Electrochemistry Electrode Potentials Electrochemistry 1 What are electrode

![Electrochemistry 2. What can we know from electrode potentials? 1. [Sign] + – 2. Electrochemistry 2. What can we know from electrode potentials? 1. [Sign] + – 2.](https://slidetodoc.com/presentation_image_h2/962d55db586b1801a1c835666195b178/image-5.jpg)

- Slides: 21

Electrochemistry Electrode Potentials

Electrochemistry 1. What are electrode potentials?

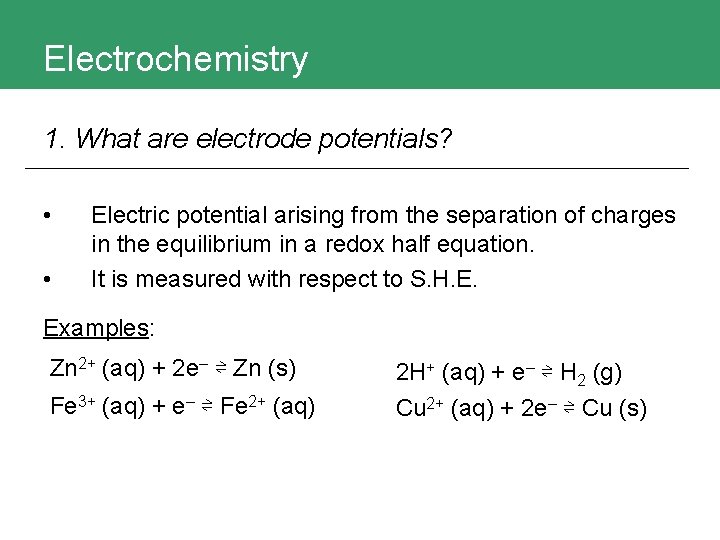
Electrochemistry 1. What are electrode potentials? • • Electric potential arising from the separation of charges in the equilibrium in a redox half equation. It is measured with respect to S. H. E. Examples: Zn 2+ (aq) + 2 e– ⇌ Zn (s) Fe 3+ (aq) + e– ⇌ Fe 2+ (aq) 2 H+ (aq) + e– ⇌ H 2 (g) Cu 2+ (aq) + 2 e– ⇌ Cu (s)

Electrochemistry 2. What can we know from electrode potentials?
![Electrochemistry 2 What can we know from electrode potentials 1 Sign 2 Electrochemistry 2. What can we know from electrode potentials? 1. [Sign] + – 2.](https://slidetodoc.com/presentation_image_h2/962d55db586b1801a1c835666195b178/image-5.jpg)
Electrochemistry 2. What can we know from electrode potentials? 1. [Sign] + – 2. forward reaction (reduction) favoured backward reaction (oxidation) favoured [Magnitude] – the extent the reaction is favoured

Electrochemistry 3. How can we measure standard electrode potentials?

Electrochemistry 3. How can we measure standard electrode potentials? - connect the half cell that you wish to study to the SHE Note: • You need to be know how to draw the cell setup. • Things to note when drawing half cell: (1) salt bridge, (2) voltmeter and (3) ensure all gases, solutions are at standard conditions.

Electrochemistry 4. How can we use standard electrode potentials?

Electrochemistry 4. How can we use standard electrode potentials? - to predict whether a redox reaction is feasible when 2 species are mixed. Examples: I 2 + Cr 2+ feasible? V 3+ + Cu 2+ feasible?

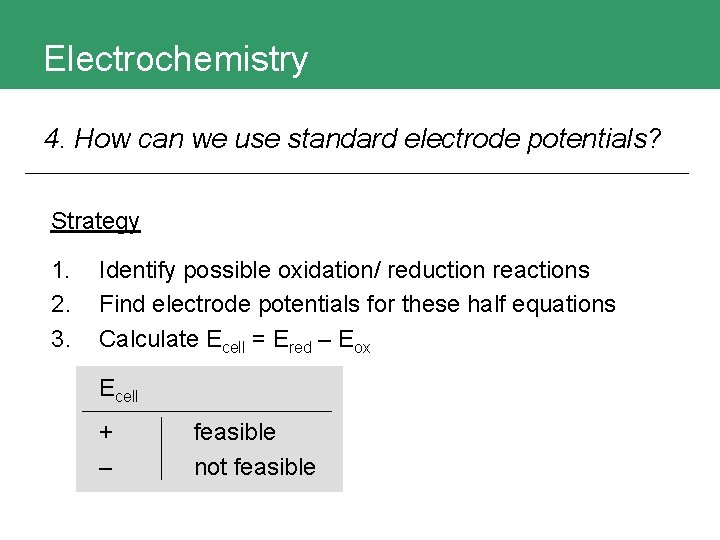
Electrochemistry 4. How can we use standard electrode potentials? Strategy 1. 2. 3. Identify possible oxidation/ reduction reactions Find electrode potentials for these half equations Calculate Ecell = Ered – Eox Ecell + – feasible not feasible

Electrochemistry Galvanic Cells

Electrochemistry 5. How to distinguish anode and cathode? Galvanic Cell

Electrochemistry 5. How to distinguish anode and cathode? Positive remove electrons Reduction Cathode Negative produce electrons Oxidation Anode Electrons flow from A C Galvanic Cell

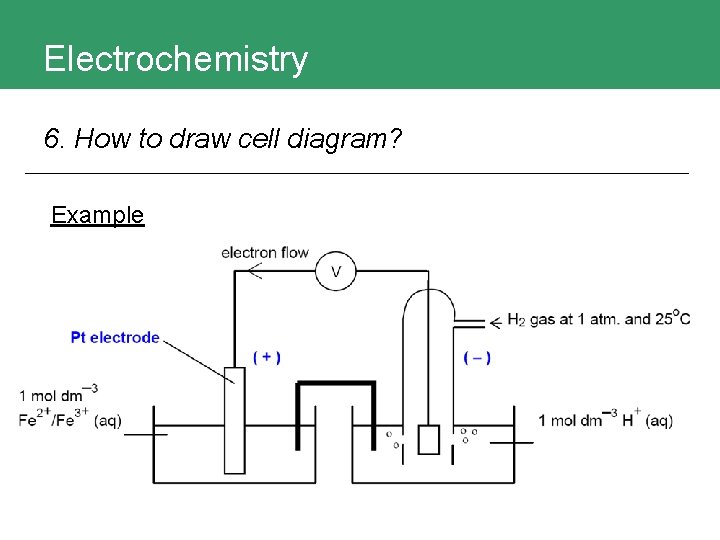
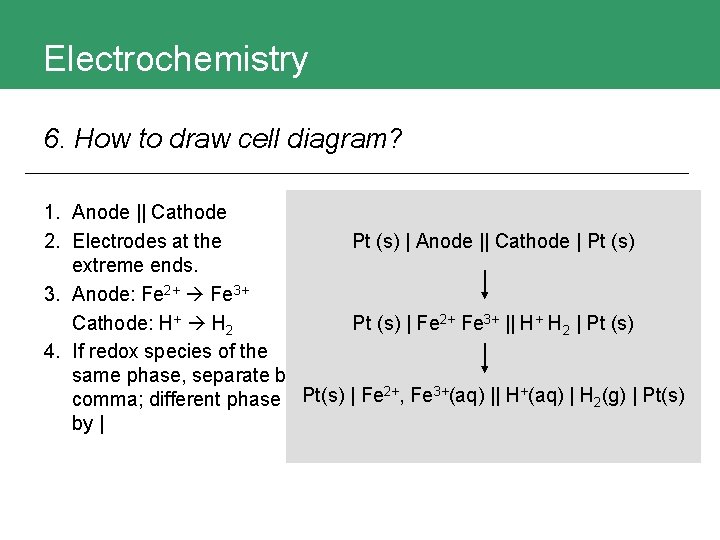
Electrochemistry 6. How to draw cell diagram?

Electrochemistry 6. How to draw cell diagram? Example

Electrochemistry 6. How to draw cell diagram? 1. Anode || Cathode 2. Electrodes at the Pt (s) | Anode || Cathode | Pt (s) extreme ends. 3. Anode: Fe 2+ Fe 3+ Cathode: H+ H 2 Pt (s) | Fe 2+ Fe 3+ || H+ H 2 | Pt (s) 4. If redox species of the same phase, separate by 2+ 3+ + comma; different phase Pt(s) | Fe , Fe (aq) || H (aq) | H 2(g) | Pt(s) by |

Electrochemistry Electrolytic Cells

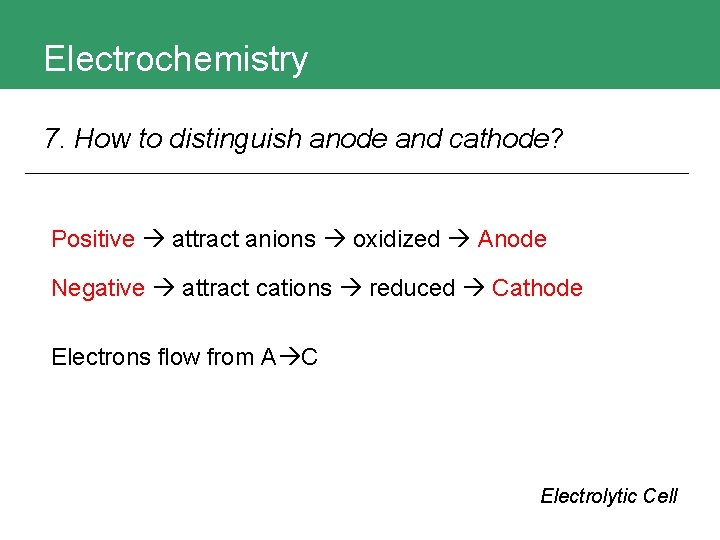
Electrochemistry 7. How to distinguish anode and cathode? Electrolytic Cell

Electrochemistry 7. How to distinguish anode and cathode? Positive attract anions oxidized Anode Negative attract cations reduced Cathode Electrons flow from A C Electrolytic Cell

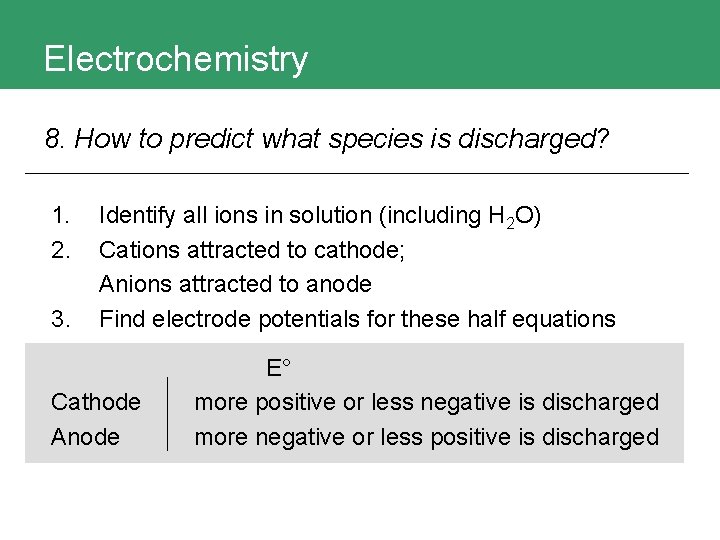
Electrochemistry 8. How to predict what species is discharged? 1. 2. 3. Identify all ions in solution (including H 2 O) Cations attracted to cathode; Anions attracted to anode Find electrode potentials for these half equations Cathode Anode E° more positive or less negative is discharged more negative or less positive is discharged

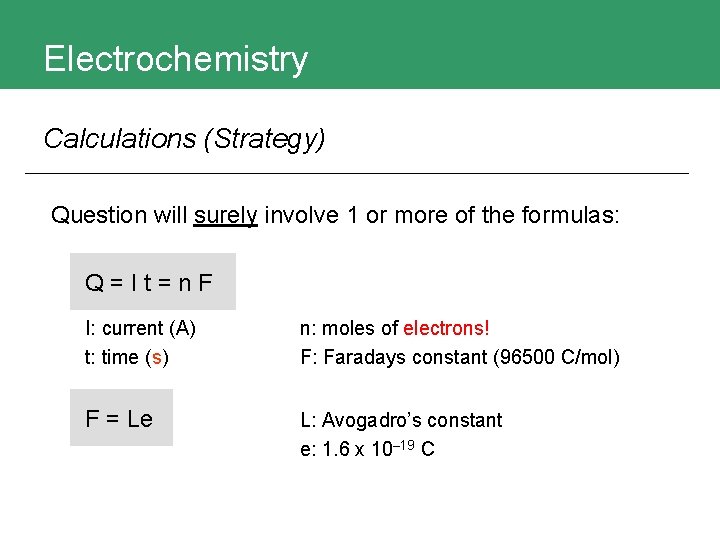
Electrochemistry Calculations (Strategy) Question will surely involve 1 or more of the formulas: Q=It=n. F I: current (A) t: time (s) n: moles of electrons! F: Faradays constant (96500 C/mol) F = Le L: Avogadro’s constant e: 1. 6 x 10– 19 C