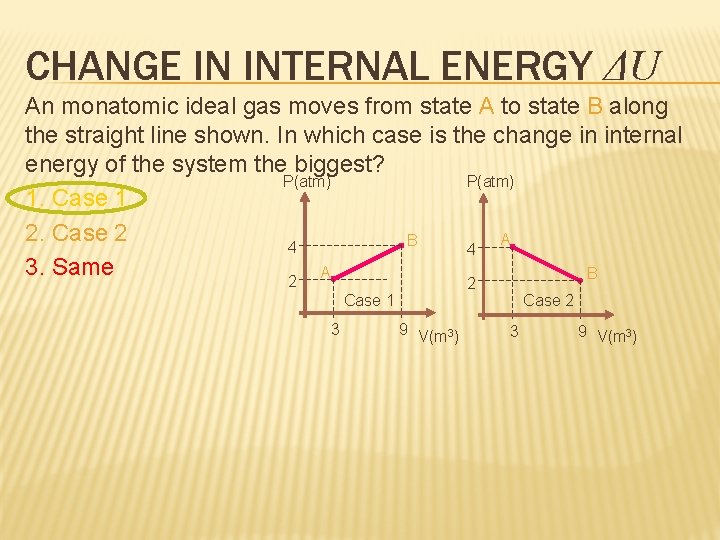
CHANGE IN INTERNAL ENERGY ΔU An monatomic ideal gas moves from state A to state B along the straight line shown. In which case is the change in internal energy of the system the biggest? P(atm) 1. Case 1 2. Case 2 A B 4 4 3. Same A B 2 2 Case 1 3 9 V(m 3) Case 2 3 9 V(m 3)

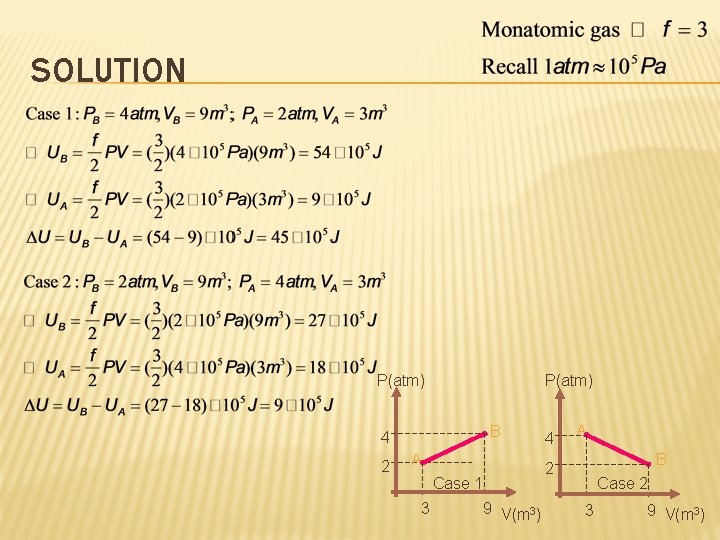
SOLUTION P(atm) B 4 2 A Case 1 3 9 V(m 3) 4 A B 2 Case 2 3 9 V(m 3)

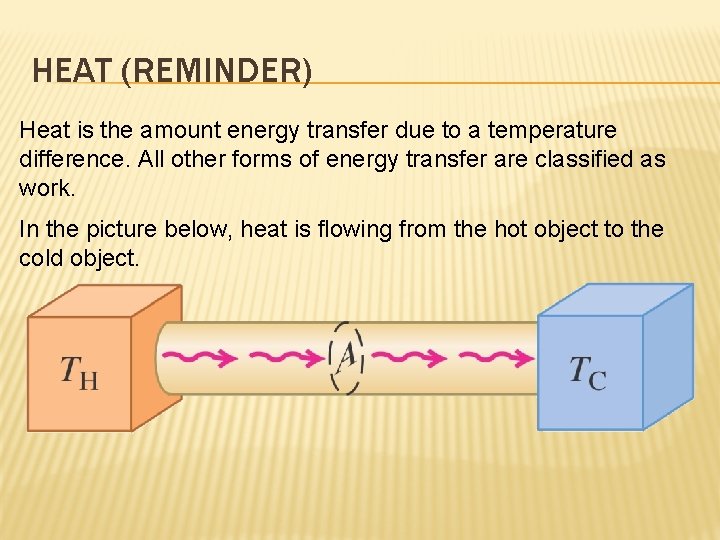
HEAT (REMINDER) Heat is the amount energy transfer due to a temperature difference. All other forms of energy transfer are classified as work. In the picture below, heat is flowing from the hot object to the cold object.

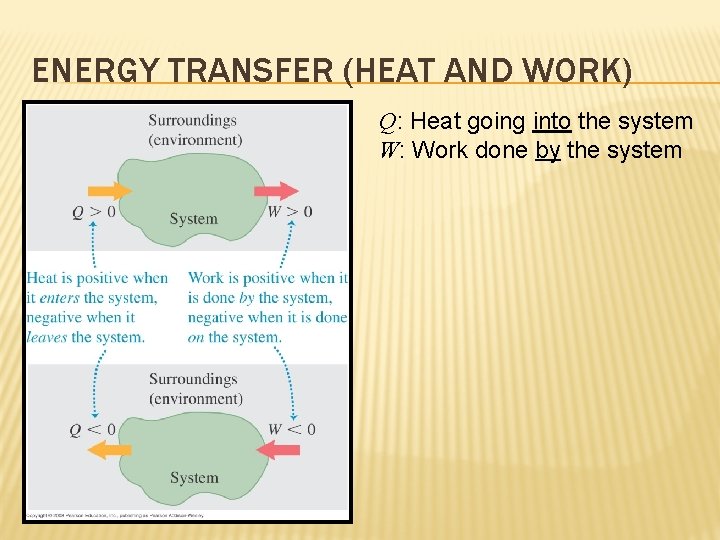
ENERGY TRANSFER (HEAT AND WORK) Q: Heat going into the system W: Work done by the system

WORK DONE ON OR BY THE SYSTEM HEAT INTO OR OUT OF THE SYSTEM (Work done by the system) = (− 1) × (Work done on the system) (Heat going into the system) = (− 1) × (Heat going out of the system) W = +100 J Work done by the system = +100 J Work done on the system = -100 J W = -150 J Work done by the system = -150 J Work done on the system = +150 J Q = +100 J Heat going into the system = +100 J Heat going out of the system = -100 J Q = -150 J Heat going into the system = -150 J Heat going out of the system = +150 J

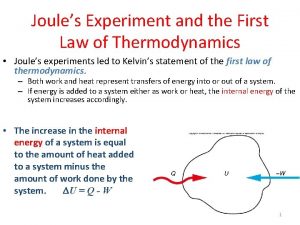
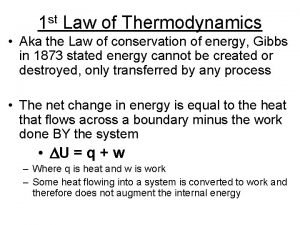
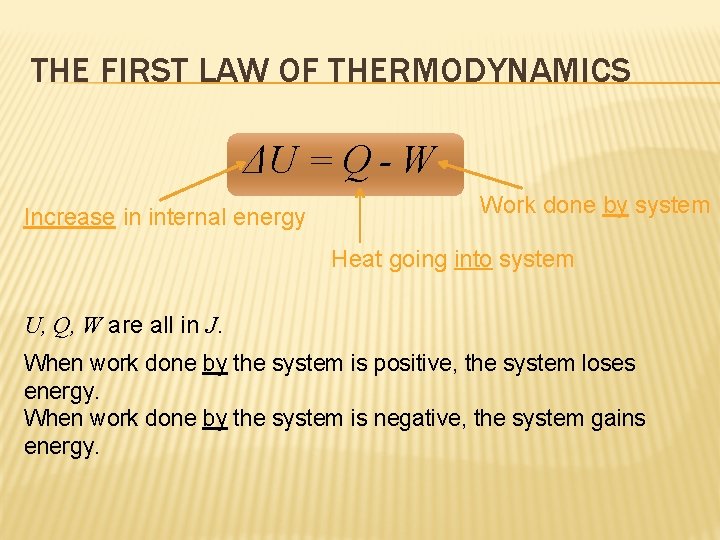
THE FIRST LAW OF THERMODYNAMICS ΔU = Q - W Increase in internal energy Work done by system Heat going into system U, Q, W are all in J. When work done by the system is positive, the system loses energy. When work done by the system is negative, the system gains energy.

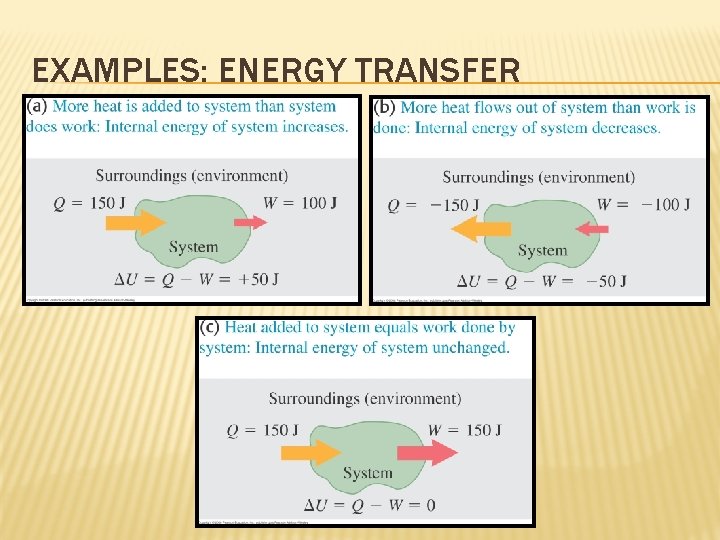
EXAMPLES: ENERGY TRANSFER

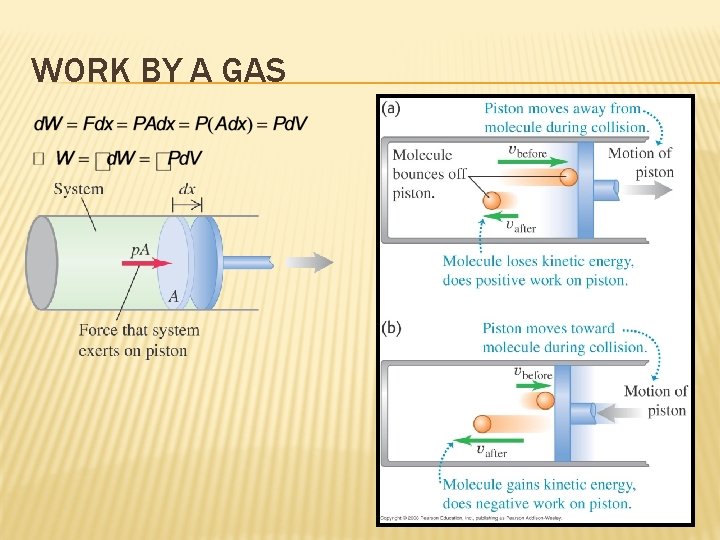
WORK BY A GAS

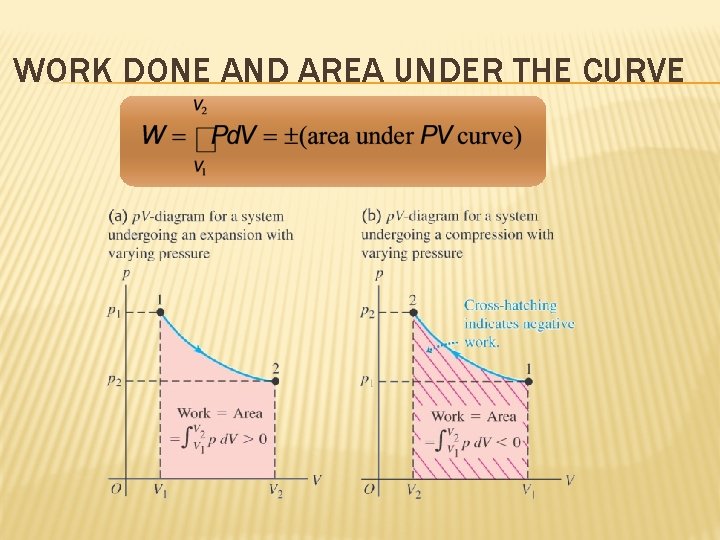
WORK DONE AND AREA UNDER THE CURVE

THE SIGN OF W If all the signs seems confusing, simply remember this: Expansion W >0 Compression W <0

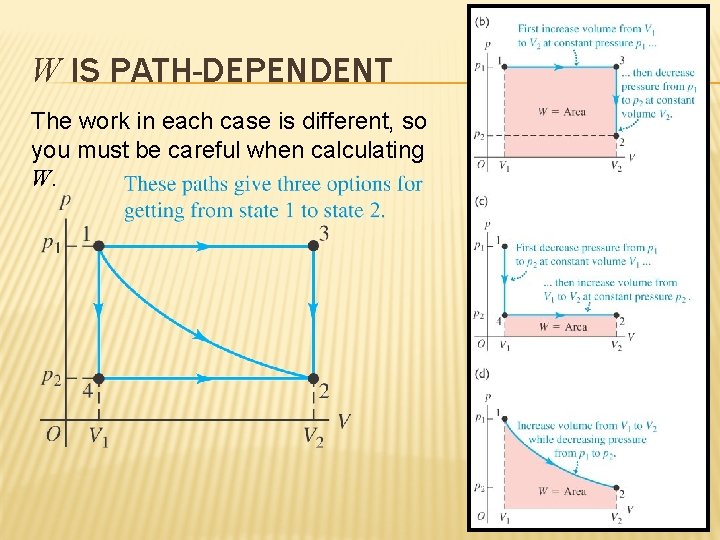
W IS PATH-DEPENDENT The work in each case is different, so you must be careful when calculating W.

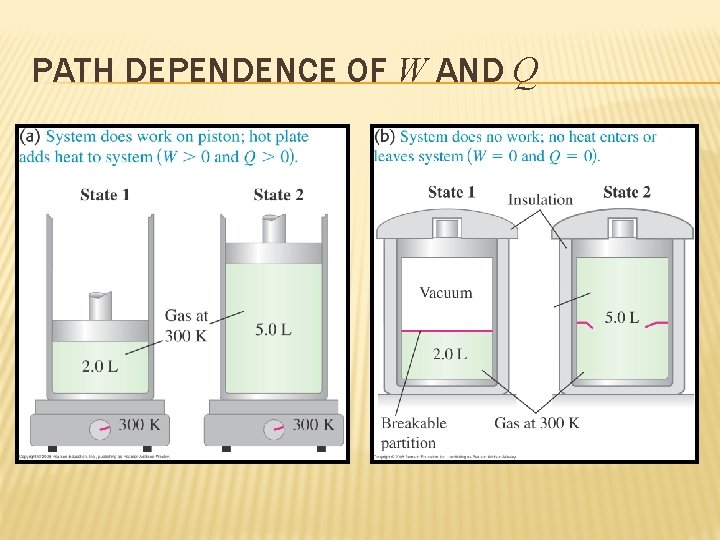
PATH DEPENDENCE OF W AND Q

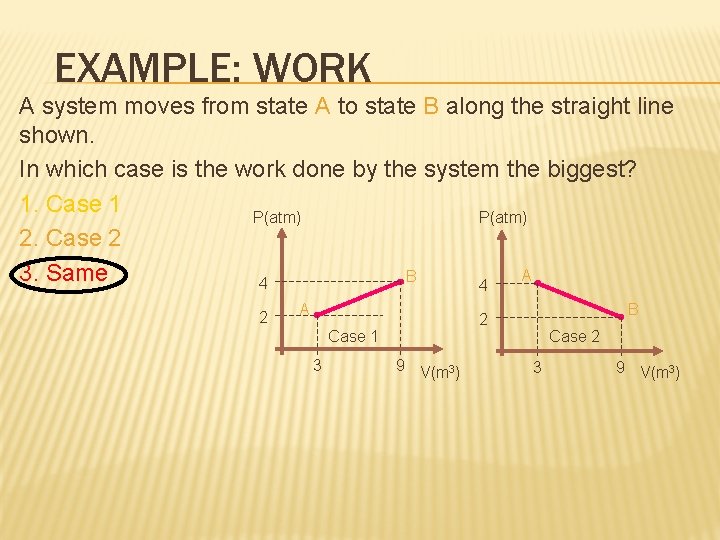
EXAMPLE: WORK A system moves from state A to state B along the straight line shown. In which case is the work done by the system the biggest? 1. Case 1 P(atm) 2. Case 2 3. Same A B 4 4 2 A Case 1 3 B 2 9 V(m 3) Case 2 3 9 V(m 3)

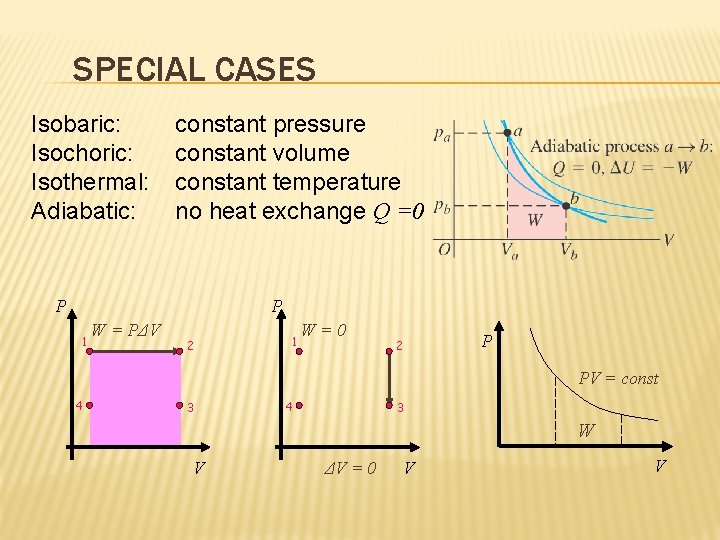
SPECIAL CASES Isobaric: Isochoric: Isothermal: Adiabatic: constant pressure constant volume constant temperature no heat exchange Q =0 P P 1 W = PΔV 2 1 W=0 2 P PV = const 4 3 W V ΔV = 0 V V

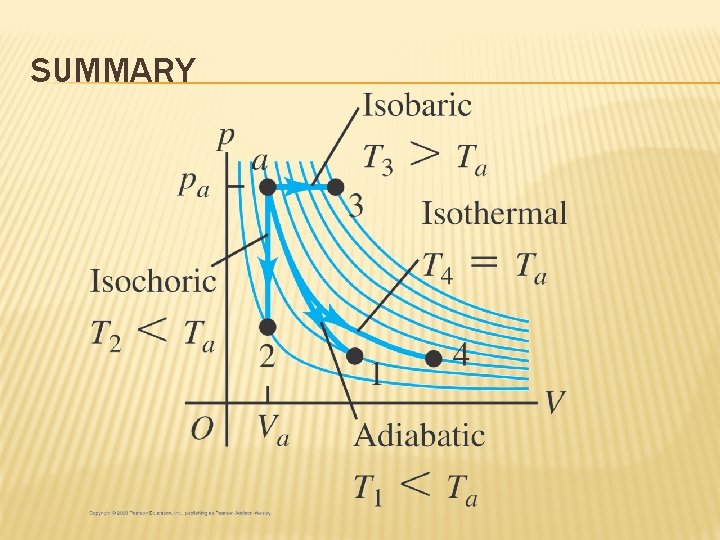
SUMMARY

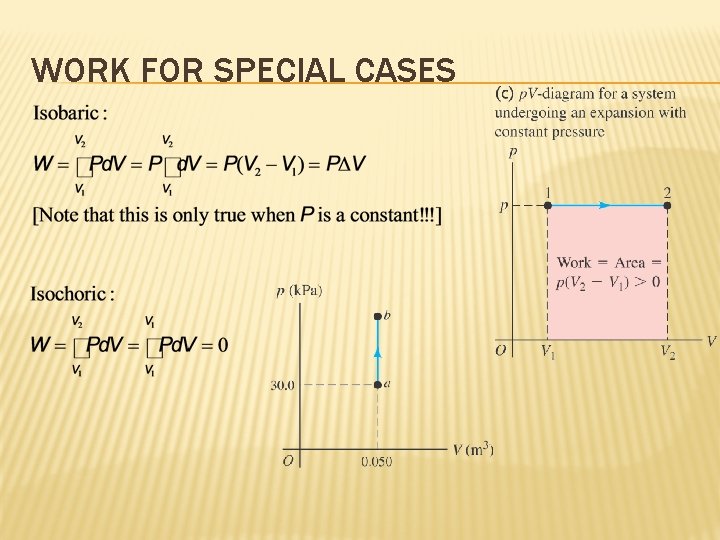
WORK FOR SPECIAL CASES

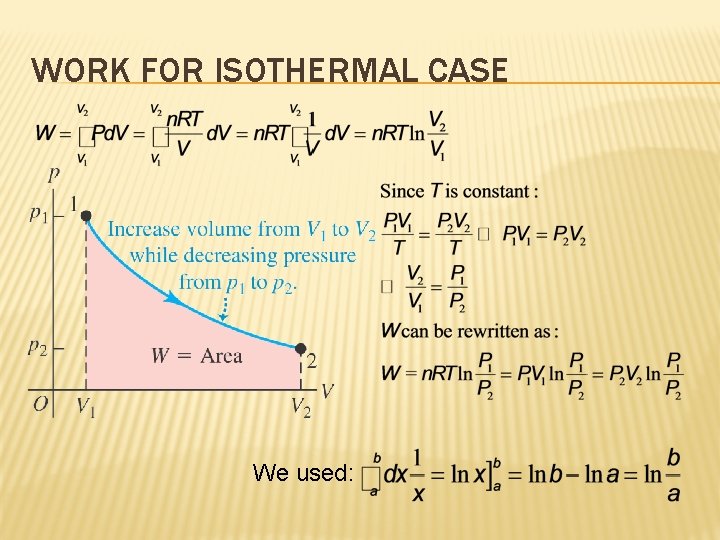
WORK FOR ISOTHERMAL CASE We used:

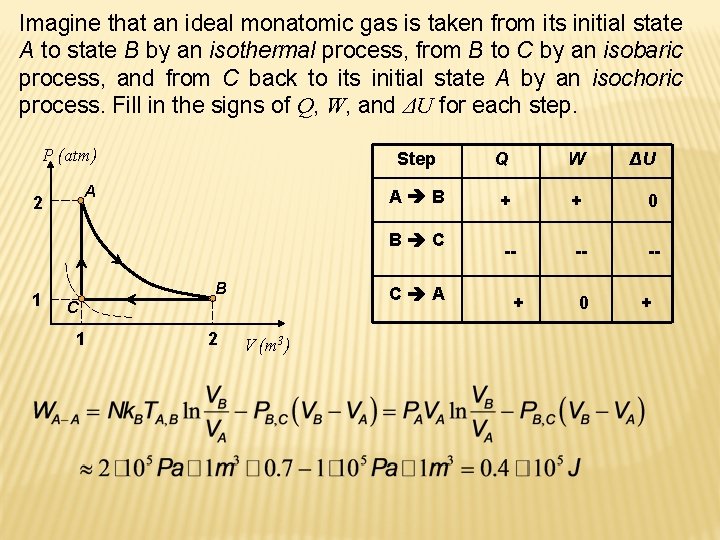
Imagine that an ideal monatomic gas is taken from its initial state A to state B by an isothermal process, from B to C by an isobaric process, and from C back to its initial state A by an isochoric process. Fill in the signs of Q, W, and ΔU for each step. P (atm) A 2 Step Q W ΔU A B + + 0 -- -- -- B C 1 B C A C 1 2 V (m 3) + 0 +

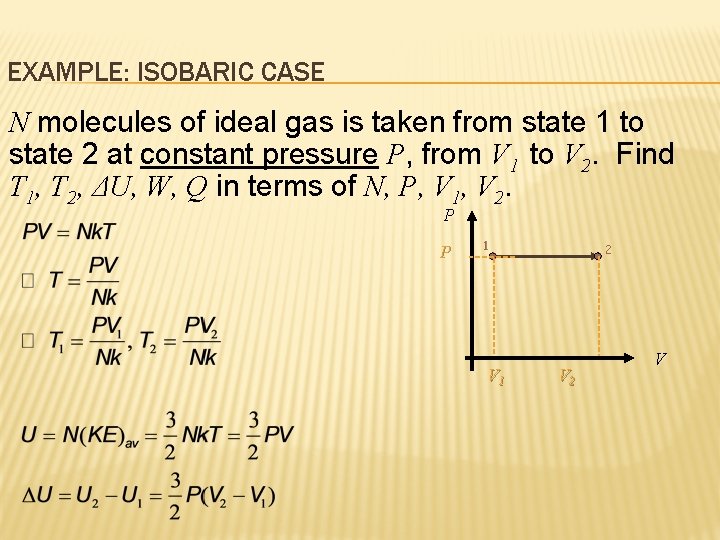
EXAMPLE: ISOBARIC CASE N molecules of ideal gas is taken from state 1 to state 2 at constant pressure P, from V 1 to V 2. Find T 1, T 2, ΔU, W, Q in terms of N, P, V 1, V 2. P P 1 V 1 2 V

FINDING W AND Q

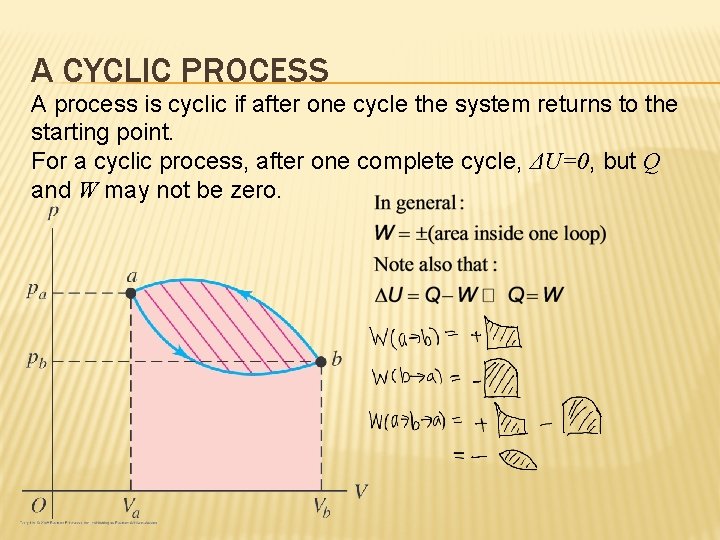
A CYCLIC PROCESS A process is cyclic if after one cycle the system returns to the starting point. For a cyclic process, after one complete cycle, ΔU=0, but Q and W may not be zero.

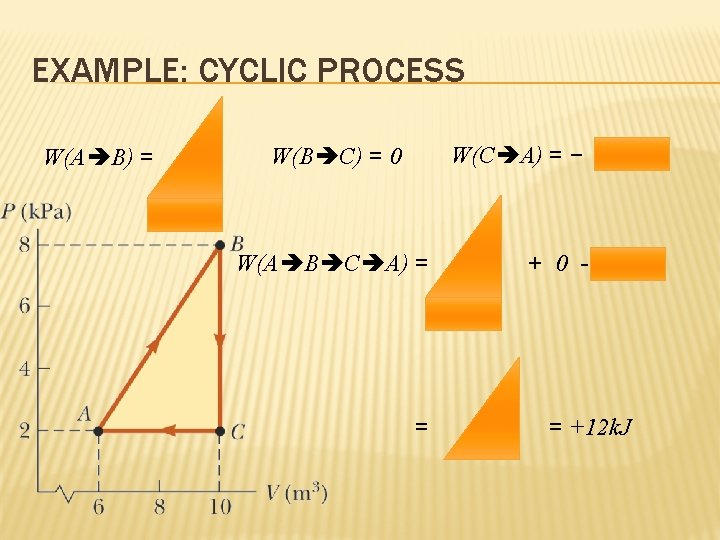
EXAMPLE: CYCLIC PROCESS W(A B) = W(C A) = − W(B C) = 0 W(A B C A) = = + 0 - = +12 k. J

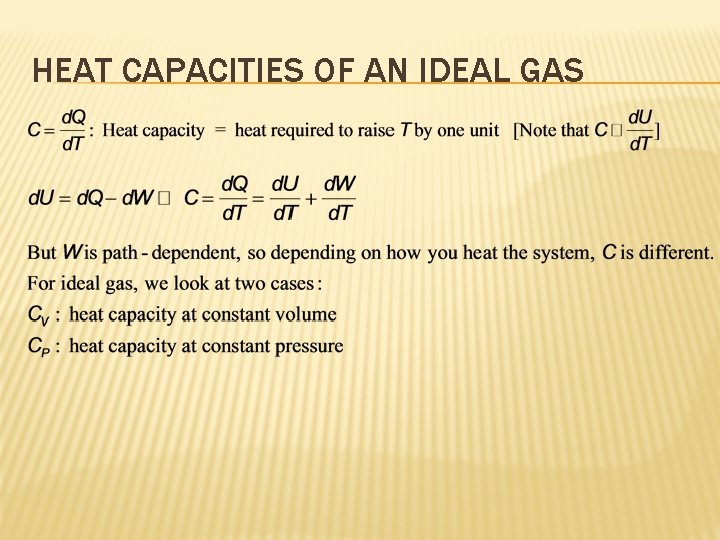
HEAT CAPACITIES OF AN IDEAL GAS

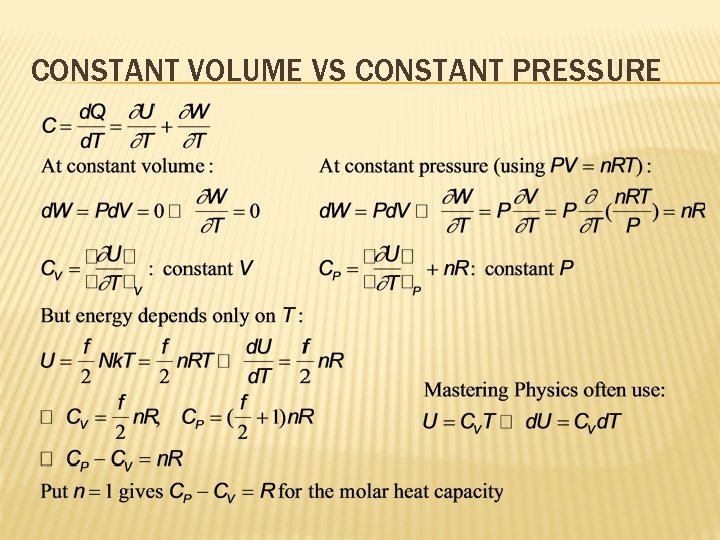
CONSTANT VOLUME VS CONSTANT PRESSURE

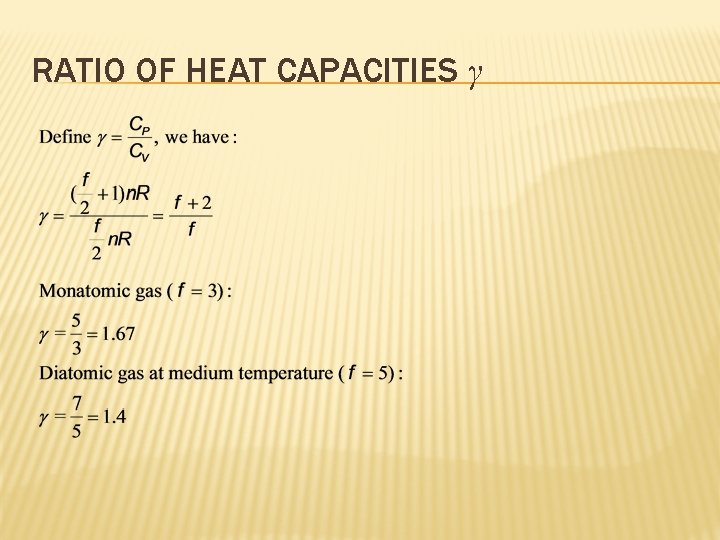
RATIO OF HEAT CAPACITIES γ

ADIABATIC PROCESS

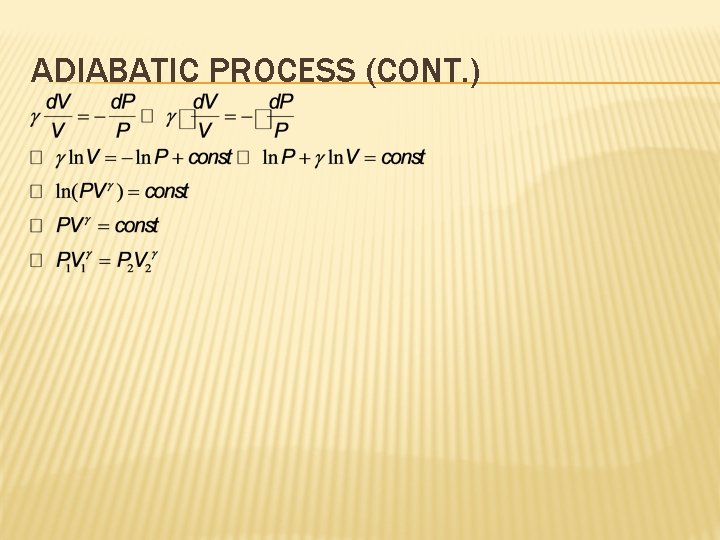
ADIABATIC PROCESS (CONT. )

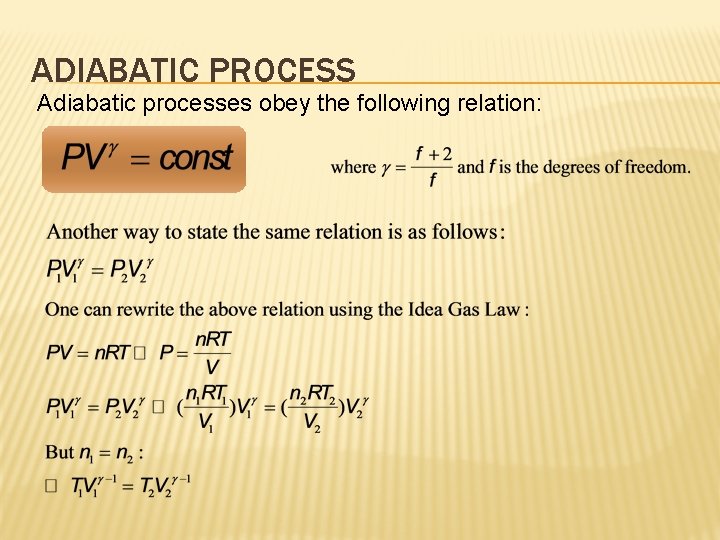
ADIABATIC PROCESS Adiabatic processes obey the following relation:

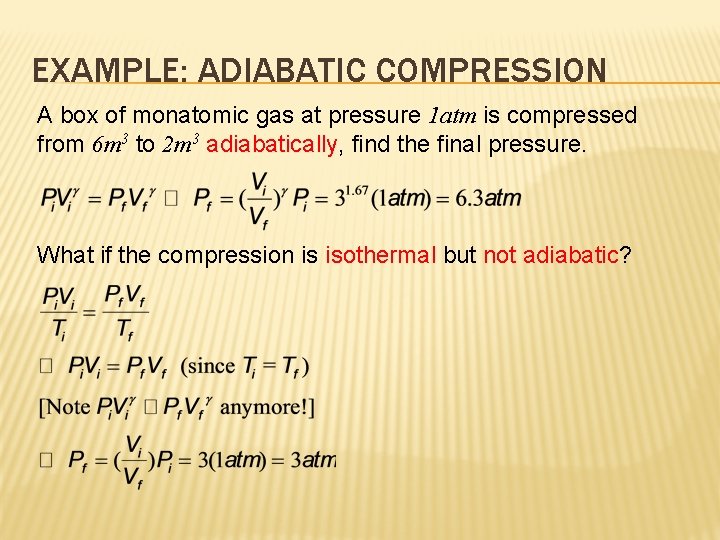
EXAMPLE: ADIABATIC COMPRESSION A box of monatomic gas at pressure 1 atm is compressed from 6 m 3 to 2 m 3 adiabatically, adiabatically find the final pressure. What if the compression is isothermal but not adiabatic? adiabatic

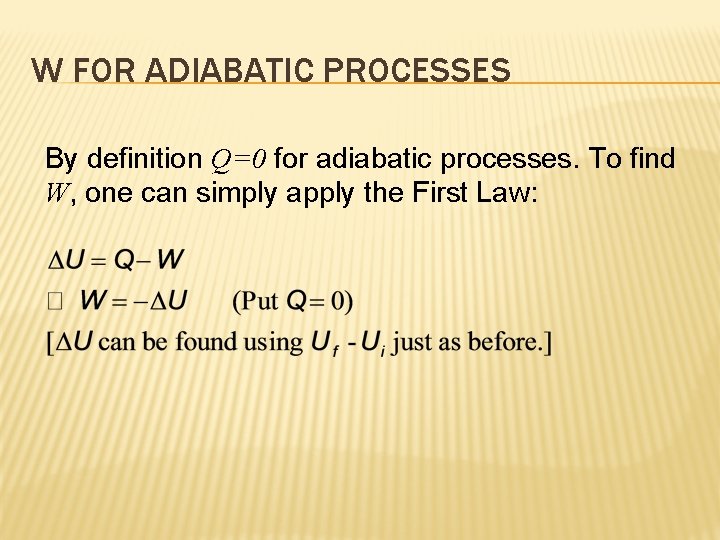
W FOR ADIABATIC PROCESSES By definition Q=0 for adiabatic processes. To find W, one can simply apply the First Law:

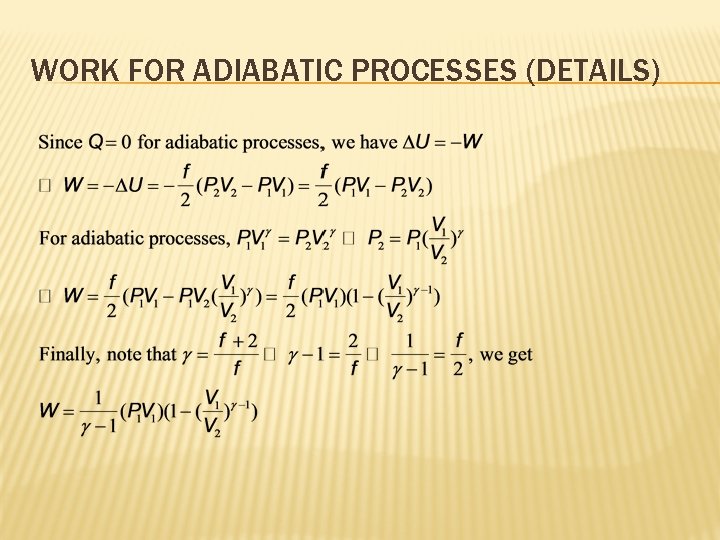
WORK FOR ADIABATIC PROCESSES (DETAILS)

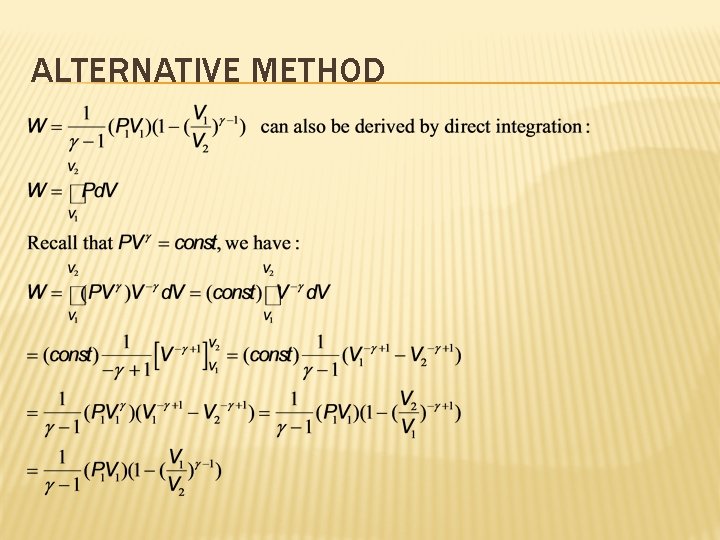
ALTERNATIVE METHOD
 Newton's first law and second law and third law
Newton's first law and second law and third law Newton's first law of motion
Newton's first law of motion Energy balance thermodynamics
Energy balance thermodynamics Isobaric process formula
Isobaric process formula Brayton cycle
Brayton cycle Joule's experiment in thermodynamics
Joule's experiment in thermodynamics Laws in thermodynamics
Laws in thermodynamics What is steady flow process in thermodynamics
What is steady flow process in thermodynamics First law of thermodynamics sign convention
First law of thermodynamics sign convention First law of thermodynamics control mass
First law of thermodynamics control mass First law of thermodynamics
First law of thermodynamics First law of thermodynamics for ideal gas
First law of thermodynamics for ideal gas Boyle's law charles law avogadro's law
Boyle's law charles law avogadro's law P=k/v
P=k/v Dr erukhimova
Dr erukhimova 0th law of thermodynamics definition
0th law of thermodynamics definition Law of thermodynamics in chemistry
Law of thermodynamics in chemistry Thermodynamics laws
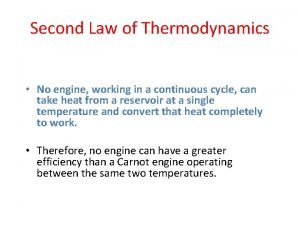
Thermodynamics laws Second law of thermodynamics
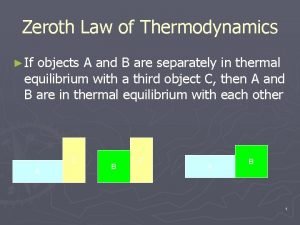
Second law of thermodynamics Zeroth law of thermodynamics
Zeroth law of thermodynamics What is second law of thermodynamics
What is second law of thermodynamics What are the laws of thermodynamics
What are the laws of thermodynamics Second law of thermodynamics
Second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics Thermodynamics enthalpy of reaction and hess's law
Thermodynamics enthalpy of reaction and hess's law Third law of thermodynamics is depend on
Third law of thermodynamics is depend on 1st law of thermodynamics
1st law of thermodynamics 1st law of thermodynamics
1st law of thermodynamics 1st law of thermodynamics
1st law of thermodynamics Zeroth law of thermodynamics
Zeroth law of thermodynamics 2nd law of thermodynamics
2nd law of thermodynamics Zeroth law of thermodynamics
Zeroth law of thermodynamics Second law of thermodynamics definition
Second law of thermodynamics definition Thermodynamics
Thermodynamics Frist law of thermodynamics
Frist law of thermodynamics Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể