Laws of Thermodynamics First Law of Thermodynamics First










- Slides: 26

Laws of Thermodynamics

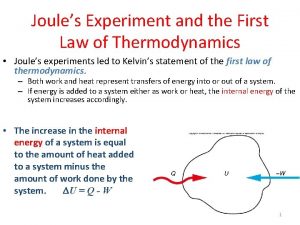
First Law of Thermodynamics • First law of thermodynamics states that energy cannot be created or destroyed it can only be transferred or transformed. • The total energy in any isolated system, such as the universe is constant. • All that can happen is the form the energy takes changes. • *This law is often called the law of conservation of energy.


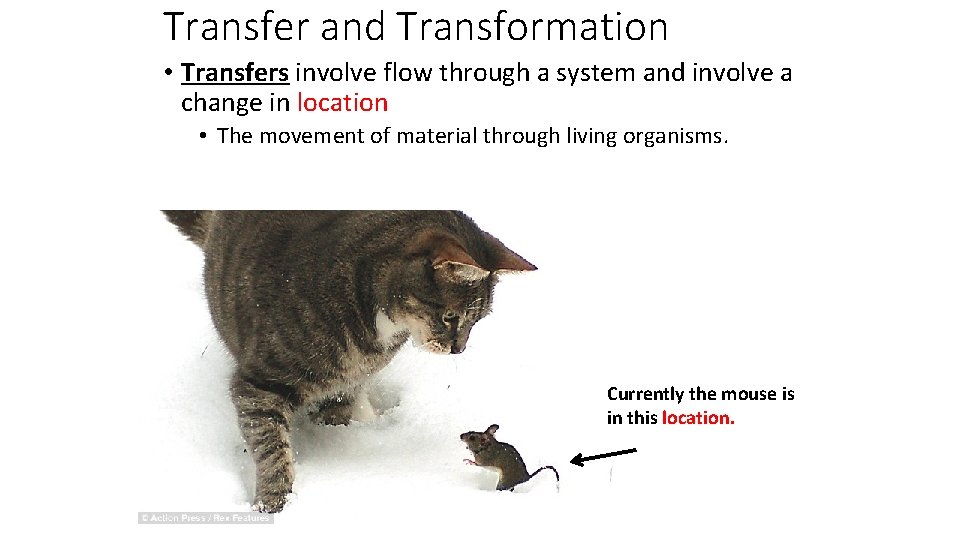
Transfer and Transformation • Transfers involve flow through a system and involve a change in location • The movement of material through living organisms. Currently the mouse is in this location.

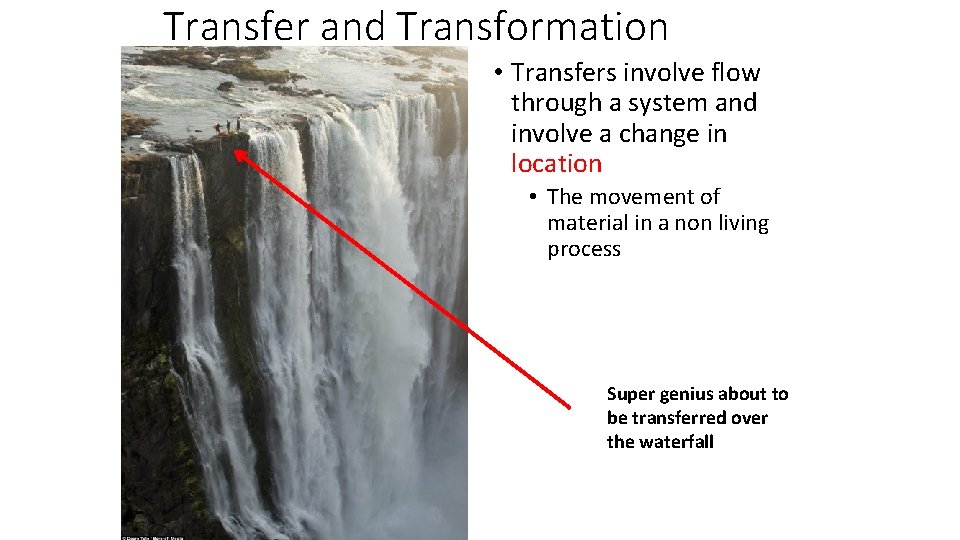
Transfer and Transformation • Transfers involve flow through a system and involve a change in location • The movement of material in a non living process Super genius about to be transferred over the waterfall

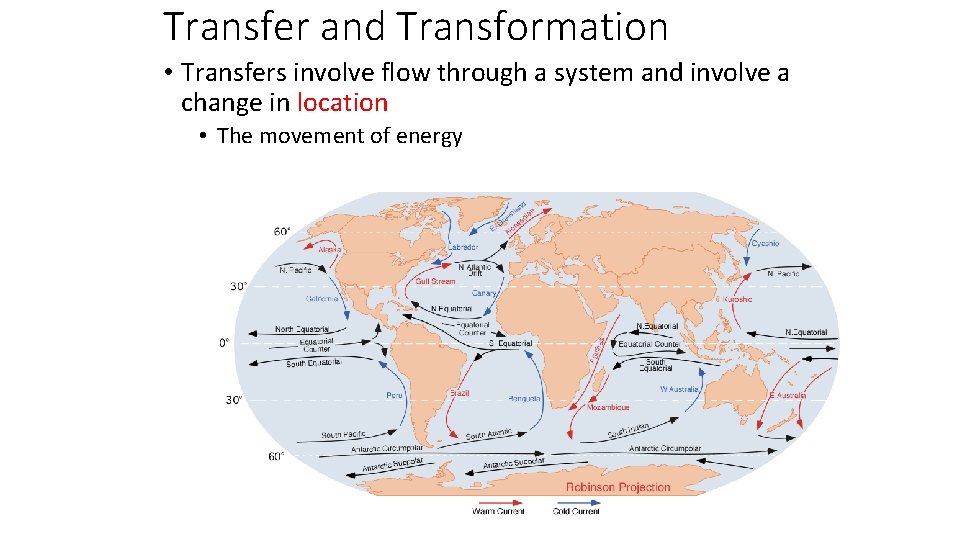
Transfer and Transformation • Transfers involve flow through a system and involve a change in location • The movement of energy Currently the mouse is in this location.

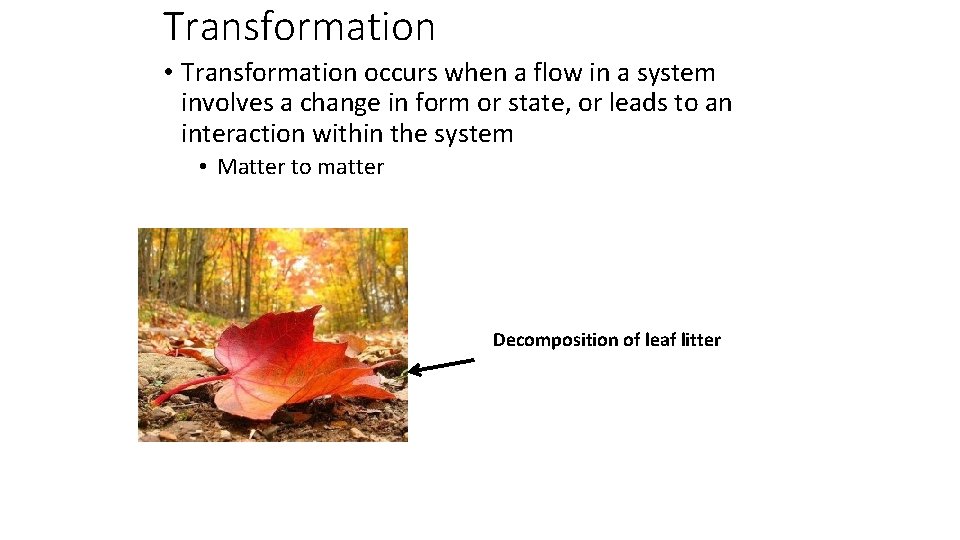
Transformation • Transformation occurs when a flow in a system involves a change in form or state, or leads to an interaction within the system • Matter to matter Decomposition of leaf litter

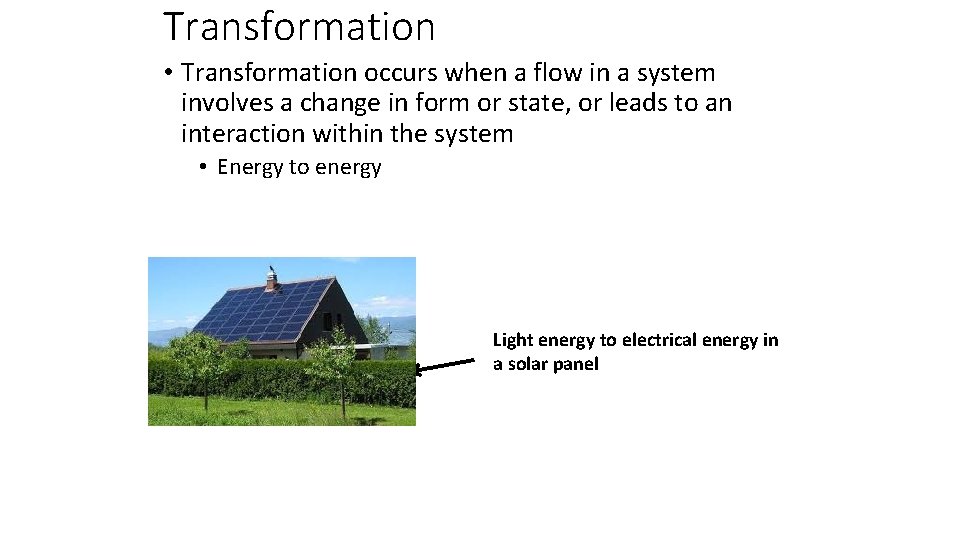
Transformation • Transformation occurs when a flow in a system involves a change in form or state, or leads to an interaction within the system • Energy to energy Light energy to electrical energy in a solar panel

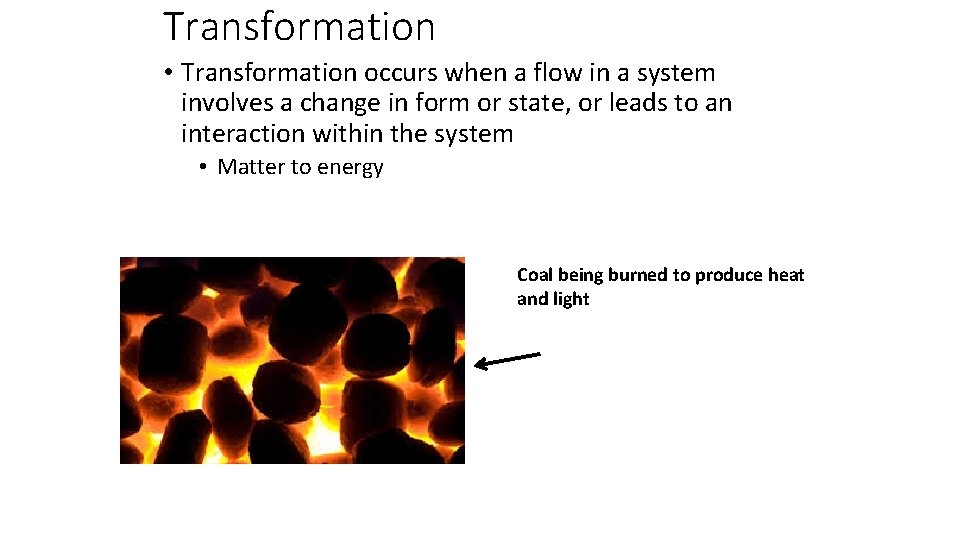
Transformation • Transformation occurs when a flow in a system involves a change in form or state, or leads to an interaction within the system • Matter to energy Coal being burned to produce heat and light

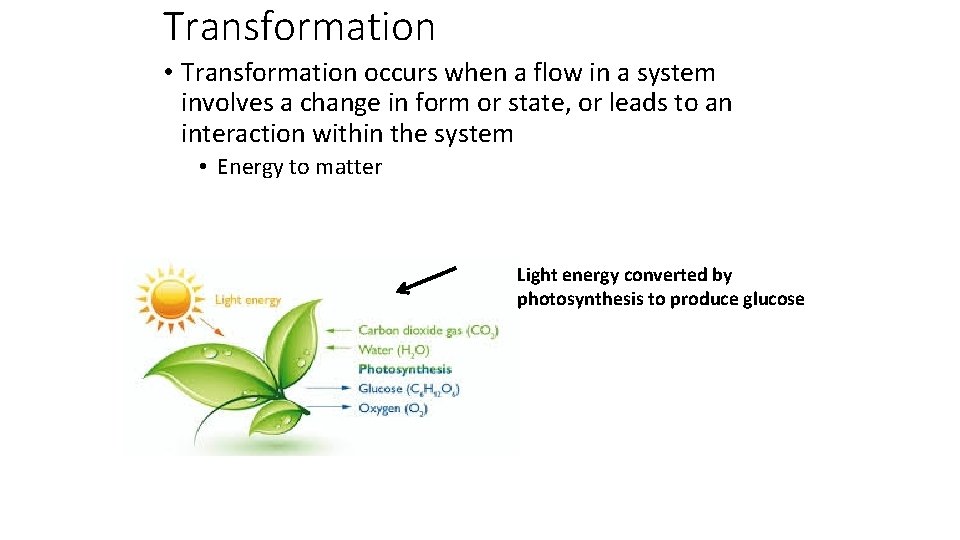
Transformation • Transformation occurs when a flow in a system involves a change in form or state, or leads to an interaction within the system • Energy to matter Light energy converted by photosynthesis to produce glucose

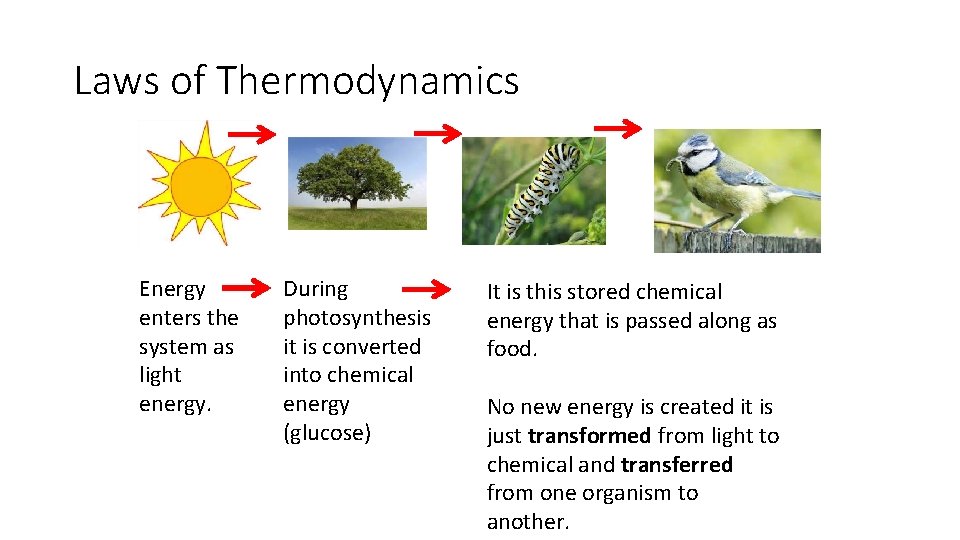
Laws of Thermodynamics Energy enters the system as light energy. During photosynthesis it is converted into chemical energy (glucose) It is this stored chemical energy that is passed along as food. No new energy is created it is just transformed from light to chemical and transferred from one organism to another.

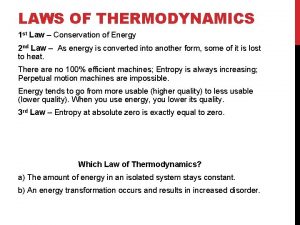
Second Law of Thermodynamics • At each stage in the transfer process, some energy is transformed into other forms including heat. • Energy leaves a system as heat. • Energy transfer and transformation are not very efficient. • Less than 10% of useable energy is transferred from one organism to the next.

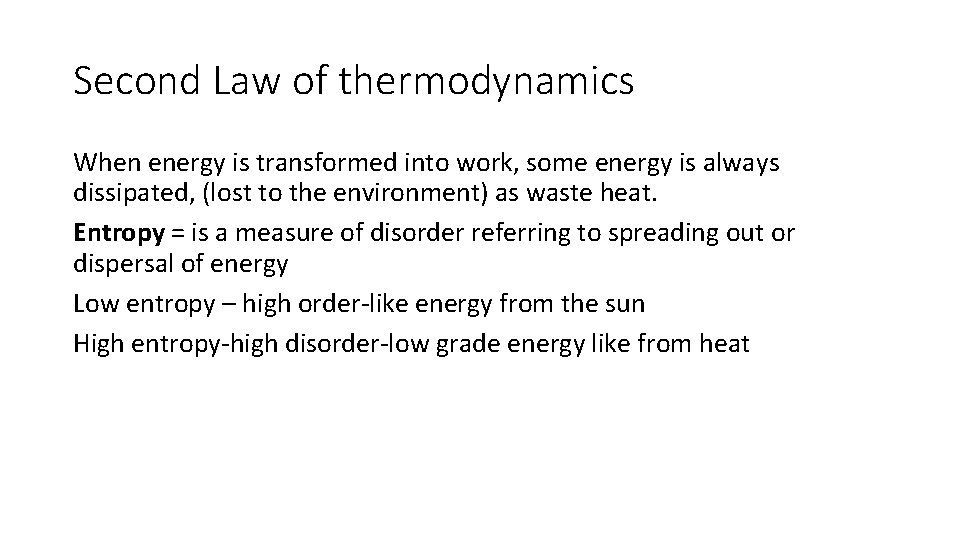
Second Law of thermodynamics When energy is transformed into work, some energy is always dissipated, (lost to the environment) as waste heat. Entropy = is a measure of disorder referring to spreading out or dispersal of energy Low entropy – high order-like energy from the sun High entropy-high disorder-low grade energy like from heat

• Life is a battle against entropy • We constantly have to maintain ourselves so our bodies can be orderly. • Without constant replenishment of energy life can not exist

High entropy High disorder Low entropy Low disorder Energy required to maintain yard

High entropy or low entropy?

High entropy or low entropy?


Energy transfer and transformation are not very efficient Efficiency transfers: Converting solar energy into chemical energy 1 -2%

Efficiency transfers • Plants giving herbivores energy they only assimilate 10% of the total plants energy

Efficiency transfers • Carnivore’s efficiency is also around 10%.

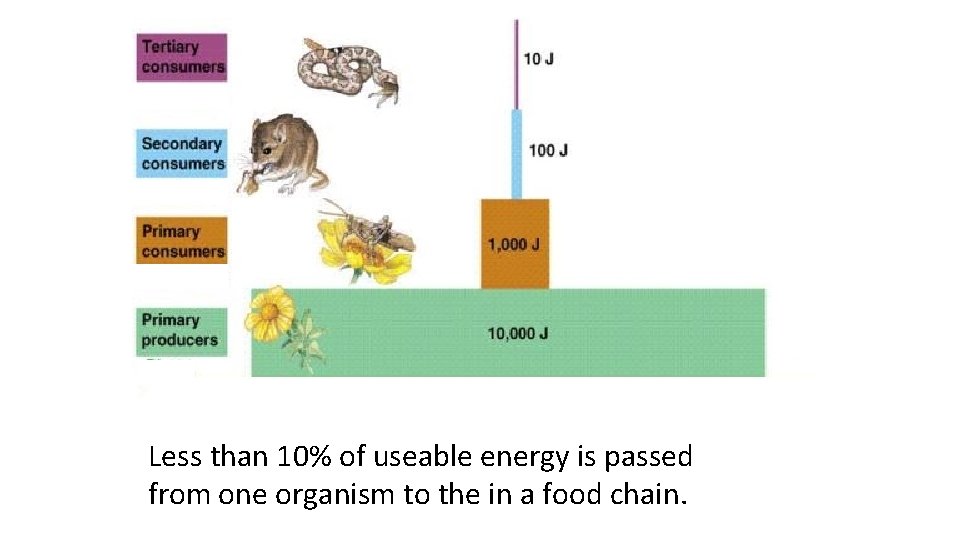
Less than 10% of useable energy is passed from one organism to the in a food chain.

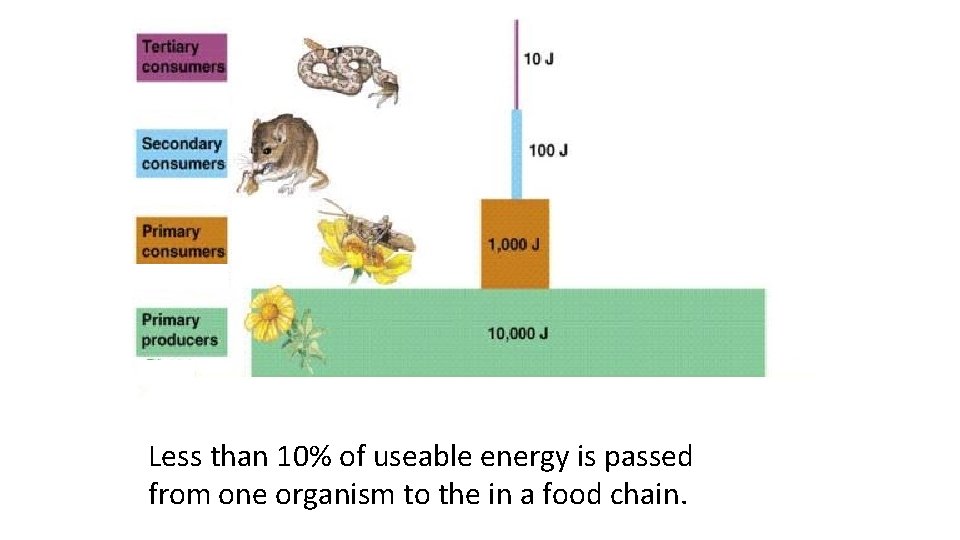
Less than 10% of useable energy is passed from one organism to the in a food chain.

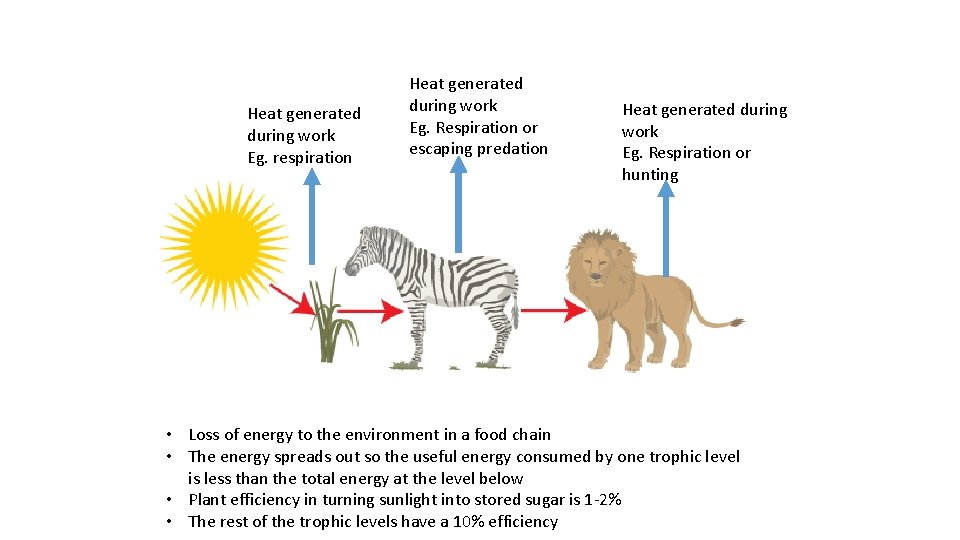
Heat generated during work Eg. respiration Heat generated during work Eg. Respiration or escaping predation Heat generated during work Eg. Respiration or hunting • Loss of energy to the environment in a food chain • The energy spreads out so the useful energy consumed by one trophic level is less than the total energy at the level below • Plant efficiency in turning sunlight into stored sugar is 1 -2% • The rest of the trophic levels have a 10% efficiency

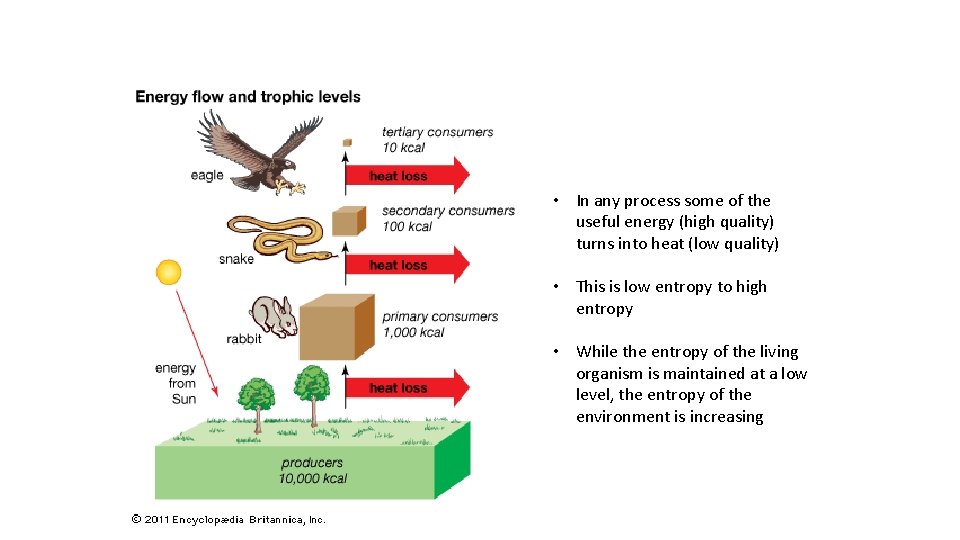
• In any process some of the useful energy (high quality) turns into heat (low quality) • This is low entropy to high entropy • While the entropy of the living organism is maintained at a low level, the entropy of the environment is increasing

 Newton's first law and second law and third law
Newton's first law and second law and third law Si unit of newton's first law
Si unit of newton's first law First law of thermodynamics in open system
First law of thermodynamics in open system Isobaric process formula
Isobaric process formula First law of thermodynamics cyclic process
First law of thermodynamics cyclic process The joule experiment
The joule experiment Laws in thermodynamics
Laws in thermodynamics First law of thermodynamics for steady state flow process
First law of thermodynamics for steady state flow process First law of thermodynamics sign convention
First law of thermodynamics sign convention Enthalpy vs internal energy
Enthalpy vs internal energy First law of thermodynamics
First law of thermodynamics First law of thermodynamics for ideal gas
First law of thermodynamics for ideal gas Laws of thermodynamics simple
Laws of thermodynamics simple Law of thermodynamics
Law of thermodynamics Pros about coal
Pros about coal Laws thermodynamics
Laws thermodynamics Zeroth law of thermodynamics statement
Zeroth law of thermodynamics statement Thermodynamics laws
Thermodynamics laws 3 laws of thermodynamics
3 laws of thermodynamics Charles de secondat
Charles de secondat Boyles law
Boyles law Constant of avogadro's law
Constant of avogadro's law Zeroth law of thermodynamics
Zeroth law of thermodynamics Newton's third law of thermodynamics
Newton's third law of thermodynamics Second law of thermodynamics
Second law of thermodynamics State second law of thermodynamics
State second law of thermodynamics Second law of thermodynamics
Second law of thermodynamics