Chapter Five First Law of Thermodynamics 1 First










- Slides: 27

Chapter Five: First Law of Thermodynamics 1

First Law of thermodynamics States that: “Energy cannot be created or destroyed BUT it can be changed from one form to another” 2

Therefore: • The first law of thermodynamics is an expression of the conservation of energy principle. • Energy can cross the boundaries of a closed system in the form of heat or work. 3

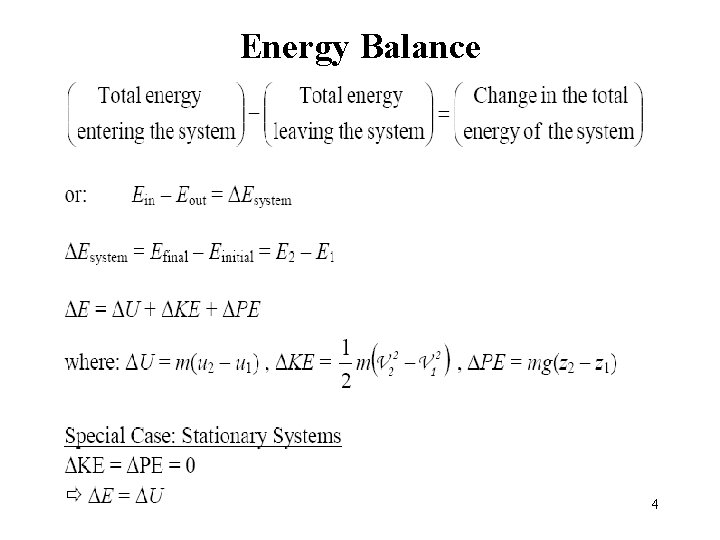
Energy Balance 4

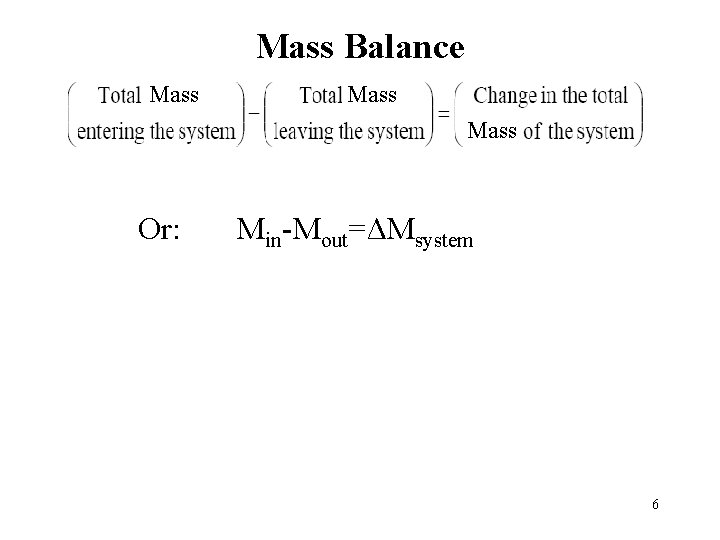
Conservation of Mass “Mass cannot be created or destroyed BUT it can be changed from one form to another” 5

Mass Balance Mass M M Or: Min-Mout=ΔMsystem 6

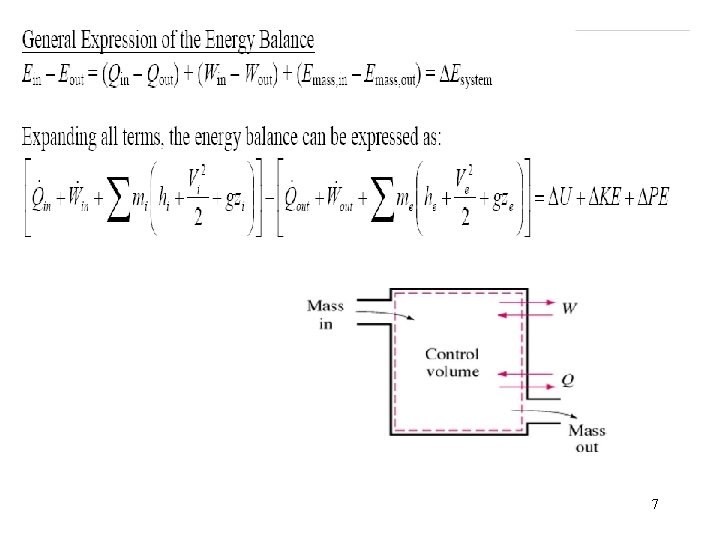
7

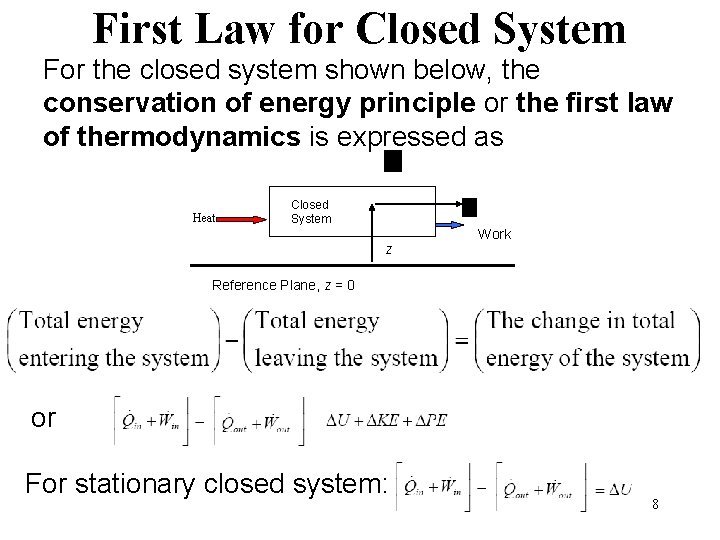
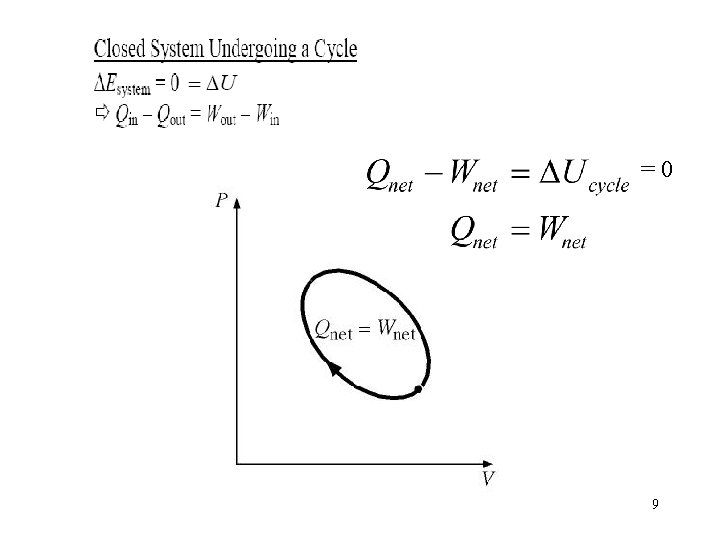
First Law for Closed System For the closed system shown below, the conservation of energy principle or the first law of thermodynamics is expressed as Heat Closed System z Work Reference Plane, z = 0 or For stationary closed system: 8

=0 9

Tutorials 1 st Law of thermodynamics 10

Problem 1 • On a hot summer day, a student turns his fan on when he leaves his room in the morning. When he returns in the evening, will the room be warmer or cooler than the neighboring rooms? Why? Assume all the doors and windows are kept closed. 11

Answer • Warmer. Because energy is added to the room air in the form of electrical work. 12

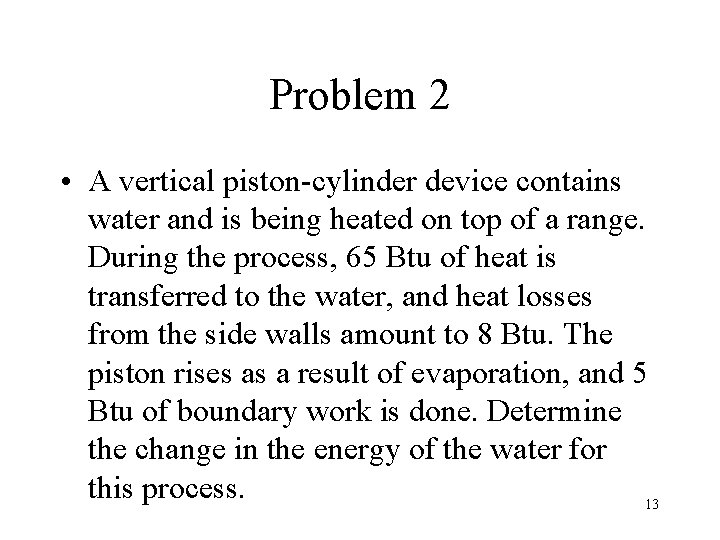
Problem 2 • A vertical piston-cylinder device contains water and is being heated on top of a range. During the process, 65 Btu of heat is transferred to the water, and heat losses from the side walls amount to 8 Btu. The piston rises as a result of evaporation, and 5 Btu of boundary work is done. Determine the change in the energy of the water for this process. 13

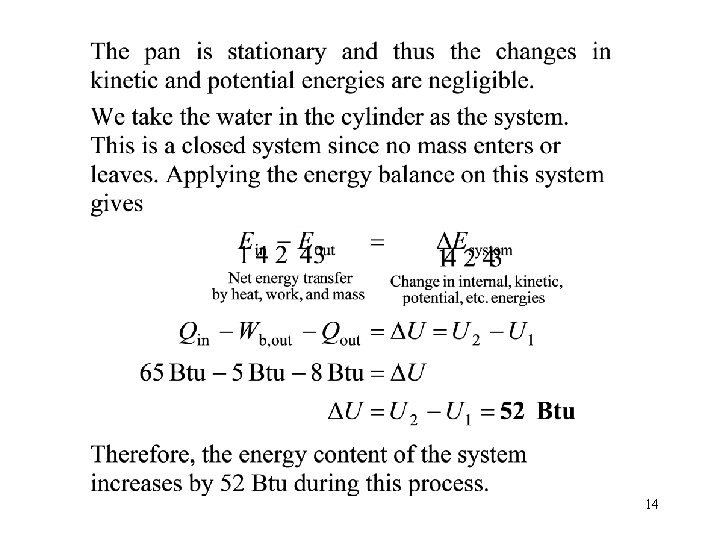
14

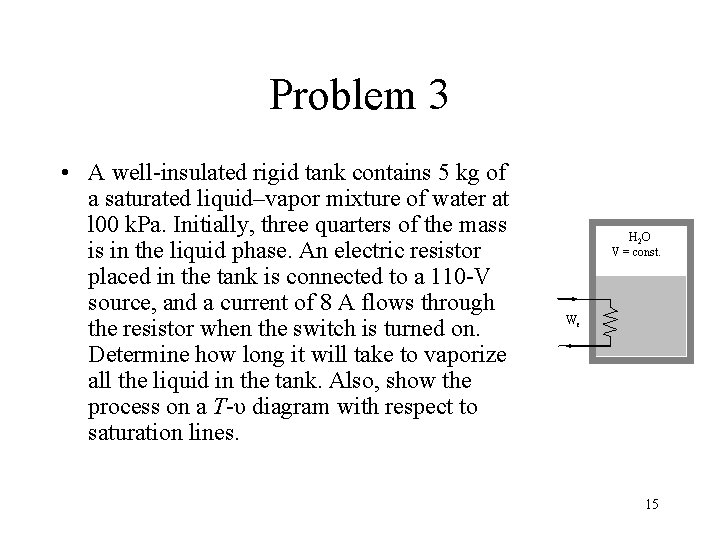
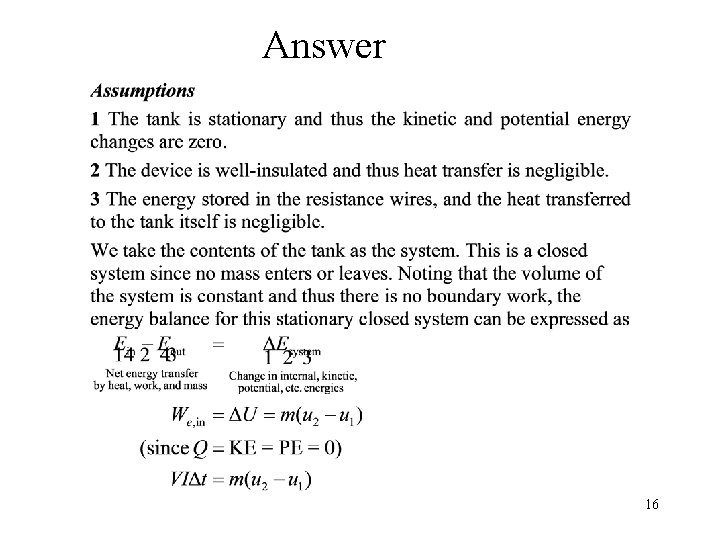
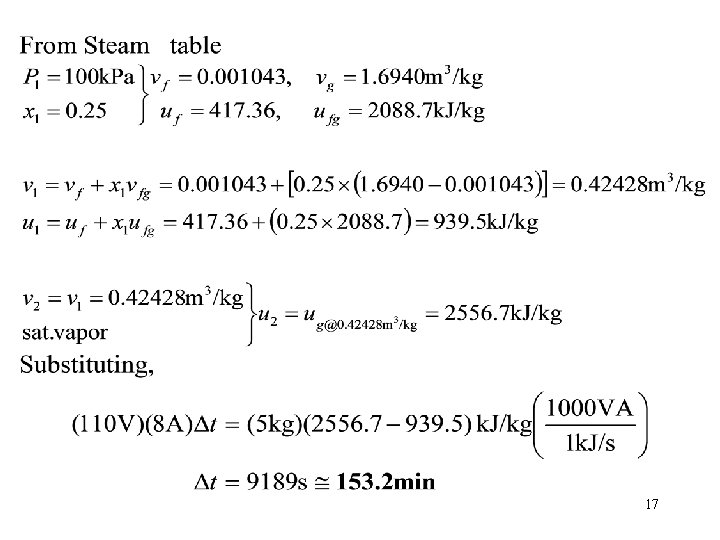
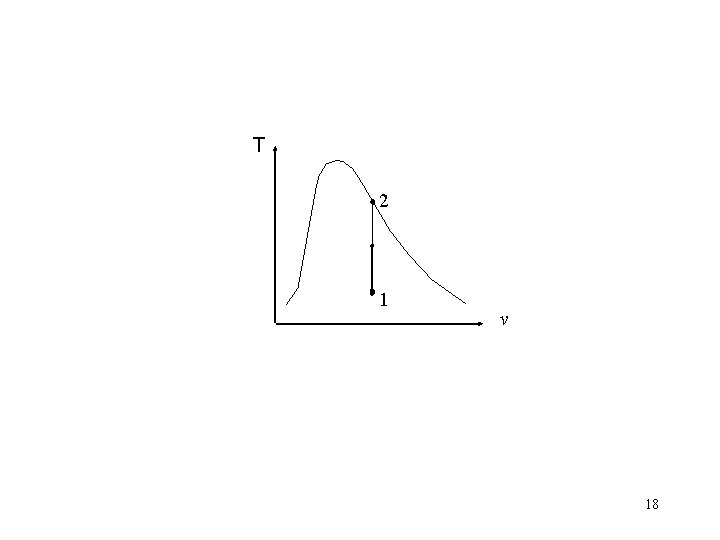
Problem 3 • A well-insulated rigid tank contains 5 kg of a saturated liquid–vapor mixture of water at l 00 k. Pa. Initially, three quarters of the mass is in the liquid phase. An electric resistor placed in the tank is connected to a 110 -V source, and a current of 8 A flows through the resistor when the switch is turned on. Determine how long it will take to vaporize all the liquid in the tank. Also, show the process on a T-υ diagram with respect to saturation lines. H 2 O V = const. We 15

Answer 16

17

T 2 1 v 18

Problem 4 • Is it possible to compress an ideal gas isothermally in an adiabatic piston-cylinder device? Explain. 19

Answer • No, it isn't. This is because the first law relation Q - W = U reduces to W = 0 in this case since the system is adiabatic (Q = 0) and U = 0 for the isothermal processes of ideal gases. Therefore, this adiabatic system cannot receive any net work at constant temperature. 20

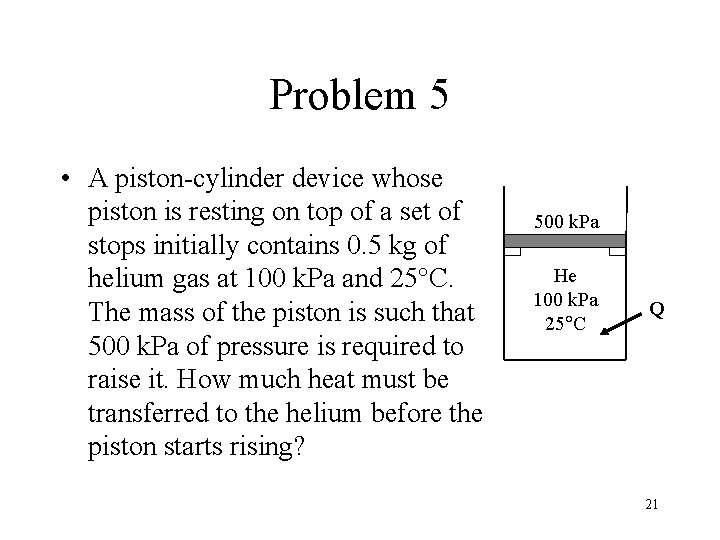
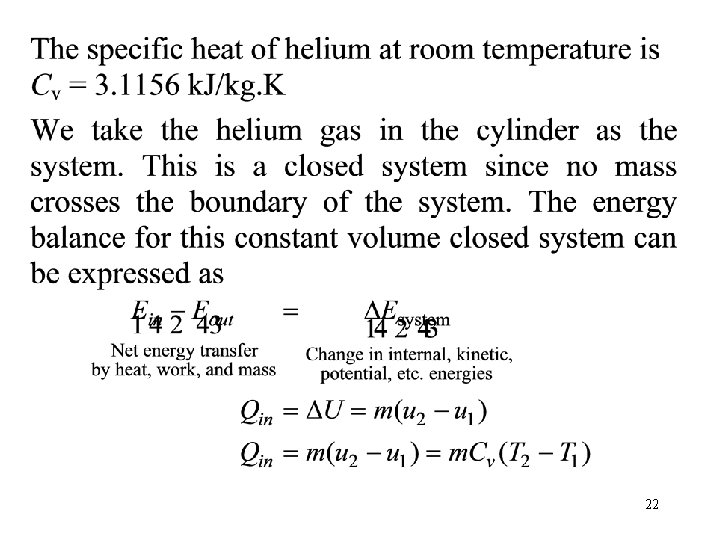
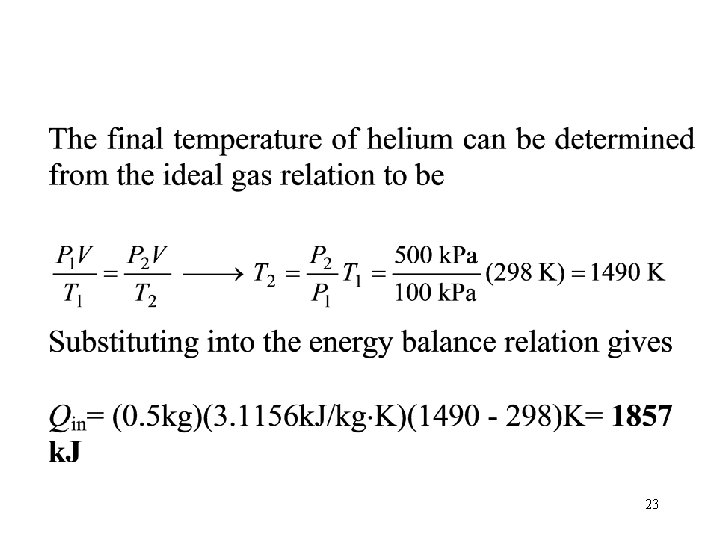
Problem 5 • A piston-cylinder device whose piston is resting on top of a set of stops initially contains 0. 5 kg of helium gas at 100 k. Pa and 25°C. The mass of the piston is such that 500 k. Pa of pressure is required to raise it. How much heat must be transferred to the helium before the piston starts rising? 500 k. Pa He 100 k. Pa 25 C Q 21

22

23

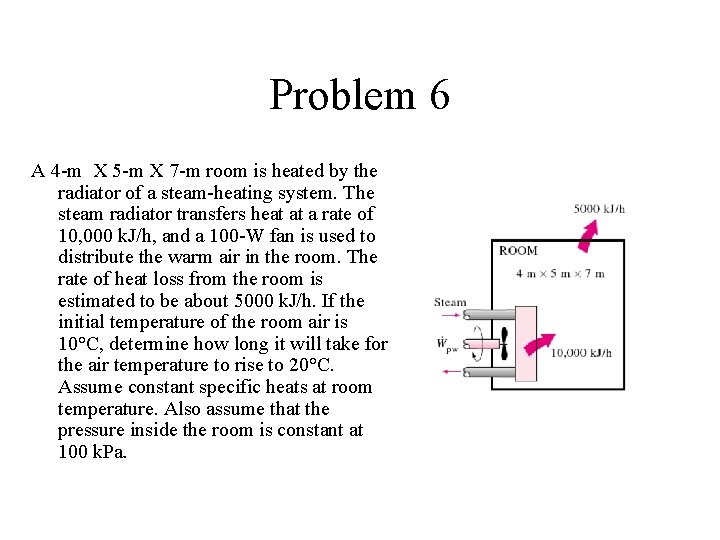
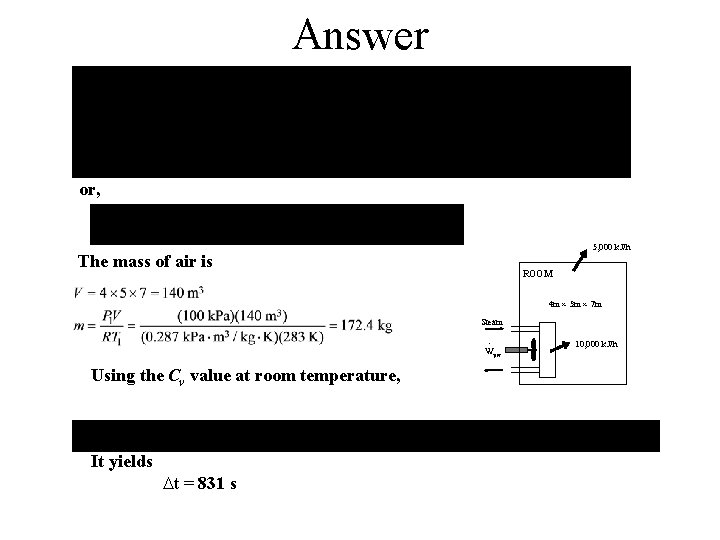
Problem 6 A 4 -m X 5 -m X 7 -m room is heated by the radiator of a steam-heating system. The steam radiator transfers heat at a rate of 10, 000 k. J/h, and a 100 -W fan is used to distribute the warm air in the room. The rate of heat loss from the room is estimated to be about 5000 k. J/h. If the initial temperature of the room air is 10°C, determine how long it will take for the air temperature to rise to 20°C. Assume constant specific heats at room temperature. Also assume that the pressure inside the room is constant at 100 k. Pa.

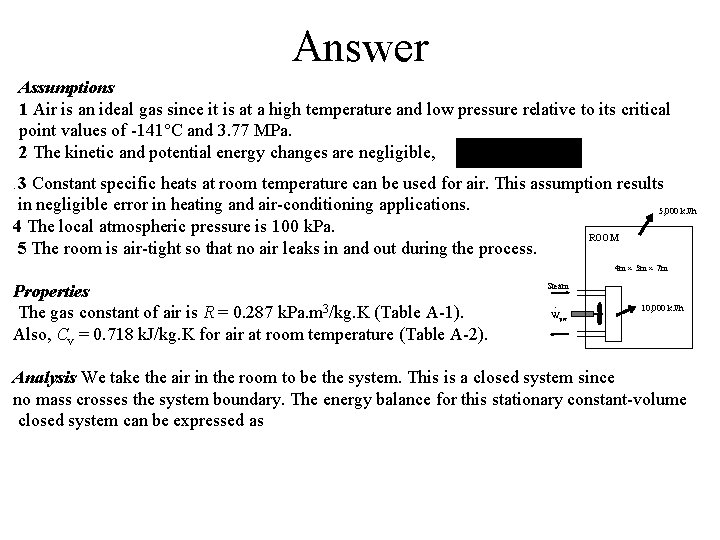
Answer Assumptions 1 Air is an ideal gas since it is at a high temperature and low pressure relative to its critical point values of -141 C and 3. 77 MPa. 2 The kinetic and potential energy changes are negligible, 3 Constant specific heats at room temperature can be used for air. This assumption results in negligible error in heating and air-conditioning applications. 5, 000 k. J/h 4 The local atmospheric pressure is 100 k. Pa. ROOM 5 The room is air-tight so that no air leaks in and out during the process. . 4 m 5 m 7 m Properties The gas constant of air is R = 0. 287 k. Pa. m 3/kg. K (Table A-1). Also, Cv = 0. 718 k. J/kg. K for air at room temperature (Table A-2). Steam · Wpw 10, 000 k. J/h Analysis We take the air in the room to be the system. This is a closed system since no mass crosses the system boundary. The energy balance for this stationary constant-volume closed system can be expressed as

Answer or, 5, 000 k. J/h The mass of air is ROOM 4 m 5 m 7 m Steam · Wpw Using the Cv value at room temperature, It yields t = 831 s 10, 000 k. J/h

End of Chapter Five 27