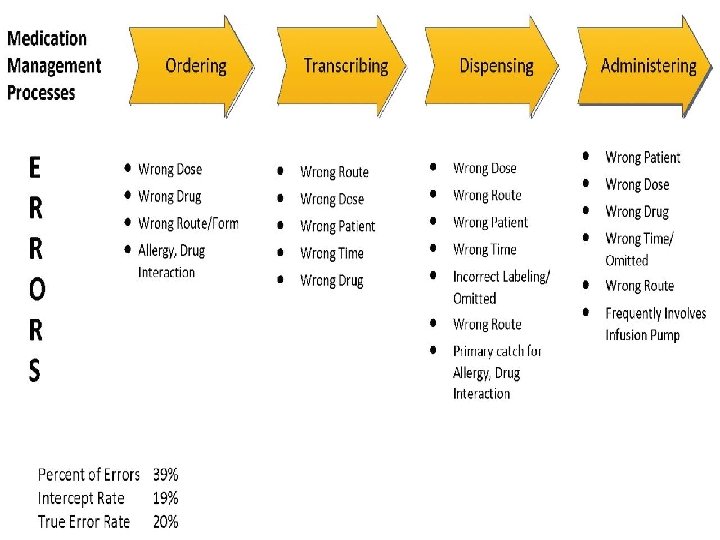
Errors of Omission Failure to counsel the patient









































- Slides: 47

Errors of Omission • Failure to counsel the patient • Failure to screen for interactions and contraindications

Mistakes and Slips • Mistake – Do things intentionally but actions are incorrect because of a knowledge or judgment deficit • Behavior in problem solving mode • Example: dose prescribed that exceeds maximum safe limit • Slip – Do things unintentionally incorrect because of an attention deficit • Behavior in automatic mode • Example: dispense chlorpromazine when prescription was clearly written for chlorpropamide

Dispensing Errors: Improving Workload • Ensure adequate staffing levels • Eliminate dispensing time limits (quotas) • Examples of limiting workload – Dispense ≤ 150 prescriptions per pharmacist per day – Require rest breaks every 2– 3 hours – Brief warm-up period before restarting work tasks – Require 30 -minute meal breaks

Dispensing Errors: Combating Distractions • Phones – Fax machines, auto refill, voice mail, priority processing, trained support personnel • Prohibit distractions during critical prescription-filling functions • Centralized filling operations • Train support personnel to answer the telephone

Dispensing Errors in the Work Area • Clutter (return used containers immediately) – Ensure adequate space – Store products with label facing forward – Choose high-use items on the basis of safety as well as convenience, use original containers – Telephone placement • Poor ergonomics • Lighting • Heat, humidity • Noise (TV, radio)

Dispensing Errors in the Work Area • Labels on bins and shelves – Failure mode: bin label may decrease chance that the actual product label will be checked when selected from bin; using bar codes will decrease chance of error • Separate by route of administration (external/injectable, etc. ) • Use auxiliary labels for externals – Amoxicillin oral suspension for ear infection thought by parents to be drops administered in child’s ear • Review published safety alerts for look-alike/ sound-alike drugs and frequent dispensing errors

Cognitive and Social Factors • Use of high-intensity task lights and magnification • Use of a device to hold prescriptions/orders at eye level • Posting alerts in strategic locations with error-prone products • Use of exaggerated, unconventional type fonts to enhance reading of drug names

Well-Designed Drug Storage • Adequate space • Label facing forward • Agents for external use should never be stored with oral medications • Separate by route of administration • Mark and/or isolate high-alert drugs • Separate sound-alike/look-alike drugs

Errors Related to Information About the Drug or Patient • Misleading or erroneous references • Ambiguity in handwritten and typed documents • Computerized prescribing • Wrong patient errors • Errors in dosage

Computerized Prescribing Errors • Computerized prescriber order entry (CPOE) improves communication and reduces some types of errors • However, this technology may have its own pitfalls: – Lower case L may look like the numeral 1 – Letter O may look like the numeral 0 (zero) – Letter Z and the numeral 2 may be misread – Wrong patient or wrong drug chosen from list

Computerized Alerts • Computer systems can be configured to flash maximum dose alerts and other safety alerts • Upgrades are necessary and usually available from software vendors

Errors in Dosage • Mathematical errors and decimal point misplacement are common causes of errors, especially in conversions between micrograms and milligrams • Oral liquid medications can be dispensed improperly because of misunderstandings with reading and labeling of oral syringes or use of such devices by parents of pediatric patients

Dispensing Errors Caused by Poor Labeling • Pharmacy computer-generated labeling and production of medication administration records should be optimized • Nonessential information should be excluded from labels and reports • Samples may be poorly labeled

Syringe and Admixture Labels • Standardization of the way labels are placed on syringes can reduce errors • Use of “For Oral Use Only” labels on oral syringes • Placement of labels on IV bags • Warning labels for special parenterals – Vinca alkaloids, other antineoplastics – Medications with specific infusion rates

Inpatient Oral Medication Label Format: Minimum Content

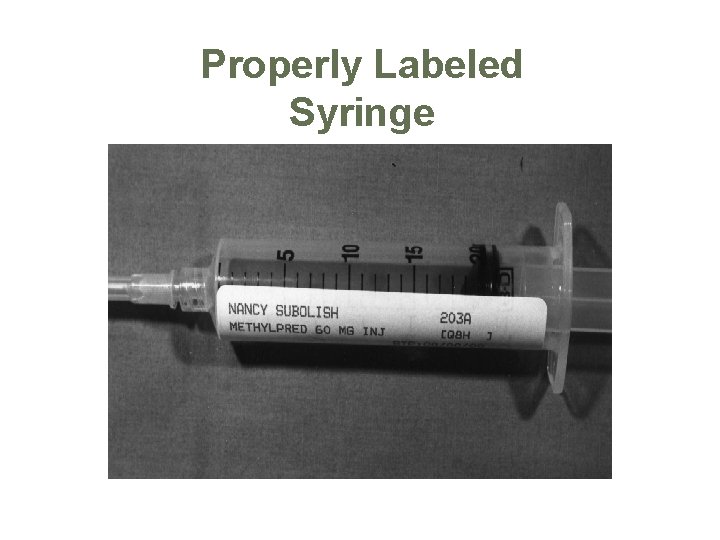
Properly Labeled Syringe

Errors Related to Dispensing Methods • 24 -hour pharmacy service reduces errors • Unit-dose dispensing should be utilized whenever feasible • Requiring multiple tablets to be taken for one dose may result in an underdose

Manual Redundancies • Independent double checks before dispensing – Original prescription order, label, and medication container should be kept together throughout the dispensing process – Pharmacist must check all of technician’s work

Manual Redundancies (continued) • Self-checking by a lone practitioner may be safer if: – Switching hands when rereading the label – Delay of self-checking – Recalculating using a different process

Manual Redundancies (continued) • Compounded products can be checked before dispensing utilizing new qualitative and quantitative analysis techniques • Use of standardized concentrations of frequently used formulations reduces errors

Dispensing Errors Caused by Poor Patient Education • Failure to adequately educate patients • Lack of pharmacist involvement in direct patient education • Failure to provide patients with understandable written instructions • Lack of involving patients in check systems • Not listening to patients when therapy is questioned or concerns are expressed

3/9/2021 22

Dispensing errors and near misses All reasonable steps need to be taken to minimise the occurrence of errors. 1. Good practice dictates that there should be a systematic approach in dealing with errors and near misses so that lessons can be learned from them and corrective action taken. 2. Pharmacists are to use barcode scanners when dispensing medicines at dispensing stations in pharmacies and pharmacy departments, and are to be used for the intended purpose. They are an aid to, but not a substitute for, minimising selection errors. 3/9/2021 23

3. Routine checking throughout the dispensing process is necessary, with particular emphasis being attached to the final check at the time of actual supply and at the time of dispatch of medication to wards. 4. Counselling of the patient or carer about their medicines provides an additional check. 5. Adequate time must be allowed to dispense properly 6. Arrangements should be in place to minimise distractions during the dispensing process, which can lead to dispensing errors. 3/9/2021 7. Pharmacists dispensing medicines 24

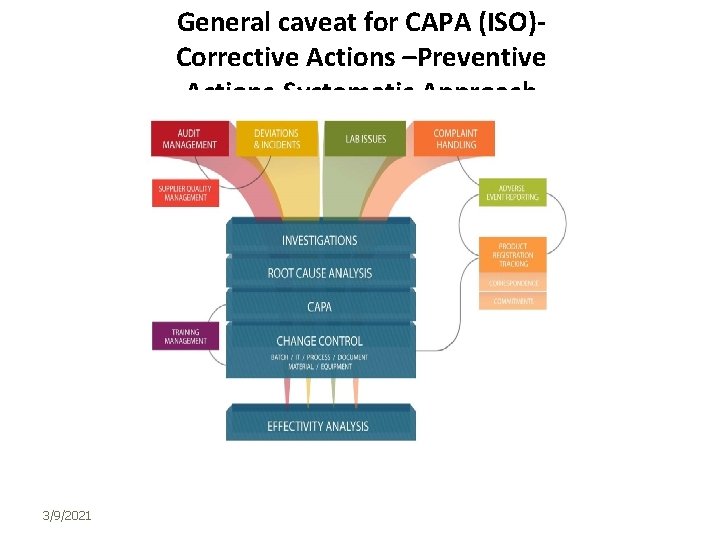
General caveat for CAPA (ISO)Corrective Actions –Preventive Actions-Systematic Approach 3/9/2021 25

CAPAs • CAPAs are based on risk, taking into account the frequency with which the nonconformity might occur and the severity of the risk if the nonconformity did occur • The objective of implementing a CAPA mechanism or system is to correct and prevent poor practices, not simply bad product. Correction and prevention of unacceptable quality system practices should result in fewer nonconformities related to product… For example, it should identify and correct improper personnel, issues of training, the failure to follow procedures, and inadequate procedures It is worth noting that there is a difference between correction and corrective action according to ISO 9000: 2005: a “Correction” ‐ action to eliminate a detected rrework example, for nonconformity. be correction can A a “Corrective action” ‐ action to eliminate the cause of a detected non‐conformity or other undesirable situation. ” Noting that there can be more than one cause for a nonconformity; and that a corrective action is taken to prevent recurrence. •

CAPA: INVESTIGATION QUESTIONS • Similarly, there is a difference between, a “Preventive action” ‐ action to eliminate the cause of a potential non‐conformity or other undesirable situation. There can be more than one cause for a potential nonconformity. • In order to establish relationships between violative conditions and responsible individuals, the following types of information, would be useful: • • Who knew of conditions? • • Who should have known of the conditions because of their specific or overall duties and positions? • • Who had the duty and power to prevent or detect the conditions, or to see they were prevented or detected? • • Who had the duty and power to correct the conditions, or to see they were corrected? What was done after person(s) learned of the conditions? Upon whose authority and instructions (be specific)?

CAPA Steps 1. Identification of the problem /dispensing error-which error 2. Identification of the cause (s) of the event 3. Proposition of solutions and plan –CAPA plan implementation 4. Monitoring of the CAPA plan 5. Documentation of the whole 1. State the problem, event, or issue identified Briefly outline the problem, event, or issue identified. This outline should be as objective as possible and include a description of: • What occurred • Where and when the event occurred • What should have occurred instead • What the source of the information was • Who discovered the information/event

Identification of the event – Collect data: Analyze the situation fully before you can move on to look at factors that contributed to the problem. To maximize the effectiveness of this step, talk to everyone with knowledge of the problem and process. You may become aware of additional information that you were not aware of at the time the event was initially discovered. – Data collection should provide you with information on: • Whether the problem truly exists, including the evidence indicating that a problem occurred • What the true scope and magnitude of the problem is, including: • How many times the event occurred, • How many subjects were affected, • Whether this event occurred in one or multiple studies under this PI's purview, with similar study team members, and/or within the program • How long the problem has existed, including: • When the event first occurred and how long it continued • What the effect or impact of the problem is and who was affected

Root cause analysis • Root cause analysis tools, including many common types of charts and graphs, can be helpful during this process. A Why Chart can be particularly helpful in determining a root cause, as it helps analyze the different layers of causes (3 or 5 Whys) During the Root Cause Analysis, ask at least the following questions: • What sequence of events lead to the problem or allowed the problem to occur? • What conditions allowed the problem to occur? • What other problems surround the occurrence of the central problem? • What were the contributing factors that led to this event? (i. e. all the items in the causal chain) • What is the underlying, root cause of the event? (i. e. the first item in the causal chain) • Identify Possible Causes: The goal of this step is to identify and document as many factors contributing to the problem as possible. There can be multiple reasons or causes that contribute to one single problem. This list may include human error, and/or process or equipment/space related factors. The focus should be on identifying underlying problems that contribute to error rather than on mistakes made by individuals.

Propose and Implement a Corrective and Preventive Action (CAPA) Plan • • Create simple, measurable solutions that address the root cause(s). These solutions should take the form of durable corrective and preventive actions that eliminate the cause and prevent it from recurring in the future, instead of just resolving the issue at hand. When developing a CAPA, consider at least the following: • What are the available solutions to prevent the event from occurring or recurring? • Are the solutions feasible? • How will the solution(s) be implemented? • What are the available resources (internal and external to the study team) to implement and monitor the CAPA plan? • Who will be responsible and accountable for implementing and monitoring the CAPA Plan? • Are the deadlines reasonable and achievable? • Can it be monitored? CAPA IMPLEMENTATION AND MONITORING

Monitor the progress and effectiveness of the CAPA Plan • • • Despite best efforts to identify root causes and solutions, the identified root causes may be incorrect and/or the solutions ineffective. Therefore, it is important that any CAPA be periodically assessed for effectiveness and modified as needed. Modifying an ineffective CAPA may require reexamining the causes of the initial event and restarting the root cause analysis process. When monitoring a CAPA, consider at least the following: • How will the CAPA plan be monitored (i. e. how will problems with the CAPA be identified and reported)? • When will you review the progress and effectiveness of the CAPA Plan? • At what intervals or frequency will you review the progress and effectiveness of the plan? • When and to whom should the outcome of the CAPA Plan be reported?

CAPA in Dispensing Processes COMPLETENESS AND CORRECTNESS APPROPRIATENESS • The prescription serves as a vehicle for communication from the licensed practitioner to the pharmacist about the pharmaceutical care of the patient • 3/9/2021 Thus, it contains or should contain the following details: • Prescriber • Patient • Product • All these details should be checked for CCA • Is the prescriber licensed, authorized to prescribe what s/he did; is the signature genuine • Is the patient the right one; are the relevant details to check appropriateness available e. g. age; sex; health status; …others • Are product details there: name of the product, dosage form, strength/potency, total amount to be dispensed, dosage and direction for use, frequency 33

Dispensing CAPAs 34

3/9/2021 35

Ethical considerations in Good Dispensing Ethical considerations include whether they require consent from the patient for treatment. Mentally ill Patients might not always understand their treatments or medication purposes. 3/9/2021 36

3/9/2021 General Ethical principles As Applied in Healthcare Beneficence is the principle that health professionals should act in the best interest of the patient. Autonomy is the principle that establishes patient rights to self-determination- to choose what will be done to them. Honesty principle states that patients have the right to the truth about their medical condition, the course of disease, the treatments recommended and the alternative treatments available. Informed Consent has occurred and treatment can be implemented if all relevant information is provided, if the patients 37

Ethical principles-Continued Confidentiality serves to assure patients that information about their medical conditions and treatments will not be given to individuals without their permission. Fidelity is the right of patients to have health professional provide services that promote patients’ interests rather than their own. Ethically, the first responsibilities of health care professionals should be directed towards the patients rather than directed at the financial well-being of their establishments. 3/9/2021 38

Specific Dispensing Ethical Rules that are punishable- RSA • • 3/9/2021 Failure to provide information to the patient on the safe and effective use of medicines dispensed or sold over the counter. This includes proper labelling Substituting or omitting to issue a medicine prescribed if this was not a request from a patient or after not having obtained the prescriber’ view, consent Failure to indicate that s/he dispensed the prescription by marking it with his signature, initials, … Failure to exercise a proper control over all connected activities such acquisition (counterfeit, poor quality, …), storage (not respecting manufacturer’s requirements), handling of products (controlled drugs) 39

3/9/2021 40

3/9/2021 41

3/9/2021 42

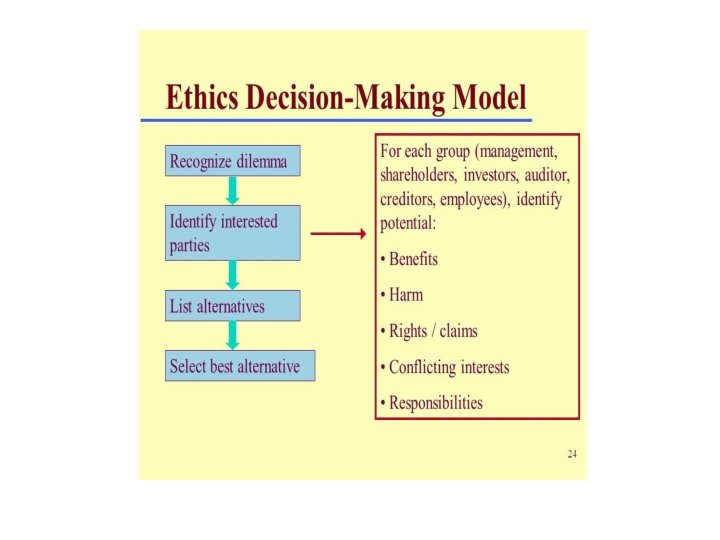
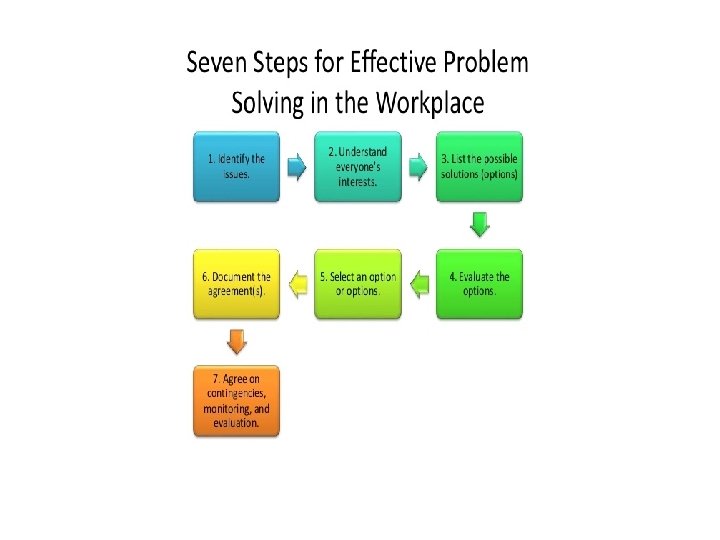
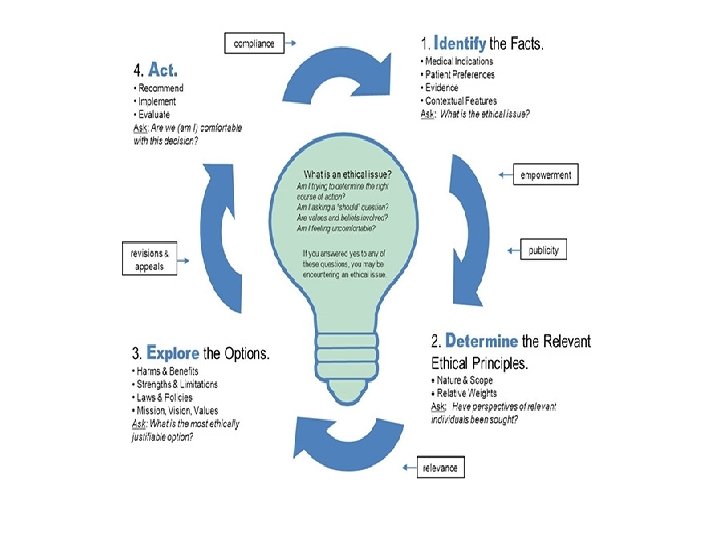
General approach to Ethical Issues 3/9/2021 43

Ethical cases for discussion

To dispense or not Emergency contraceptives 3/9/2021 45

3/9/2021 46

Concluding remarks on ethics discussions 3/9/2021 47
 Brittle fracture vs ductile fracture
Brittle fracture vs ductile fracture Non conducted pac ecg
Non conducted pac ecg Failure to pace vs failure to capture
Failure to pace vs failure to capture Patient 2 patient
Patient 2 patient Legal department structure chart
Legal department structure chart Www.gucci.com sale
Www.gucci.com sale Outside counsel billing guideline creation
Outside counsel billing guideline creation Organigram general counsel
Organigram general counsel Marcus evans enterprise in-house counsel
Marcus evans enterprise in-house counsel Iit general counsel
Iit general counsel Dhs cisa general counsel
Dhs cisa general counsel A wise man has many counselors
A wise man has many counselors Denovo advokatbyrå
Denovo advokatbyrå Yakima county superior court clerk
Yakima county superior court clerk Preach the whole counsel of god
Preach the whole counsel of god Stacking the deck fallacy examples
Stacking the deck fallacy examples Classical conditioning ap psychology
Classical conditioning ap psychology Omission of articles
Omission of articles Bias through placement examples
Bias through placement examples Apostrophes of omission
Apostrophes of omission Sin of commission
Sin of commission Bias through selection and omission guiding questions
Bias through selection and omission guiding questions Fixation omission emphasis
Fixation omission emphasis Bias through statistics and crowd counts examples
Bias through statistics and crowd counts examples Sin of omission and commission
Sin of omission and commission Bias through selection and omission example
Bias through selection and omission example Instrument scan techniques
Instrument scan techniques Welcome to all of you
Welcome to all of you 6 types of media bias
6 types of media bias Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Bổ thể
Bổ thể Từ ngữ thể hiện lòng nhân hậu
Từ ngữ thể hiện lòng nhân hậu Tư thế ngồi viết
Tư thế ngồi viết Diễn thế sinh thái là
Diễn thế sinh thái là Thế nào là giọng cùng tên? *
Thế nào là giọng cùng tên? * Thể thơ truyền thống
Thể thơ truyền thống Phép trừ bù
Phép trừ bù Bài hát chúa yêu trần thế alleluia
Bài hát chúa yêu trần thế alleluia Hươu thường đẻ mỗi lứa mấy con
Hươu thường đẻ mỗi lứa mấy con đại từ thay thế
đại từ thay thế Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Công thức tính độ biến thiên đông lượng
Công thức tính độ biến thiên đông lượng Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Thế nào là mạng điện lắp đặt kiểu nổi
Thế nào là mạng điện lắp đặt kiểu nổi Hình ảnh bộ gõ cơ thể búng tay
Hình ảnh bộ gõ cơ thể búng tay Lời thề hippocrates
Lời thề hippocrates Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra