Thermodynamics and Statistical Mechanics First Law of Thermodynamics

- Slides: 27

Thermodynamics and Statistical Mechanics First Law of Thermodynamics Thermo & Stat Mech Spring 2006 Class 3

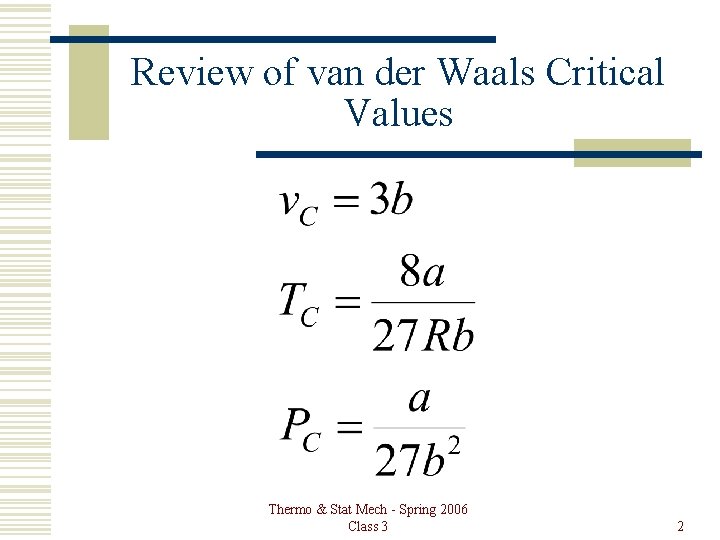
Review of van der Waals Critical Values Thermo & Stat Mech - Spring 2006 Class 3 2

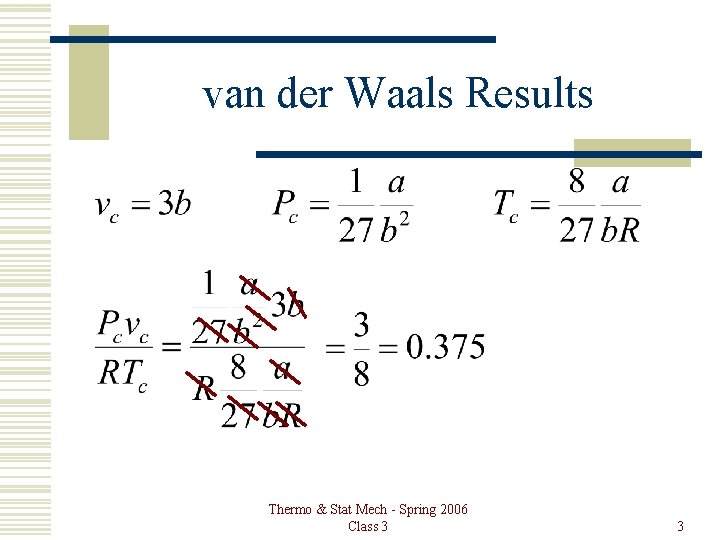
van der Waals Results Thermo & Stat Mech - Spring 2006 Class 3 3

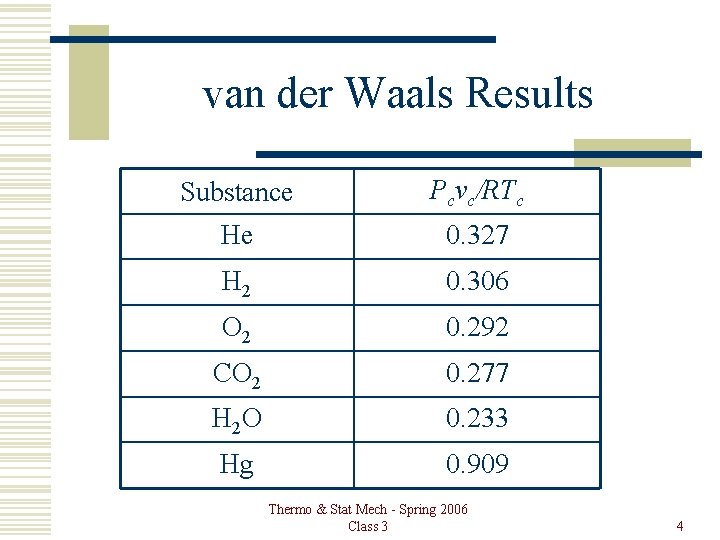
van der Waals Results Substance Pcvc/RTc He 0. 327 H 2 0. 306 O 2 0. 292 CO 2 0. 277 H 2 O 0. 233 Hg 0. 909 Thermo & Stat Mech - Spring 2006 Class 3 4

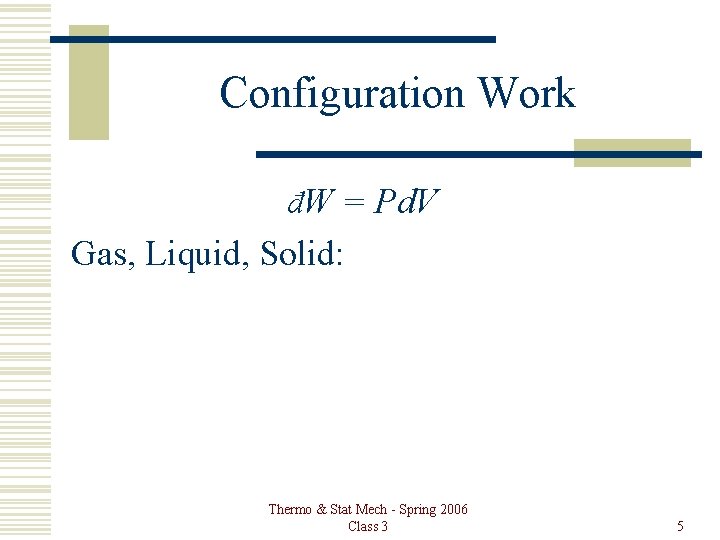
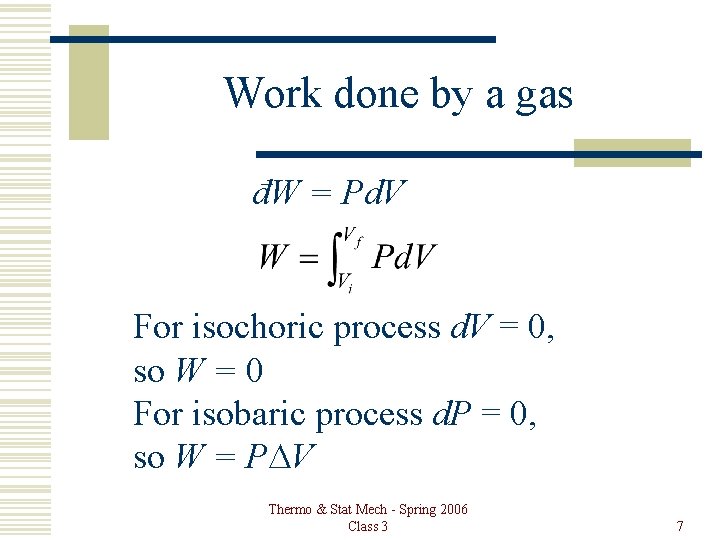
Configuration Work đW = Pd. V Gas, Liquid, Solid: Thermo & Stat Mech - Spring 2006 Class 3 5

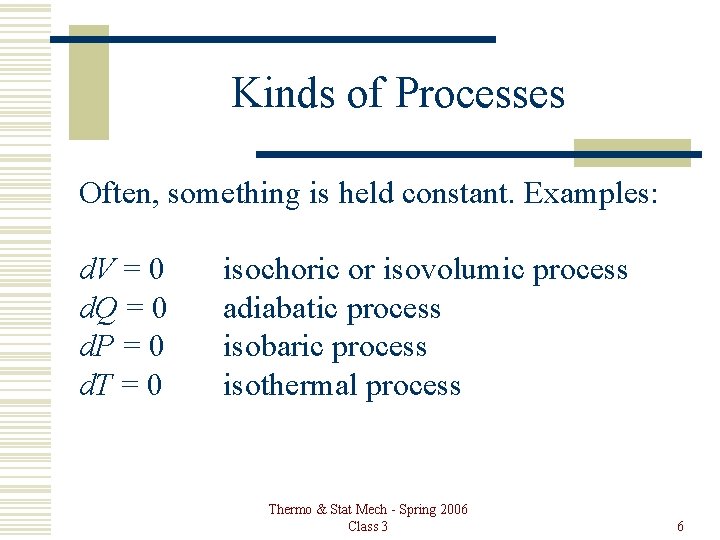
Kinds of Processes Often, something is held constant. Examples: d. V = 0 d. Q = 0 d. P = 0 d. T = 0 isochoric or isovolumic process adiabatic process isobaric process isothermal process Thermo & Stat Mech - Spring 2006 Class 3 6

Work done by a gas đW = Pd. V For isochoric process d. V = 0, so W = 0 For isobaric process d. P = 0, so W = PDV Thermo & Stat Mech - Spring 2006 Class 3 7

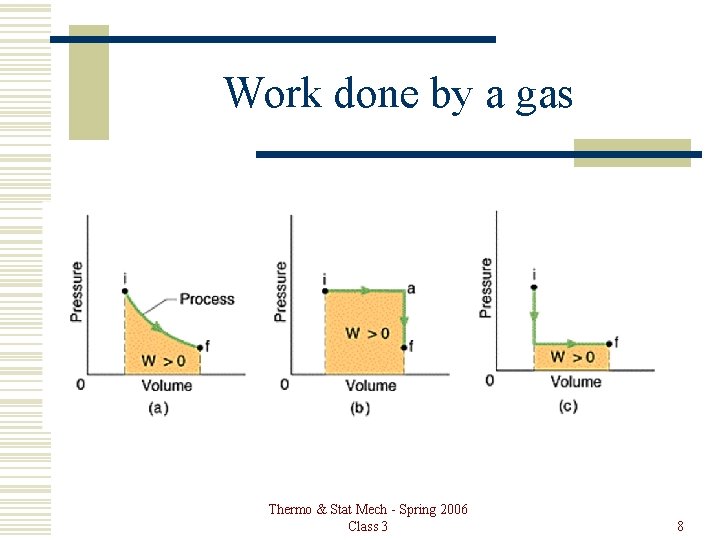
Work done by a gas Thermo & Stat Mech - Spring 2006 Class 3 8

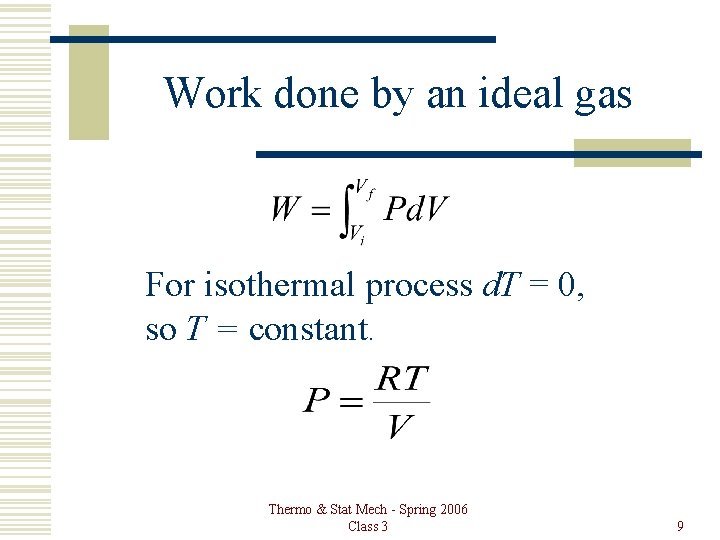
Work done by an ideal gas For isothermal process d. T = 0, so T = constant. Thermo & Stat Mech - Spring 2006 Class 3 9

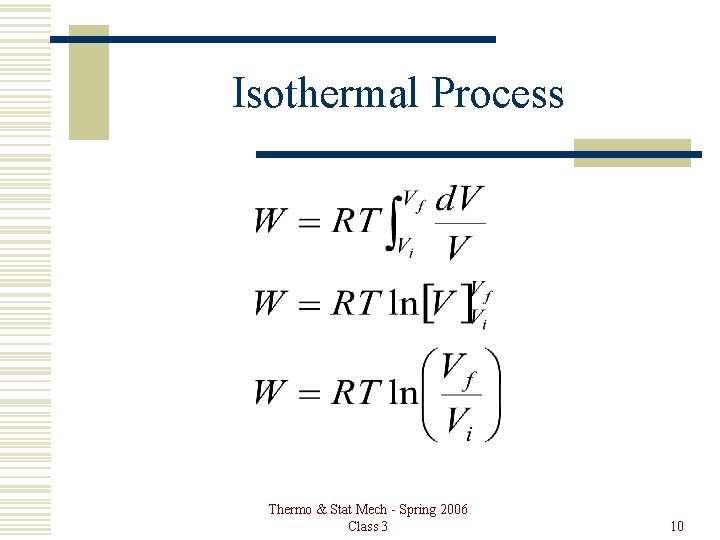
Isothermal Process Thermo & Stat Mech - Spring 2006 Class 3 10

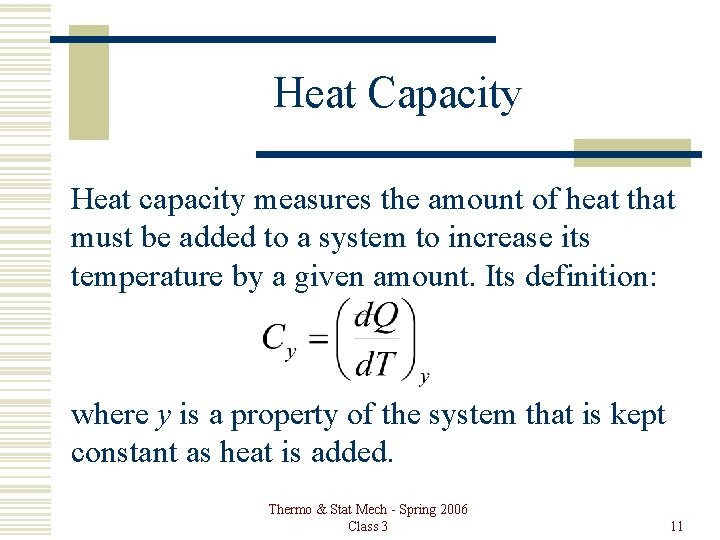
Heat Capacity Heat capacity measures the amount of heat that must be added to a system to increase its temperature by a given amount. Its definition: where y is a property of the system that is kept constant as heat is added. Thermo & Stat Mech - Spring 2006 Class 3 11

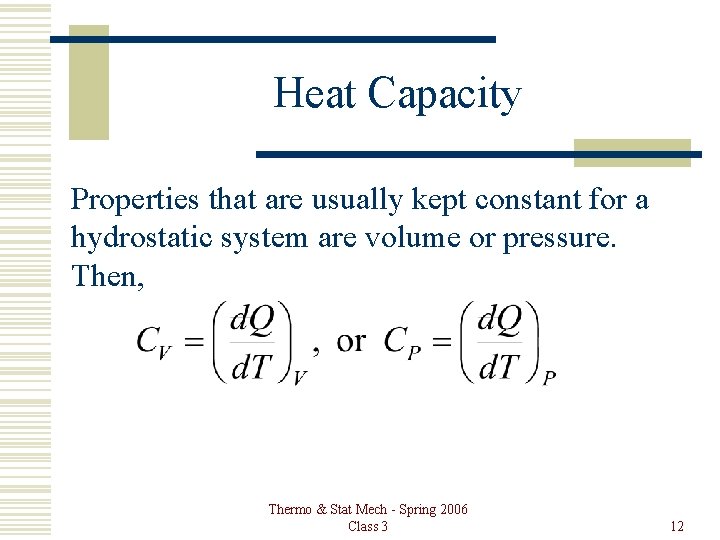
Heat Capacity Properties that are usually kept constant for a hydrostatic system are volume or pressure. Then, Thermo & Stat Mech - Spring 2006 Class 3 12

Heat Capacity Clearly, the heat capacity depends on the size of the system under consideration. To get rid of that effect, and have a heat capacity that depends only on the properties of the substance being studied, two other quantities are defined: specific heat capacity, and molar heat capacity. Thermo & Stat Mech - Spring 2006 Class 3 13

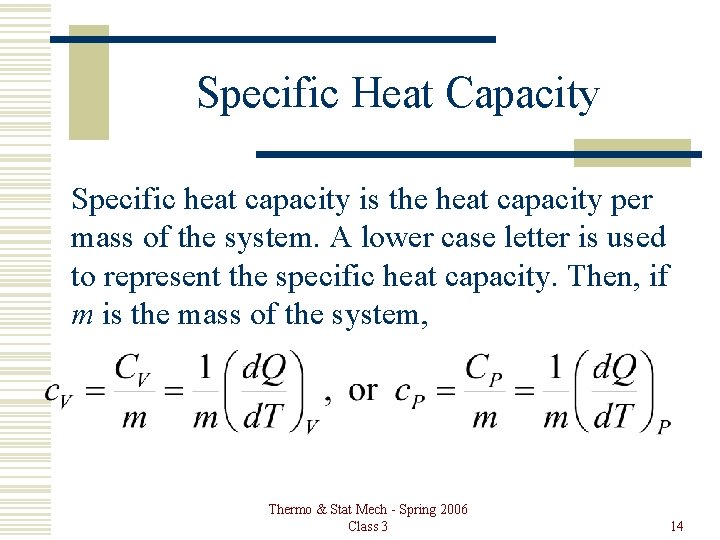
Specific Heat Capacity Specific heat capacity is the heat capacity per mass of the system. A lower case letter is used to represent the specific heat capacity. Then, if m is the mass of the system, Thermo & Stat Mech - Spring 2006 Class 3 14

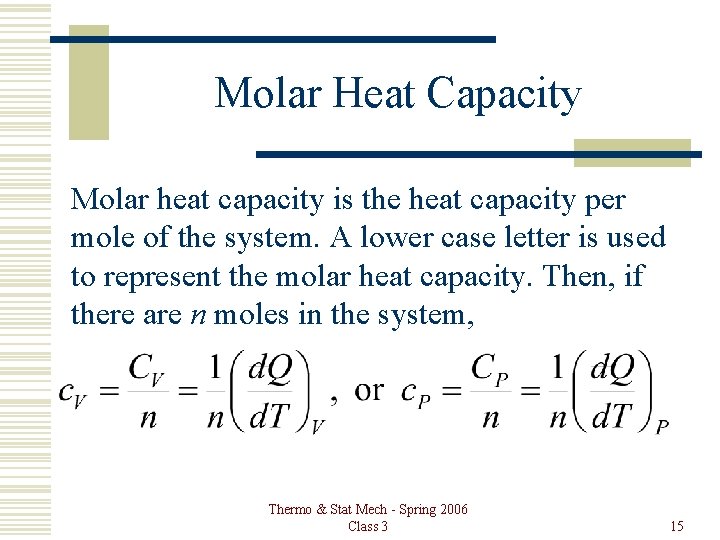
Molar Heat Capacity Molar heat capacity is the heat capacity per mole of the system. A lower case letter is used to represent the molar heat capacity. Then, if there are n moles in the system, Thermo & Stat Mech - Spring 2006 Class 3 15

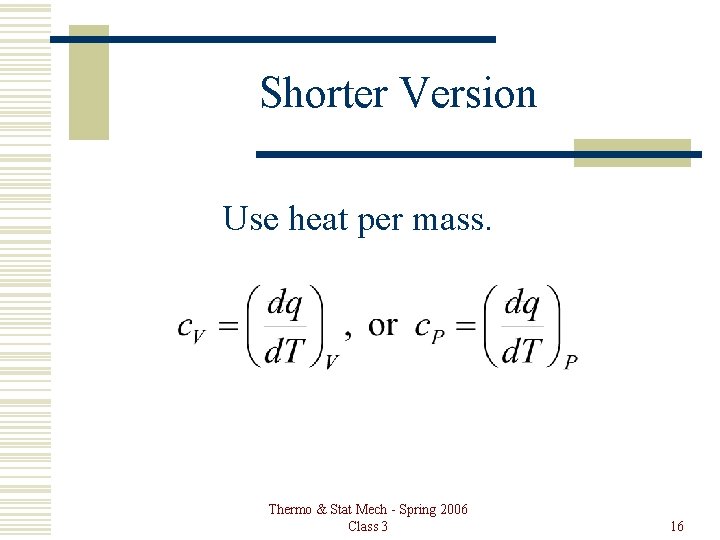
Shorter Version Use heat per mass. Thermo & Stat Mech - Spring 2006 Class 3 16

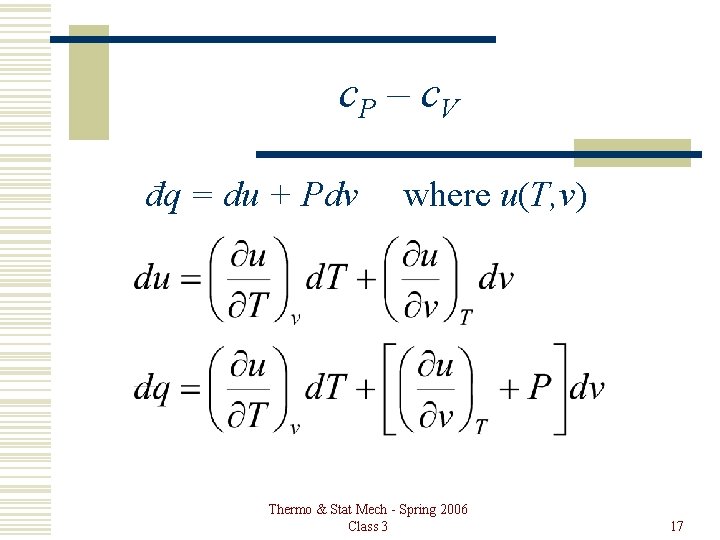
c. P – c. V đq = du + Pdv where u(T, v) Thermo & Stat Mech - Spring 2006 Class 3 17

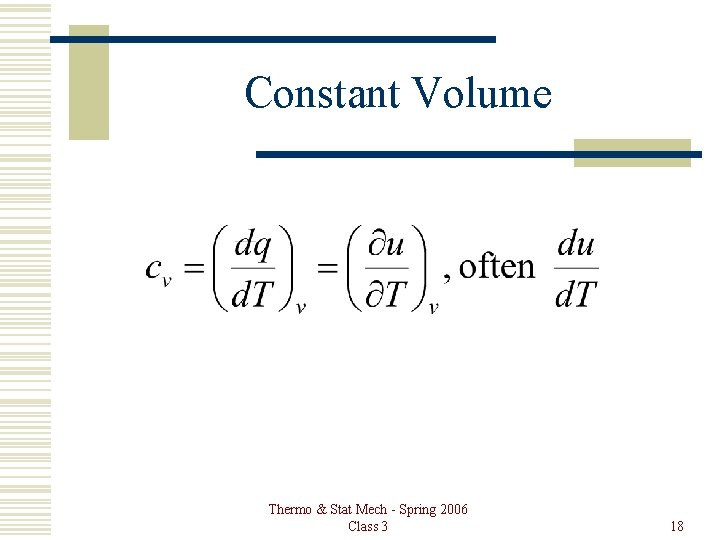
Constant Volume Thermo & Stat Mech - Spring 2006 Class 3 18

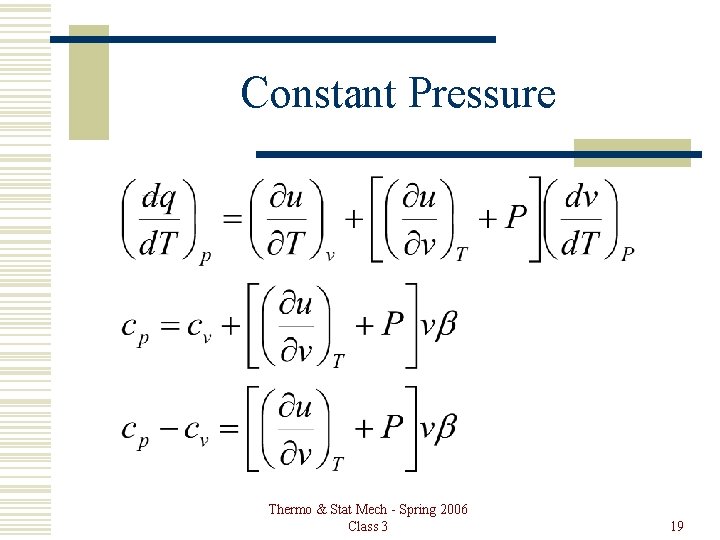
Constant Pressure Thermo & Stat Mech - Spring 2006 Class 3 19

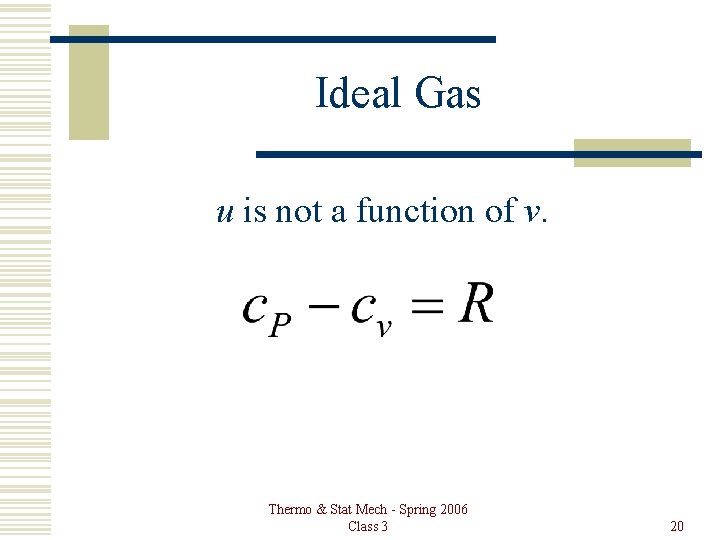
Ideal Gas u is not a function of v. Thermo & Stat Mech - Spring 2006 Class 3 20

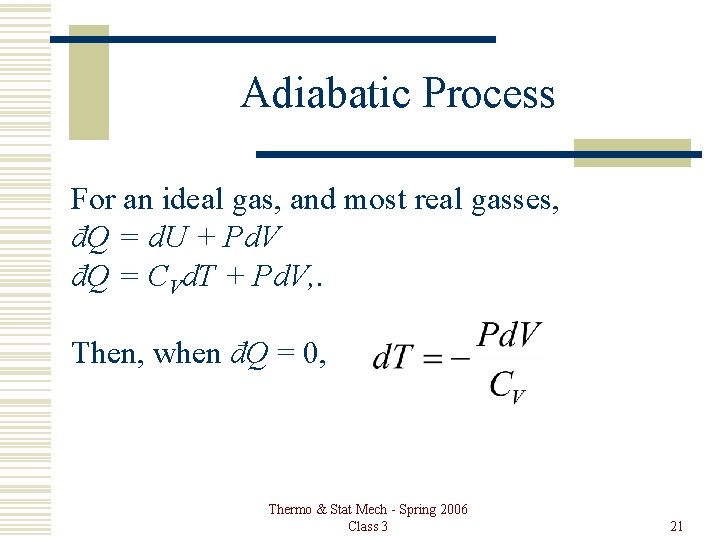
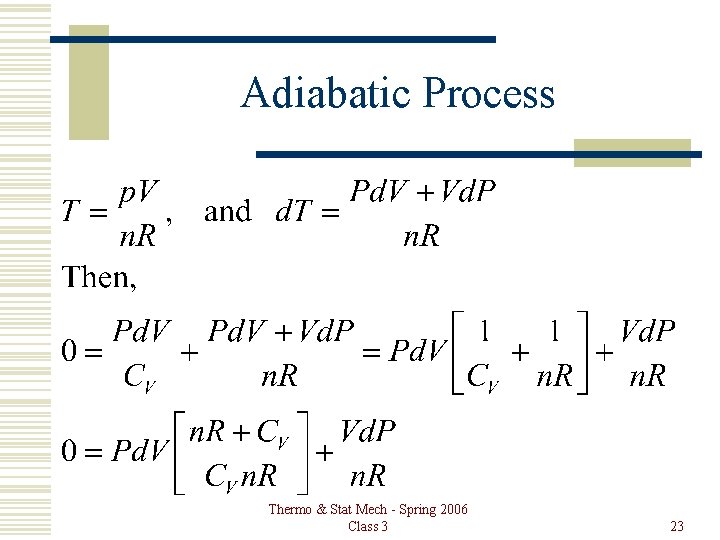
Adiabatic Process For an ideal gas, and most real gasses, đQ = d. U + Pd. V đQ = CVd. T + Pd. V, . Then, when đQ = 0, Thermo & Stat Mech - Spring 2006 Class 3 21

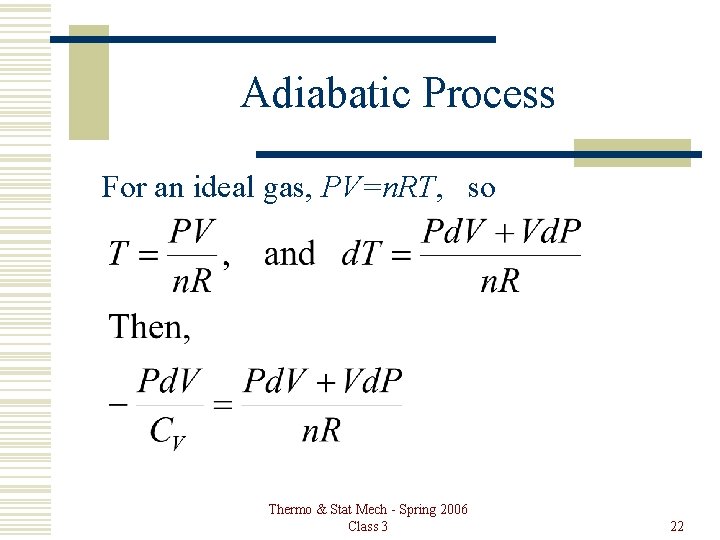
Adiabatic Process For an ideal gas, PV=n. RT, so Thermo & Stat Mech - Spring 2006 Class 3 22

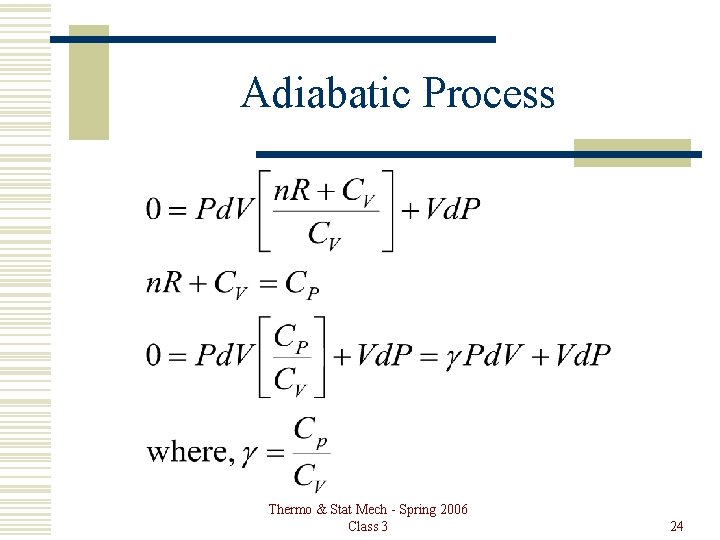
Adiabatic Process Thermo & Stat Mech - Spring 2006 Class 3 23

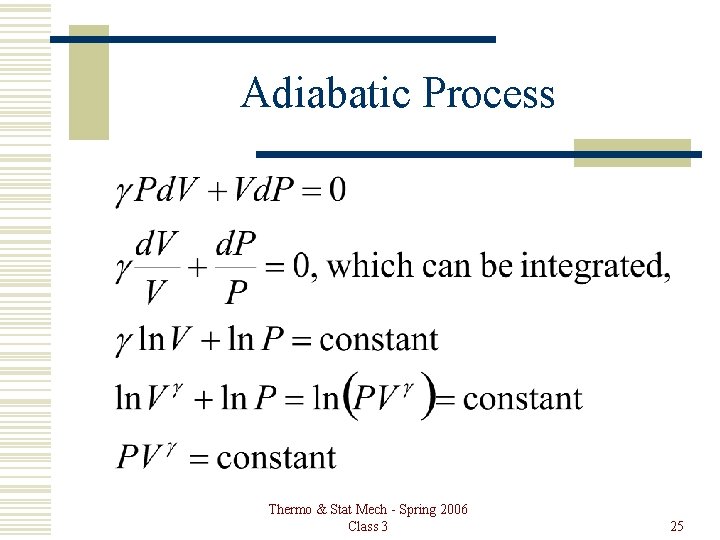
Adiabatic Process Thermo & Stat Mech - Spring 2006 Class 3 24

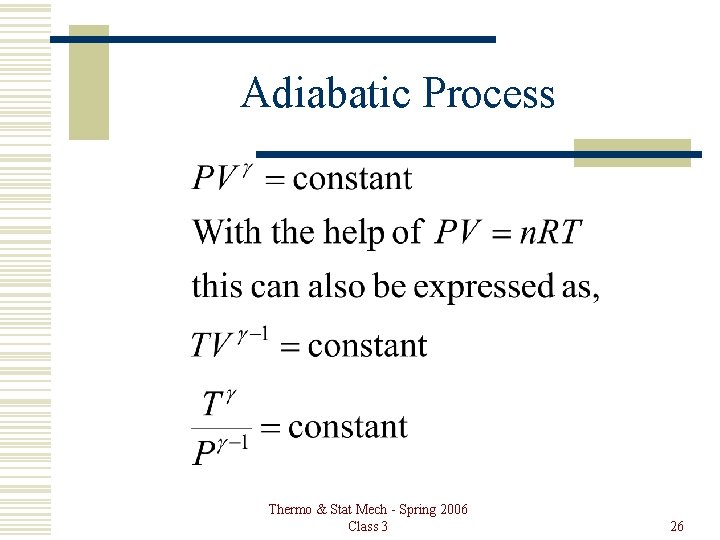
Adiabatic Process Thermo & Stat Mech - Spring 2006 Class 3 25

Adiabatic Process Thermo & Stat Mech - Spring 2006 Class 3 26

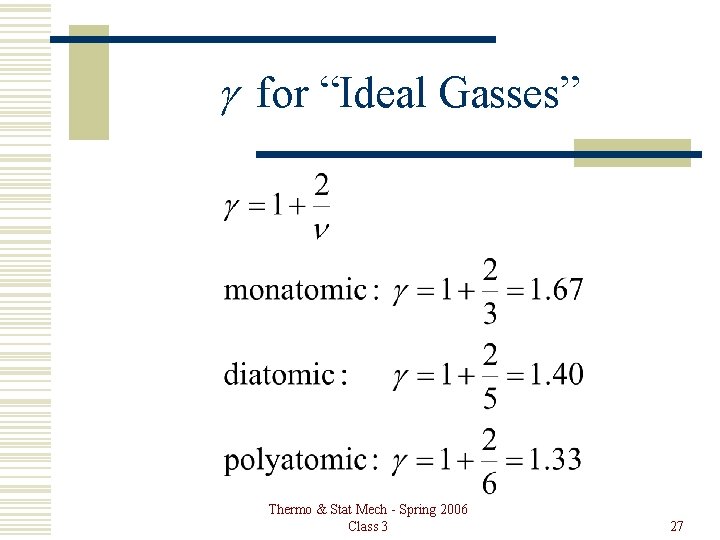
g for “Ideal Gasses” Thermo & Stat Mech - Spring 2006 Class 3 27
 Thermodynamics and statistical mechanics
Thermodynamics and statistical mechanics Thermodynamics and statistical mechanics
Thermodynamics and statistical mechanics Newton's first law and second law and third law
Newton's first law and second law and third law Si unit of newton's first law
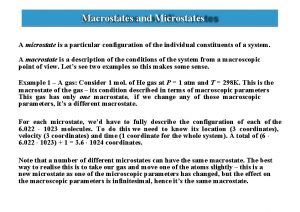
Si unit of newton's first law Microstate and macrostate
Microstate and macrostate Energy balance thermodynamics
Energy balance thermodynamics Isobaric process formula
Isobaric process formula 1th law of thermodynamics
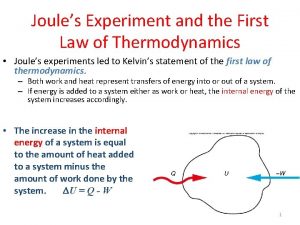
1th law of thermodynamics Joule's experiment first law of thermodynamics
Joule's experiment first law of thermodynamics Laws in thermodynamics
Laws in thermodynamics First law applied to flow process
First law applied to flow process First law of thermodynamics sign convention
First law of thermodynamics sign convention First law of thermodynamics control mass
First law of thermodynamics control mass First law of thermodynamics
First law of thermodynamics First law of thermodynamics for ideal gas
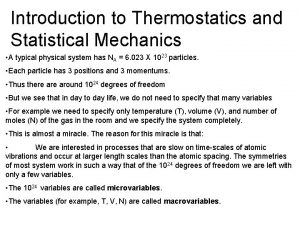
First law of thermodynamics for ideal gas Statistical thermodynamics in chemistry
Statistical thermodynamics in chemistry Statistical thermodynamics
Statistical thermodynamics Partition function in statistical mechanics
Partition function in statistical mechanics Statistical mechanics
Statistical mechanics Classical equipartition theorem
Classical equipartition theorem Partition function in statistical mechanics
Partition function in statistical mechanics Relation between partition function and internal energy
Relation between partition function and internal energy Partition function in statistical mechanics
Partition function in statistical mechanics Introduction to quantum statistical mechanics
Introduction to quantum statistical mechanics Statistical mechanics of deep learning
Statistical mechanics of deep learning Statistical mechanics
Statistical mechanics Boyle's law charles law avogadro's law
Boyle's law charles law avogadro's law Avogadro's law constants
Avogadro's law constants