ELECTROCHEMISTRY Chapter 21 redox reactions electrochemical cells electrode
![Cells at Non-standard Conditions (2) Eo only applies to [ ] = 1 M Cells at Non-standard Conditions (2) Eo only applies to [ ] = 1 M](https://slidetodoc.com/presentation_image_h/d29053ad0026930229820079822bf0e7/image-11.jpg)
- Slides: 35

ELECTROCHEMISTRY Chapter 21 • redox reactions • electrochemical cells • electrode processes • construction • notation Electric automobile • cell potential and Go • standard reduction potentials (Eo) • non-equilibrium conditions (Q) • batteries • corrosion 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 1

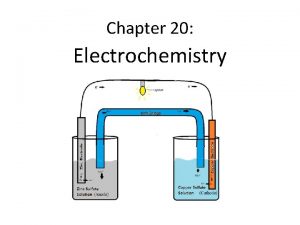
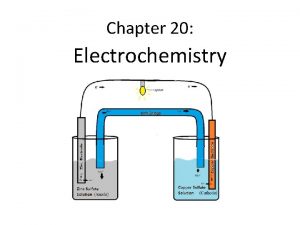
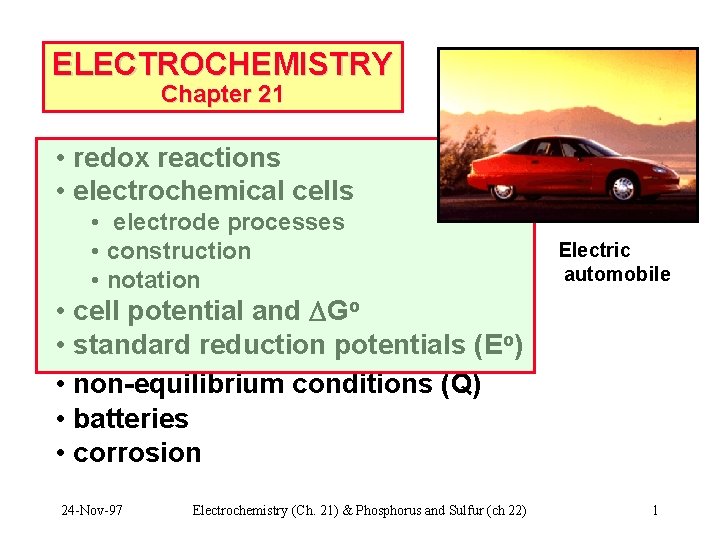
CHEMICAL CHANGE ELECTRIC CURRENT With time, Cu plates out onto Zn metal strip, and Zn strip “disappears. ” • Zn is oxidized and is the reducing agent Zn(s) Zn 2+(aq) + 2 e • Cu 2+ is reduced and is the oxidizing agent Cu 2+(aq) + 2 e- Cu(s) 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 2

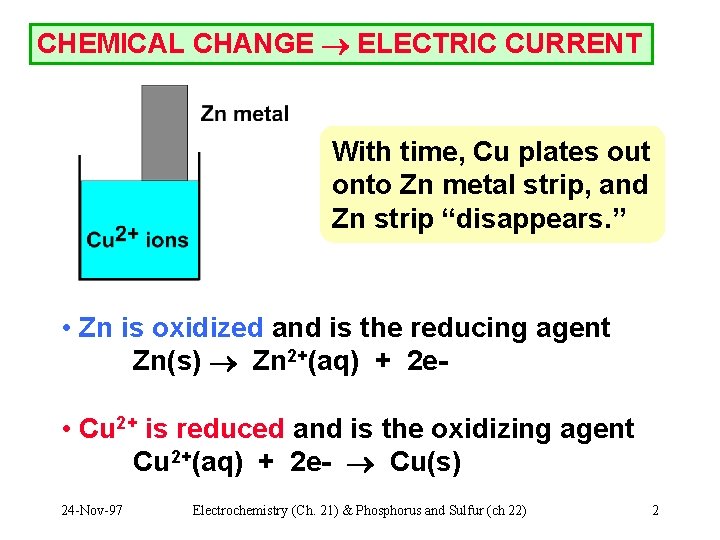
ANODE CATHODE OXIDATION REDUCTION • Electrons travel thru external wire. • Salt bridge allows anions and cations to move between electrode compartments. • This maintains electrical neutrality. 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 3

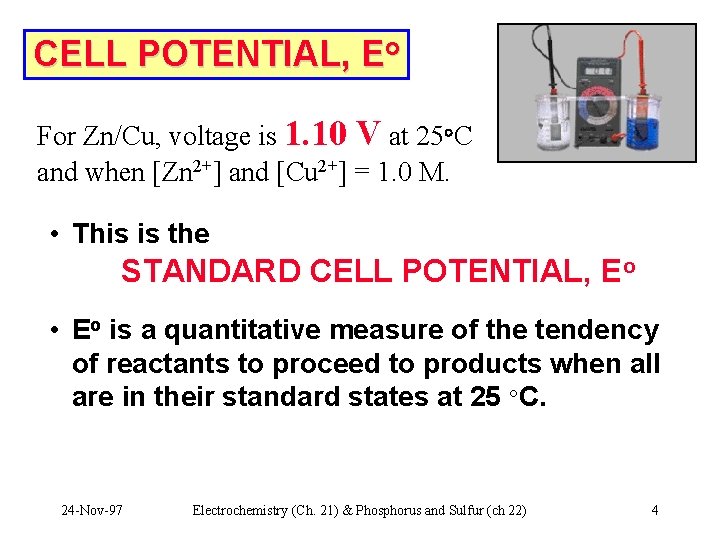
CELL POTENTIAL, Eo For Zn/Cu, voltage is 1. 10 V at 25°C and when [Zn 2+] and [Cu 2+] = 1. 0 M. • This is the STANDARD CELL POTENTIAL, Eo • Eo is a quantitative measure of the tendency of reactants to proceed to products when all are in their standard states at 25 °C. 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 4

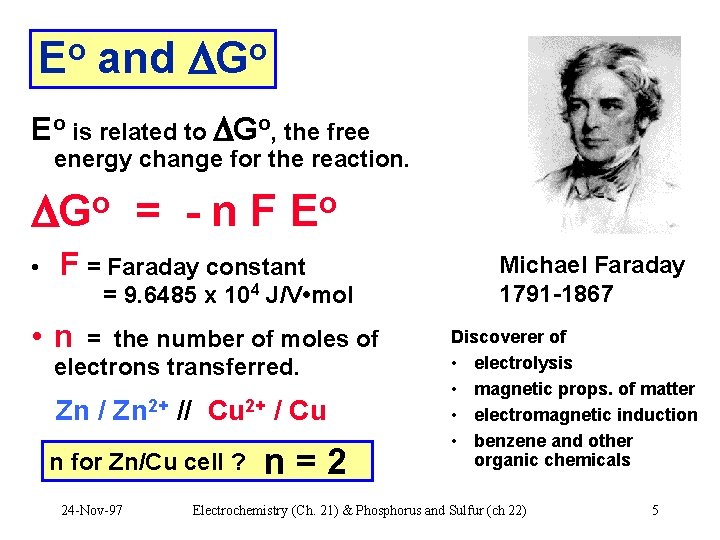
Eo and Go Eo is related to Go, the free energy change for the reaction. Go = - n F Eo • F = Faraday constant = 9. 6485 x 104 J/V • mol • n = the number of moles of electrons transferred. Zn / Zn 2+ // Cu 2+ / Cu n for Zn/Cu cell ? 24 -Nov-97 n=2 Michael Faraday 1791 -1867 Discoverer of • electrolysis • magnetic props. of matter • electromagnetic induction • benzene and other organic chemicals Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 5

Eo and Go (2) Go = - n F Eo • For a product-favored reaction – battery or voltaic cell: Chemistry electric current Reactants Products Go < 0 and so Eo > 0 (Eo is positive) • For a reactant-favored reaction - electrolysis cell: Electric current chemistry Reactants Products Go > 0 and so Eo < 0 (Eo is negative) 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 6

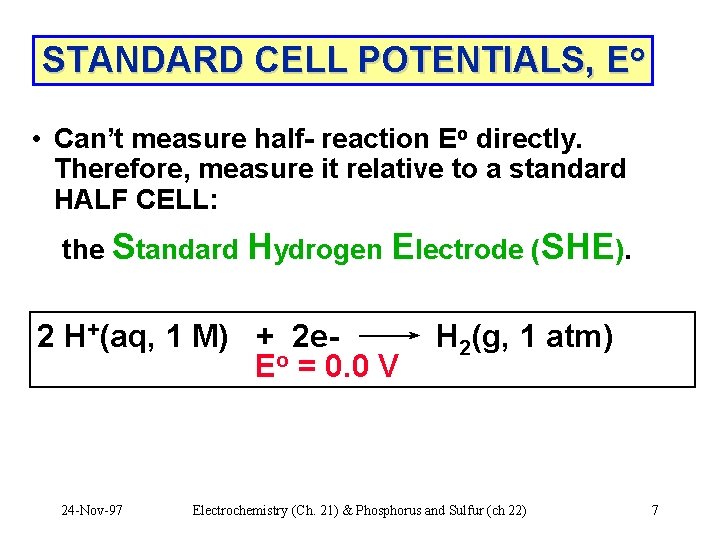
STANDARD CELL POTENTIALS, Eo • Can’t measure half- reaction Eo directly. Therefore, measure it relative to a standard HALF CELL: the Standard Hydrogen Electrode (SHE). 2 H+(aq, 1 M) + 2 e. Eo = 0. 0 V 24 -Nov-97 H 2(g, 1 atm) Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 7

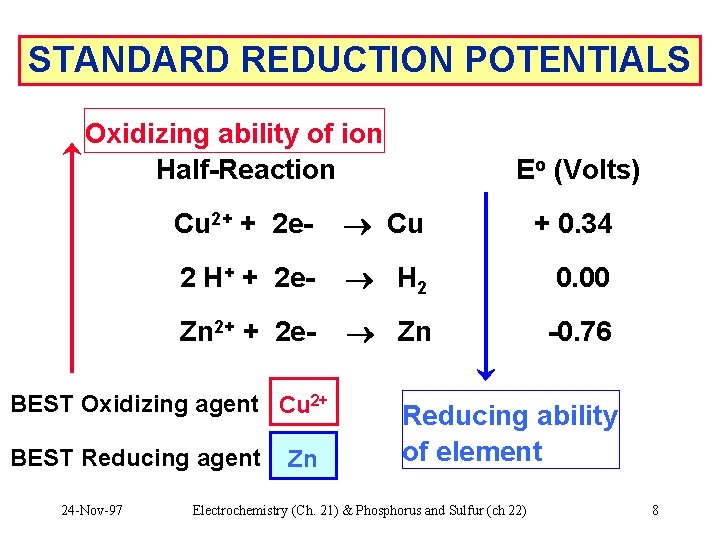
STANDARD REDUCTION POTENTIALS Oxidizing ability of ion Half-Reaction Cu 2+ + 2 e- Cu + 0. 34 2 H+ + 2 e- H 2 0. 00 Zn 2+ + 2 e- Zn -0. 76 BEST Oxidizing agent Cu ? ? 2+ BEST Reducing agent ? Zn ? 24 -Nov-97 Eo (Volts) Reducing ability of element Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 8

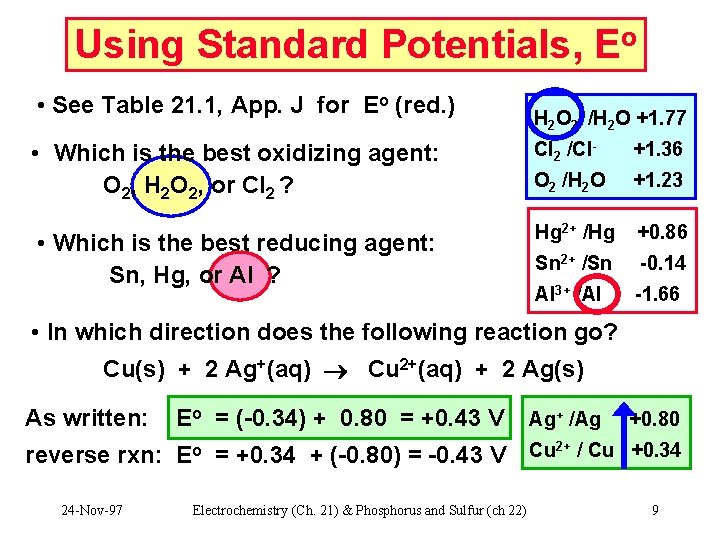
Using Standard Potentials, Eo • See Table 21. 1, App. J for Eo (red. ) • Which is the best oxidizing agent: O 2, H 2 O 2, or Cl 2 ? • Which is the best reducing agent: Sn, Hg, or Al ? H 2 O 2 /H 2 O +1. 77 Cl 2 /Cl- +1. 36 O 2 /H 2 O +1. 23 Hg 2+ /Hg +0. 86 Sn 2+ /Sn -0. 14 Al 3+ /Al -1. 66 • In which direction does the following reaction go? Cu(s) + 2 Ag+(aq) Cu 2+(aq) + 2 Ag(s) As written: Eo = (-0. 34) + 0. 80 = +0. 43 V Ag+ /Ag +0. 80 2+ reverse rxn: Eo = +0. 34 + (-0. 80) = -0. 43 V Cu / Cu +0. 34 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 9

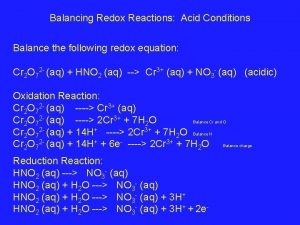
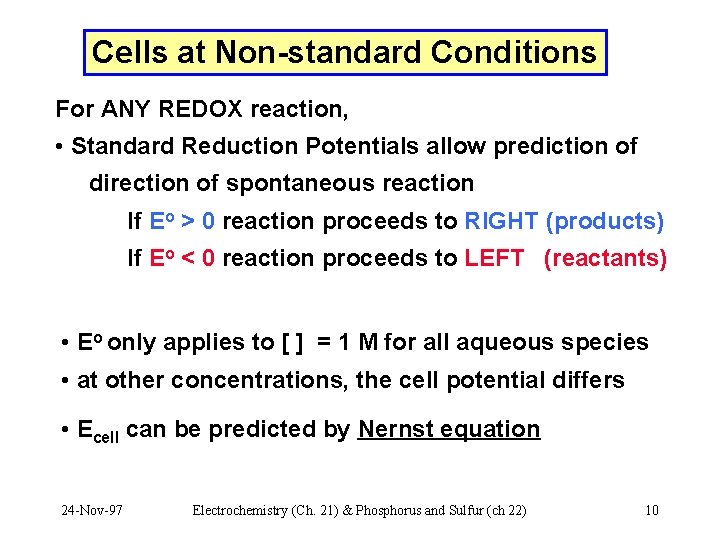
Cells at Non-standard Conditions For ANY REDOX reaction, • Standard Reduction Potentials allow prediction of direction of spontaneous reaction If Eo > 0 reaction proceeds to RIGHT (products) If Eo < 0 reaction proceeds to LEFT (reactants) • Eo only applies to [ ] = 1 M for all aqueous species • at other concentrations, the cell potential differs • Ecell can be predicted by Nernst equation 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 10
![Cells at Nonstandard Conditions 2 Eo only applies to 1 M Cells at Non-standard Conditions (2) Eo only applies to [ ] = 1 M](https://slidetodoc.com/presentation_image_h/d29053ad0026930229820079822bf0e7/image-11.jpg)
Cells at Non-standard Conditions (2) Eo only applies to [ ] = 1 M for all aqueous species at other concentrations, the cell potential differs Ecell can be predicted by Nernst equation E = Eo - RT ln (Q) n. F n = # e- transferred F = Faraday’s constant = 9. 6485 x 104 J/V • mol Q is the REACTION QUOTIENT (recall ch. 16, 20) Go, Eo At equilibrium refer to ALL REACTANTS relative to G = 0 E= 0 Q=K ALL PRODUCTS 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 11

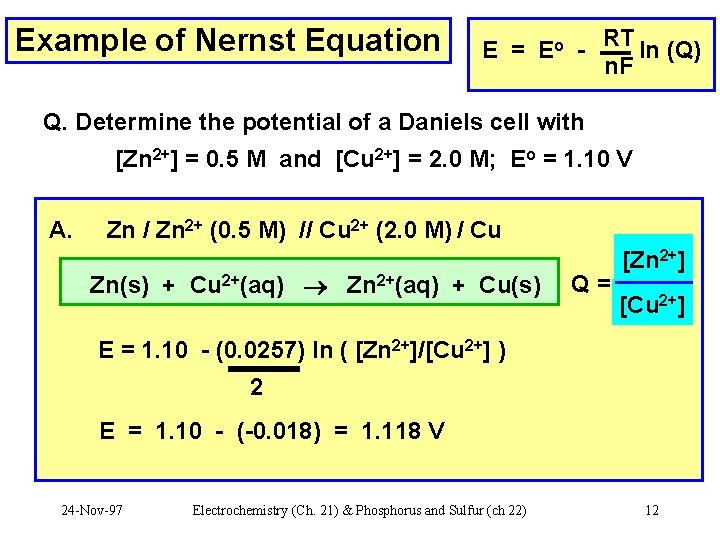
Example of Nernst Equation E = Eo - RT ln (Q) n. F Q. Determine the potential of a Daniels cell with [Zn 2+] = 0. 5 M and [Cu 2+] = 2. 0 M; Eo = 1. 10 V A. Zn / Zn 2+ (0. 5 M) // Cu 2+ (2. 0 M) / Cu Zn(s) + Cu 2+(aq) Zn 2+(aq) + Cu(s) [Zn 2+] Q=? [Cu 2+] E = 1. 10 - (0. 0257) ln ( [Zn 2+]/[Cu 2+] ) 2 E = 1. 10 - (-0. 018) = 1. 118 V 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 12

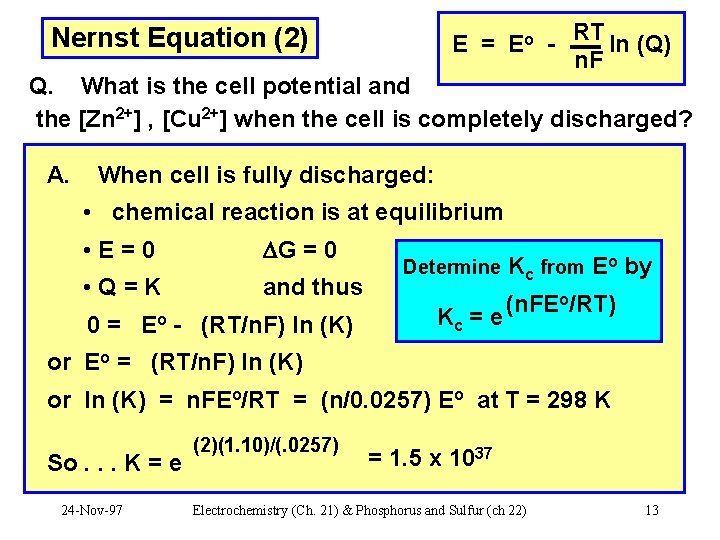
Nernst Equation (2) E = Eo RT ln (Q) n. F Q. What is the cell potential and the [Zn 2+] , [Cu 2+] when the cell is completely discharged? A. When cell is fully discharged: • chemical reaction is at equilibrium • E=0 G = 0 • Q=K and thus 0 = Eo - (RT/n. F) ln (K) Determine Kc = e Kc from Eo by (n. FEo/RT) or Eo = (RT/n. F) ln (K) or ln (K) = n. FEo/RT = (n/0. 0257) Eo at T = 298 K So. . . K = e 24 -Nov-97 (2)(1. 10)/(. 0257) = 1. 5 x 1037 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 13

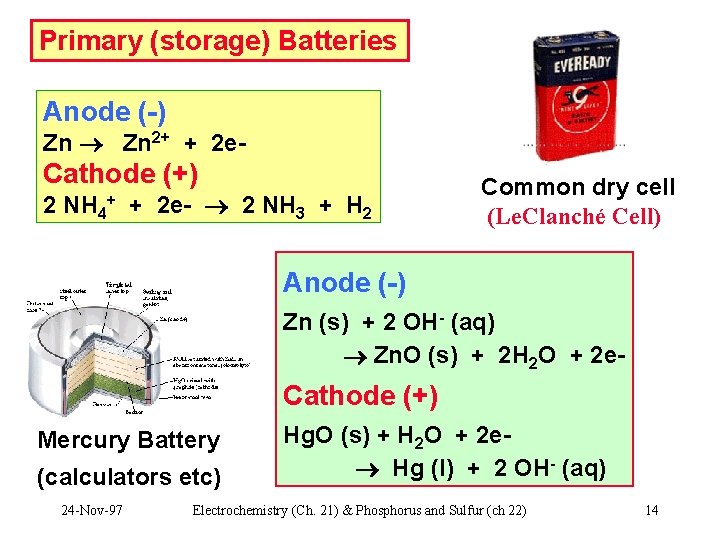
Primary (storage) Batteries Anode (-) Zn 2+ + 2 e- Cathode (+) 2 NH 4 + 2 e- 2 NH 3 + H 2 + Common dry cell (Le. Clanché Cell) Anode (-) Zn (s) + 2 OH- (aq) Zn. O (s) + 2 H 2 O + 2 e- Cathode (+) Mercury Battery (calculators etc) 24 -Nov-97 Hg. O (s) + H 2 O + 2 e Hg (l) + 2 OH- (aq) Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 14

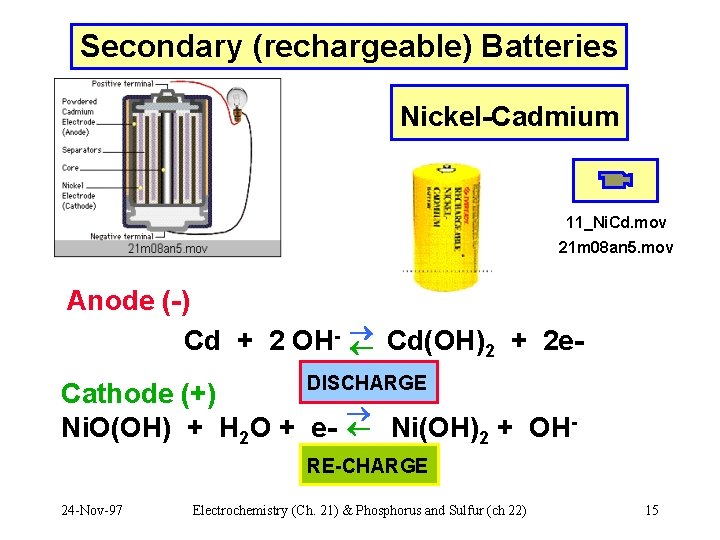
Secondary (rechargeable) Batteries Nickel-Cadmium 11_Ni. Cd. mov 21 m 08 an 5. mov Anode (-) Cd + 2 OH- Cd(OH)2 + 2 e. DISCHARGE Cathode (+) Ni. O(OH) + H 2 O + e- Ni(OH)2 + OHRE-CHARGE 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 15

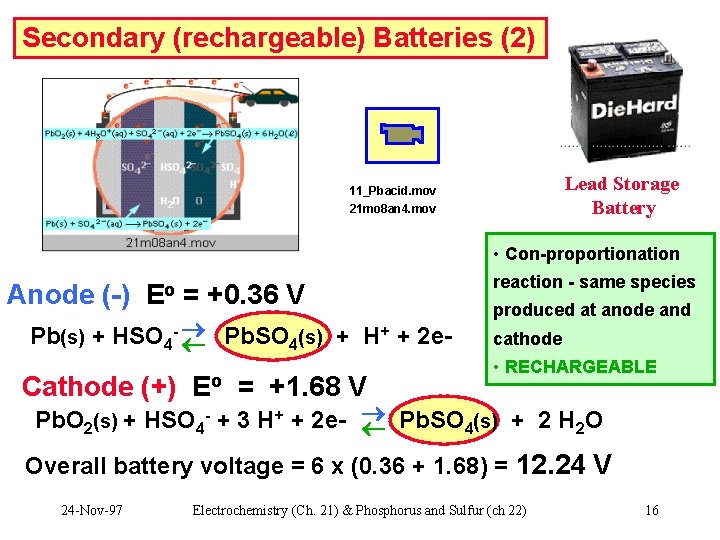
Secondary (rechargeable) Batteries (2) Lead Storage Battery 11_Pbacid. mov 21 mo 8 an 4. mov • Con-proportionation Anode (-) Eo = +0. 36 V + Pb(s) + HSO 4 - Pb. SO 4(s) + H + 2 e- Cathode (+) Eo = +1. 68 V reaction - same species produced at anode and cathode • RECHARGEABLE Pb. O 2(s) + HSO 4 - + 3 H+ + 2 e- Pb. SO 4(s) + 2 H 2 O Overall battery voltage = 6 x (0. 36 + 1. 68) = 12. 24 V 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 16

Corrosion - an electrochemical reaction Electrochemical or redox reactions are tremendously damaging to modern society e. g. - rusting of cars, etc: EOX = +0. 44 anode: Fe - Fe 2+ + 2 e. ERED = +0. 40 cathode: O 2 + 2 H 2 O + 4 e- 4 OHnet: 2 Fe(s) + O 2 (g) + 2 H 2 O (l) 2 Fe(OH)2 (s) Ecell = +0. 84 Mechanisms for minimizing corrosion • sacrificial anodes (cathodic protection) (e. g. Mg) • coatings - e. g. galvanized steel • - Zn layer forms (Zn(OH)2. x. Zn. CO 3) • this is INERT (like Al 2 O 3); if breaks, Zn is sacrificial 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 17

Electrolysis of Aqueous Na. OH Electric Energy Chemical Change Anode : Eo = -0. 40 V 4 OH- O 2(g) + 2 H 2 O + 2 e- Cathode : Eo = -0. 83 V 11_electrolysis. mov 21 m 10 vd 1. mov 4 H 2 O + 4 e- 2 H 2 + 4 OH- Eo for cell = -1. 23 V since Eo < 0 , Go > 0 - not spontaneous ! - ONLY occurs if Eexternal > 1. 23 V is applied 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 18

ELECTROCHEMISTRY Chapter 21 • redox reactions • electrochemical cells • construction • electrode processes • notation Electric automobile • cell potential and Go • standard reduction potentials (Eo) • non-equilibrium conditions (Q) • batteries • corrosion 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 19

Phosphorus and Sulfur Chemistry Kotz, Ch 22 • the elements • physical properties • chemical reactions • redox chemistry • acid/base chemistry 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 20

Elemental Sulfur - Obtained from: - free element in volcanic vents ‘mined’ by Frasch process - minerals : Fe. S 2 (pyrite), Pb. S 2 (galena) Cu 2 S (chalcocite) (S produced as by-product of metal extraction) - natural gas and oil processing desulfurization: 2 H 2 S (g) + SO 2 (g) 3 S (s) + 2 H 2 O (g) 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 21

Elemental Phosphorus - not found free in nature - too easily oxidized “phosphate rock” Ca 3 (PO 4)2 Ca 5 (PO 4)3 F Ca 5 (PO 4)3 OH Ca 5 (PO 4)3 Cl calcium phosphate fluoro apatite hydroxy apatite (teeth etc) chloro apatite • Isolate phosphorus from these ‘rocks’ by burning with charcoal and sand 2 Ca 3 (PO 4)2 (l) + 6 Si. O 2 (s) P 4 O 10 (g) + 6 Ca. Si. O 3 (l) P 4 O 10 (g) + 10 C (s) P 4 + 10 CO (g) 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 22

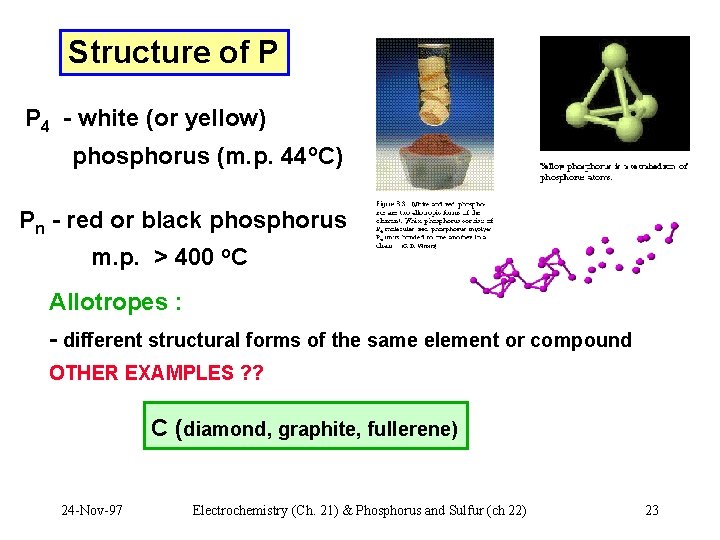
Structure of P P 4 - white (or yellow) phosphorus (m. p. 44 o. C) Pn - red or black phosphorus m. p. > 400 o. C Allotropes : - different structural forms of the same element or compound OTHER EXAMPLES ? ? C (diamond, graphite, fullerene) 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 23

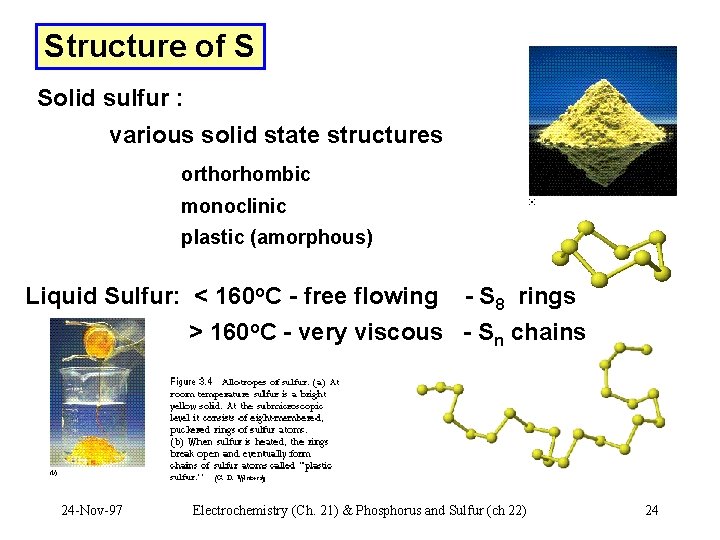
Structure of S Solid sulfur : various solid state structures orthorhombic monoclinic plastic (amorphous) Liquid Sulfur: < 160 o. C - free flowing - S 8 rings > 160 o. C - very viscous - Sn chains 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 24

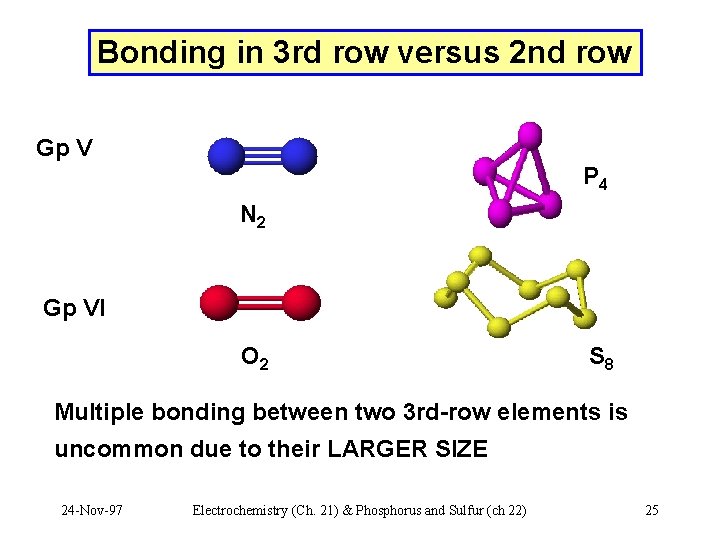
Bonding in 3 rd row versus 2 nd row Gp V P 4 N 2 Gp VI O 2 S 8 Multiple bonding between two 3 rd-row elements is uncommon due to their LARGER SIZE 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 25

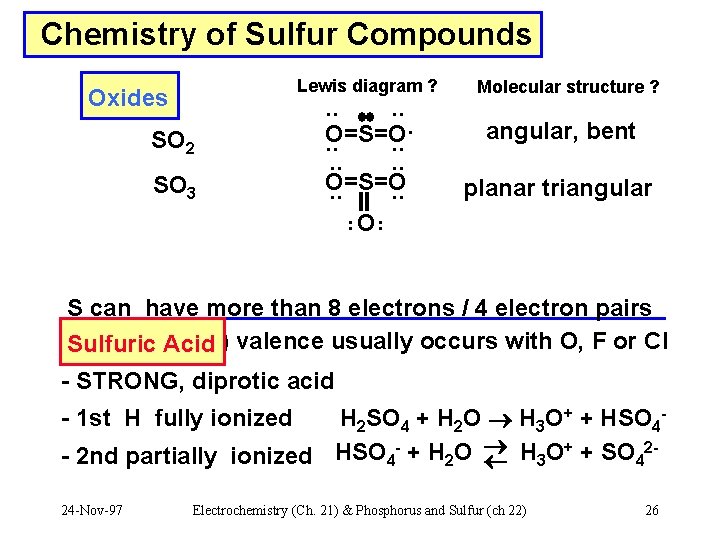
Chemistry of Sulfur Compounds Lewis diagram ? Oxides SO 2 SO 3 . . O=S=O. . . O. . Molecular structure ? angular, bent planar triangular S can have more than 8 electrons / 4 electron pairs expanded (>4) valence usually occurs with O, F or Cl Sulfuric Acid - STRONG, diprotic acid H 2 SO 4 + H 2 O H 3 O+ + HSO 4+ 2 - 2 nd partially ionized HSO 4 - + H 2 O H 3 O + SO 4 - 1 st H fully ionized 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 26

Reactions of Sulfuric Acid 1. Strong acid Na. NO 3 + H 2 SO 4 HNO 3 + Na. HSO 4 2. Dehydrating agent C 11 H 22 O 11 + H 2 SO 4 12 C + 11 H 3 O+ 11 HSO 43. Strong oxidizing agent 2 Br- + 2 H 2 SO 4 (conc. ) 2 Br 2 + SO 42 - + SO 2 + 2 H 2 O 4. Useful solvent : m. p. 10 o. C b. p. 338 o. C 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 27

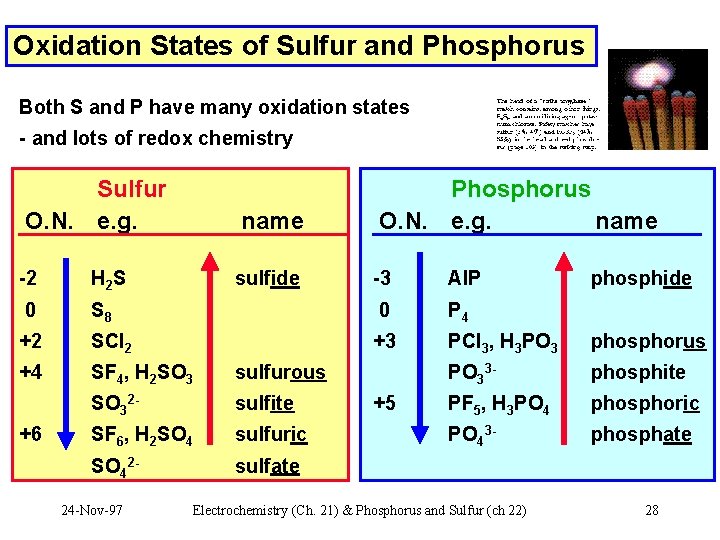
Oxidation States of Sulfur and Phosphorus Both S and P have many oxidation states - and lots of redox chemistry Sulfur O. N. e. g. -2 H 2 S 0 name -3 Al. P S 8 0 P 4 +2 SCl 2 +3 PCl 3, H 3 PO 3 phosphorus +4 SF 4, H 2 SO 3 sulfurous PO 33 - phosphite SO 32 - sulfite PF 5, H 3 PO 4 phosphoric SF 6, H 2 SO 4 sulfuric PO 43 - phosphate SO 42 - sulfate +6 24 -Nov-97 sulfide Phosphorus O. N. e. g. name +5 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) phosphide 28

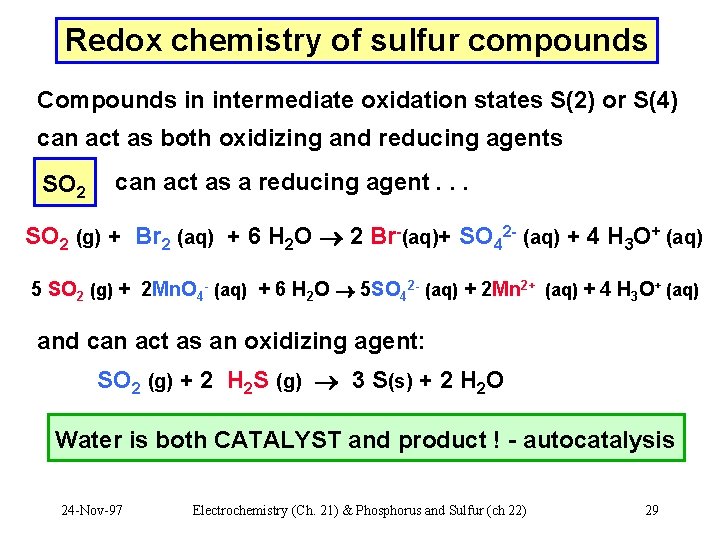
Redox chemistry of sulfur compounds Compounds in intermediate oxidation states S(2) or S(4) can act as both oxidizing and reducing agents SO 2 can act as a reducing agent. . . SO 2 (g) + Br 2 (aq) + 6 H 2 O 2 Br-(aq)+ SO 42 - (aq) + 4 H 3 O+ (aq) 5 SO 2 (g) + 2 Mn. O 4 - (aq) + 6 H 2 O 5 SO 42 - (aq) + 2 Mn 2+ (aq) + 4 H 3 O+ (aq) and can act as an oxidizing agent: SO 2 (g) + 2 H 2 S (g) 3 S(s) + 2 H 2 O Water is both CATALYST and product ! - autocatalysis 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 29

Chemistry of phosphorus compounds OXIDES P 4 + 3 O 2 P 4 O 6 P 4 + 5 O 2 P 4 O 10 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 30

Phosphoric acid P 4 O 10 + 6 H 2 O 4 H 3 PO 4 - phosphoric acid H 3 PO 4 is a weak tri-protic acid - even 1 st H+ not fully ionized H 3 PO 4 (aq) + H 2 O Kc (eq) H 3 O+(aq) + H 2 PO 4 - (aq) dihydrogen phosphate 7. 5 x 10 -3 + 2 H 2 PO 4 - (aq) + H 2 O H 3 O (aq) + HPO 4 (aq) hydrogen 6. 2 x 10 -8 HPO 42 - (aq) + H 2 O H 3 O+(aq) + PO 43 - (aq) 3. 6 x 10 -13 phosphate 24 -Nov-97 phosphate Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 31

Phosphorus Chemistry (2) P 4 O 6 + 6 H 2 O 4 H 3 PO 3 - phosphorus acid H 3 PO 3 is a weak di-protic acid WHY ONLY 2 IONIZABLE hydrogens ? The P-H bond is strong and non-polar - not ionizable P (III) oxide and its acid are easily oxidized to P (V) so they act as REDUCING agents: - 2 e. Cu 2+(aq) + H 3 PO 3(aq) + 3 H 2 O Cu (s) + H 3 PO 4(aq) + 2 H 3 O+ - 2 e 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 32

Phosphorus Chemistry (3) P 3 - Phosphine. PH 3 - like NH 3 but weaker base Phosphide - ionic compounds with some metals 6 Ca + P 4 2 Ca 3 P 2 P 5+ (Ca 2+)3 ( P 3 -) 2 Phosphoric acid, phosphate compounds Polyphosphates - condensation of hydroxy-acids X-O-H + H-O-X X-O-X + H 2 O O O e. g. 2 H 3 PO 4 H-O-P-O-H O 24 -Nov-97 O + H 2 O di-phosphoric acid Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 33

Phosphorus Chemistry (4) Phosphate condensation/hydrolysis important in Biochemistry: + H 2 O + R enzymes [R-O-(PO 2)-O-PO 3]3 -(aq) + H 2 O [R-O-(PO 3)]2 -(aq)+ H 2 PO 4 -(aq) ATP 3 - + H 2 O AMP 2 - + H 2 PO 4 -(aq) Go = -30. 5 k. J/mol Energy from - removal of e--e- repulsion in reactant (ATP) - P-O bond converted to P=O bond - more resonance stabilization in products 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 34

P and S Chemistry Kotz, Ch 22 • Physical properties • Chemical reactions • redox chemistry • acid/base chemistry 24 -Nov-97 Electrochemistry (Ch. 21) & Phosphorus and Sulfur (ch 22) 35
 How to write a balanced redox reaction
How to write a balanced redox reaction Chapter 19 redox reactions study guide answers
Chapter 19 redox reactions study guide answers Balancing redox reactions
Balancing redox reactions Oxidation number of no2-
Oxidation number of no2- Reactions that produce gas
Reactions that produce gas Reduction and oxidation
Reduction and oxidation Spontaneity electrochemistry
Spontaneity electrochemistry Downs cell
Downs cell Leo says ger
Leo says ger Electrochemistry balancing equations
Electrochemistry balancing equations Balancing redox reactions in acid
Balancing redox reactions in acid Balance redox reactions
Balance redox reactions Balancing redox reactions
Balancing redox reactions How to balance equation in acidic solution
How to balance equation in acidic solution How to write redox half reactions
How to write redox half reactions Balancing redox reactions
Balancing redox reactions Redox reactions ncea level 2
Redox reactions ncea level 2 Chemsheets combining half equations
Chemsheets combining half equations How redox reactions work
How redox reactions work Concept map of oxidative phosphorylation
Concept map of oxidative phosphorylation Khan academy electrochemistry
Khan academy electrochemistry What are spectator ions
What are spectator ions Voltaic cell components
Voltaic cell components Predicting redox reactions
Predicting redox reactions Chapter 8 cellular reproduction cells from cells
Chapter 8 cellular reproduction cells from cells Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Types of reactions
Types of reactions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Enzymes affect the reaction in living cells by changing the
Enzymes affect the reaction in living cells by changing the Ap chemistry chapter 18 electrochemistry test
Ap chemistry chapter 18 electrochemistry test Introduction to electrochemistry
Introduction to electrochemistry Chapter 20 review electrochemistry
Chapter 20 review electrochemistry Chapter 21 electrochemistry
Chapter 21 electrochemistry Chapter 21 electrochemistry
Chapter 21 electrochemistry Electrons flow from anode to cathode
Electrons flow from anode to cathode