Standard Reference Electrode Standard Hydrogen Electrode SHE SHE
- Slides: 14

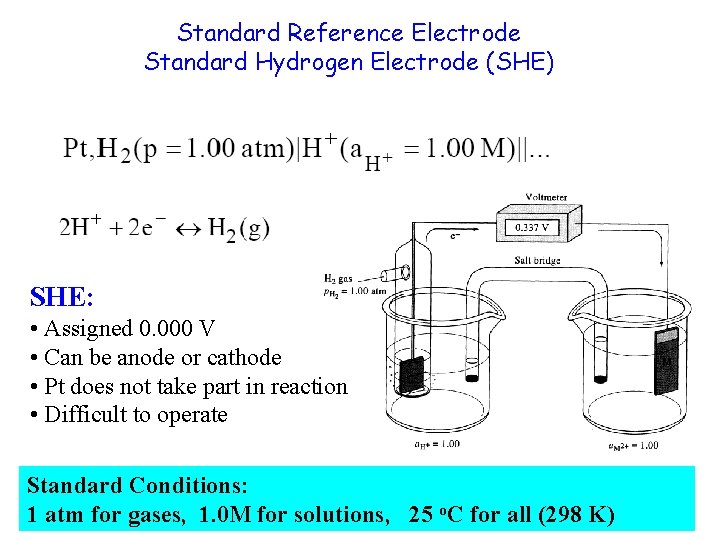
Standard Reference Electrode Standard Hydrogen Electrode (SHE) SHE: • Assigned 0. 000 V • Can be anode or cathode • Pt does not take part in reaction • Difficult to operate Standard Conditions: 1 atm for gases, 1. 0 M for solutions, 25 o. C for all (298 K)

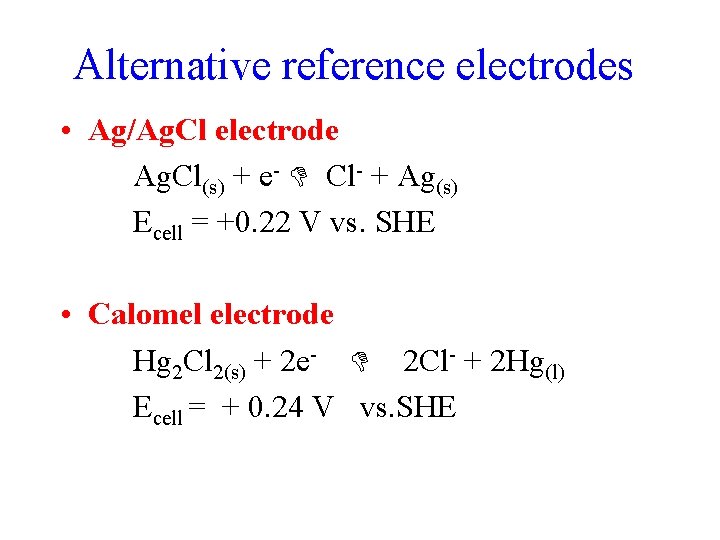
Alternative reference electrodes • Ag/Ag. Cl electrode Ag. Cl(s) + e- Cl- + Ag(s) Ecell = +0. 22 V vs. SHE • Calomel electrode Hg 2 Cl 2(s) + 2 e- 2 Cl- + 2 Hg(l) Ecell = + 0. 24 V vs. SHE

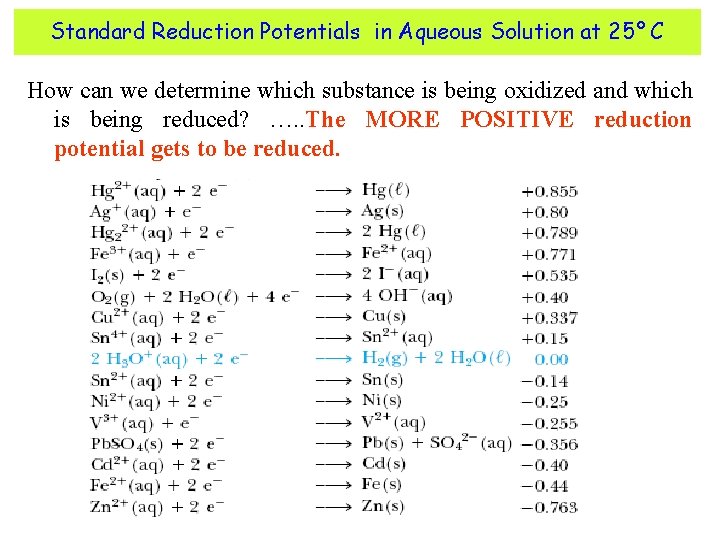
Standard Reduction Potentials in Aqueous Solution at 25° C How can we determine which substance is being oxidized and which is being reduced? …. . The MORE POSITIVE reduction potential gets to be reduced.

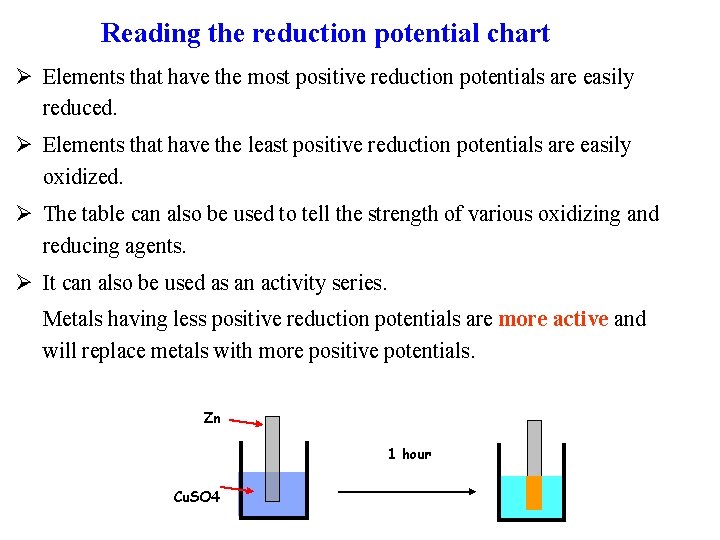
Reading the reduction potential chart Ø Elements that have the most positive reduction potentials are easily reduced. Ø Elements that have the least positive reduction potentials are easily oxidized. Ø The table can also be used to tell the strength of various oxidizing and reducing agents. Ø It can also be used as an activity series. Metals having less positive reduction potentials are more active and will replace metals with more positive potentials. Zn 1 hour Cu. SO 4

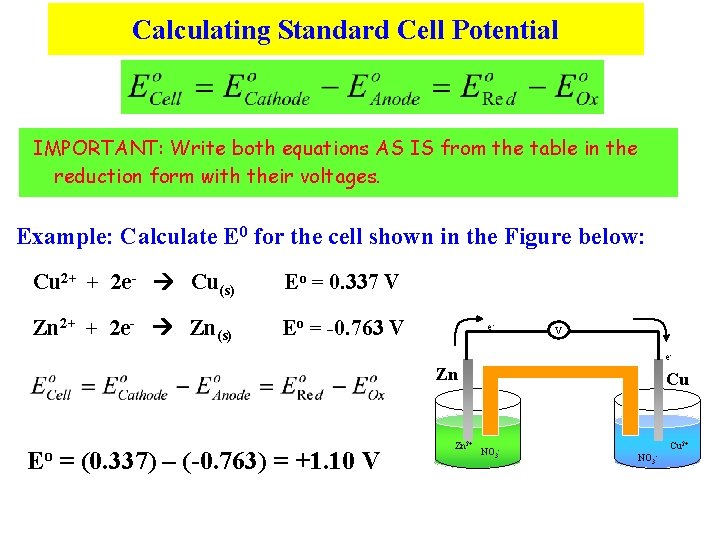
Calculating Standard Cell Potential IMPORTANT: Write both equations AS IS from the table in the reduction form with their voltages. Example: Calculate E 0 for the cell shown in the Figure below: Cu 2+ + 2 e- Cu(s) Eo = 0. 337 V Zn 2+ + 2 e- Zn(s) Eo = -0. 763 V e- Zn Eo = (0. 337) – (-0. 763) = +1. 10 V Zn 2+ Cu NO 3 - Cu 2+ NO 3 -

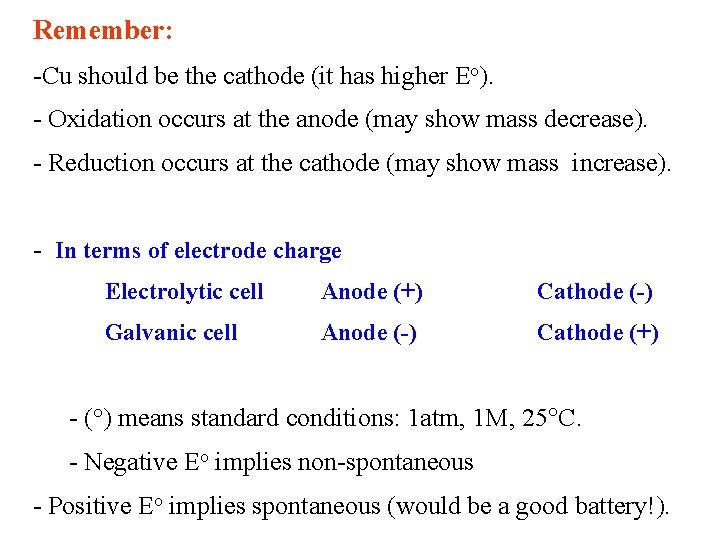
Remember: -Cu should be the cathode (it has higher Eo). - Oxidation occurs at the anode (may show mass decrease). - Reduction occurs at the cathode (may show mass increase). - In terms of electrode charge Electrolytic cell Anode (+) Cathode (-) Galvanic cell Anode (-) Cathode (+) - (°) means standard conditions: 1 atm, 1 M, 25 C. - Negative Eo implies non-spontaneous - Positive Eo implies spontaneous (would be a good battery!).

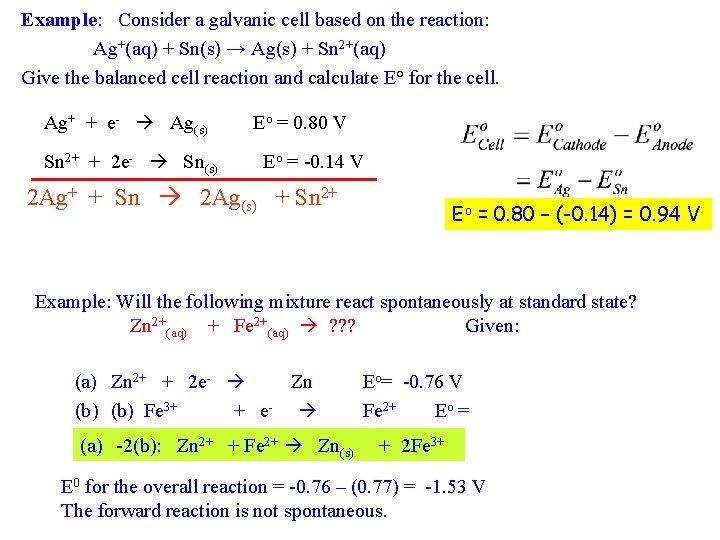
Example: Consider a galvanic cell based on the reaction: Ag+(aq) + Sn(s) → Ag(s) + Sn 2+(aq) Give the balanced cell reaction and calculate E° for the cell. Ag+ + e- Ag(s) Sn 2+ + 2 e- Sn(s) Eo = 0. 80 V Eo = -0. 14 V 2 Ag+ + Sn 2 Ag(s) + Sn 2+ Eo = 0. 80 – (-0. 14) = 0. 94 V Example: Will the following mixture react spontaneously at standard state? Zn 2+(aq) + Fe 2+(aq) ? ? ? Given: (a) Zn 2+ + 2 e- Zn Eo= -0. 76 V (b) Fe 3+ + e- Fe 2+ Eo = V Zn 2+ + Fe 2+ Zn (a) 0. 77 -2(b): + 2 Fe 3+ (s) E 0 for the overall reaction = -0. 76 – (0. 77) = -1. 53 V The forward reaction is not spontaneous.

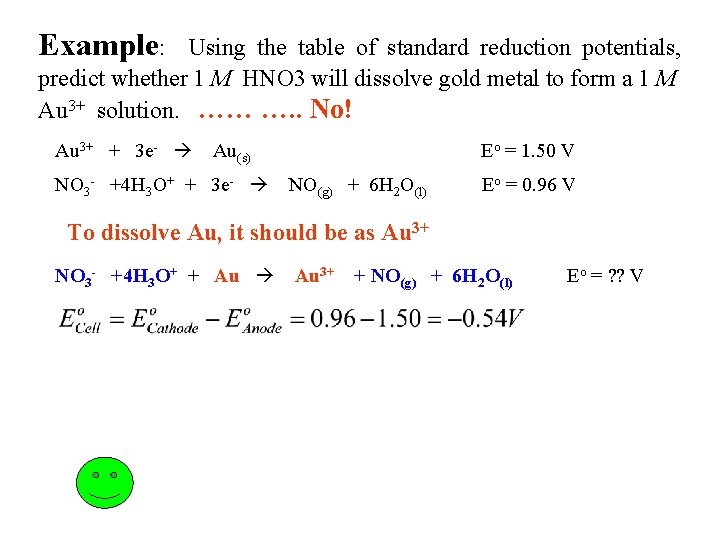
Example: Using the table of standard reduction potentials, predict whether 1 M HNO 3 will dissolve gold metal to form a 1 M Au 3+ solution. …… …. . No! Au 3+ + 3 e- Au(s) NO 3 - +4 H 3 O+ + 3 e- Eo = 1. 50 V NO(g) + 6 H 2 O(l) Eo = 0. 96 V To dissolve Au, it should be as Au 3+ NO 3 - +4 H 3 O+ + Au 3+ + NO(g) + 6 H 2 O(l) Eo = ? ? V

Dependence of Cell Potential on Concentration Voltaic cells at non-standard conditions -- Le. Chatlier’s principle can be applied. An increase in the concentration of a reactant will favor the forward reaction and the cell potential will increase. The converse is also true! Example: For the cell reaction: 2 Al + 3 Mn 2+ → 2 Al 3+ + 3 Mn E°cell = ? ? Predict whether Ecell is larger or smaller than E°cell for the following cases: a. [Al 3+ ] = 2. 0 M, [Mn 2+ ] = 1. 0 M A: Ecell < E°cell b. [Al 3+ ] = 1. 0 M, [Mn 2+] = 3. 0 M B: Ecell > E°cell

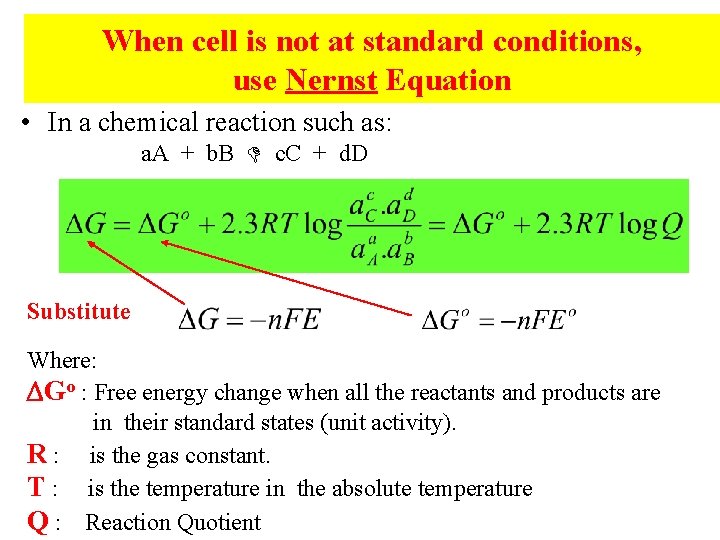
When cell is not at standard conditions, use Nernst Equation • In a chemical reaction such as: a. A + b. B c. C + d. D Substitute Where: Go : Free energy change when all the reactants and products are in their standard states (unit activity). R : is the gas constant. T : is the temperature in the absolute temperature Q : Reaction Quotient

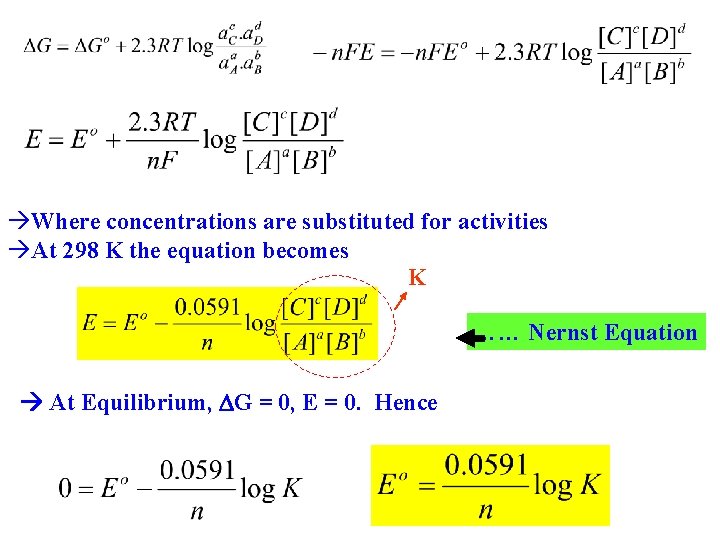
Where concentrations are substituted for activities At 298 K the equation becomes K …… Nernst Equation At Equilibrium, G = 0, E = 0. Hence

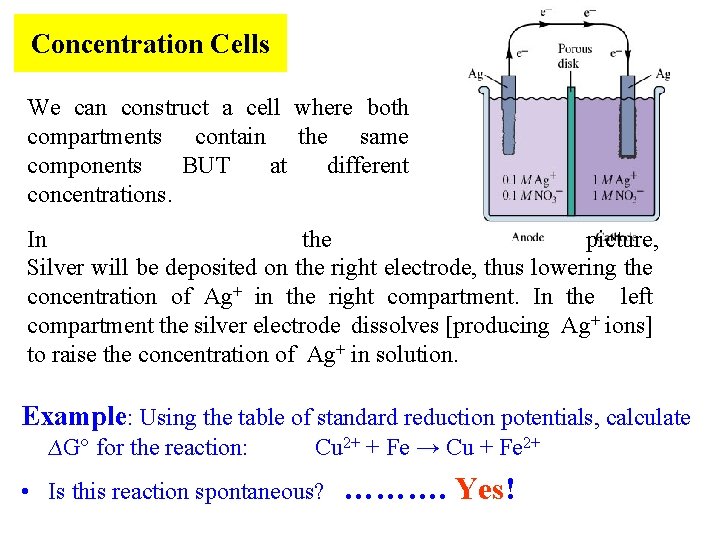
Concentration Cells We can construct a cell where both compartments contain the same components BUT at different concentrations. In the picture, Silver will be deposited on the right electrode, thus lowering the concentration of Ag+ in the right compartment. In the left compartment the silver electrode dissolves [producing Ag+ ions] to raise the concentration of Ag+ in solution. Example: Using the table of standard reduction potentials, calculate ∆G° for the reaction: Cu 2+ + Fe → Cu + Fe 2+ • Is this reaction spontaneous? ………. Yes!

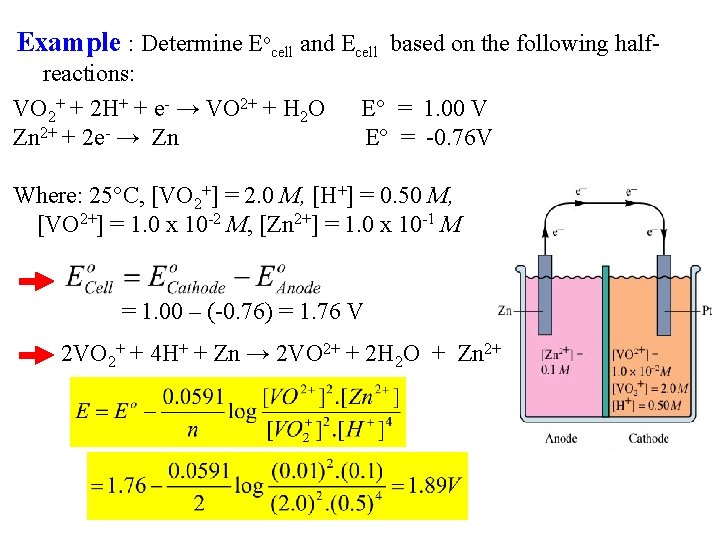
Example : Determine Eocell and Ecell based on the following half- reactions: VO 2+ + 2 H+ + e- → VO 2+ + H 2 O Zn 2+ + 2 e- → Zn E° = 1. 00 V E° = -0. 76 V Where: 25°C, [VO 2+] = 2. 0 M, [H+] = 0. 50 M, [VO 2+] = 1. 0 x 10 -2 M, [Zn 2+] = 1. 0 x 10 -1 M = 1. 00 – (-0. 76) = 1. 76 V 2 VO 2+ + 4 H+ + Zn → 2 VO 2+ + 2 H 2 O + Zn 2+

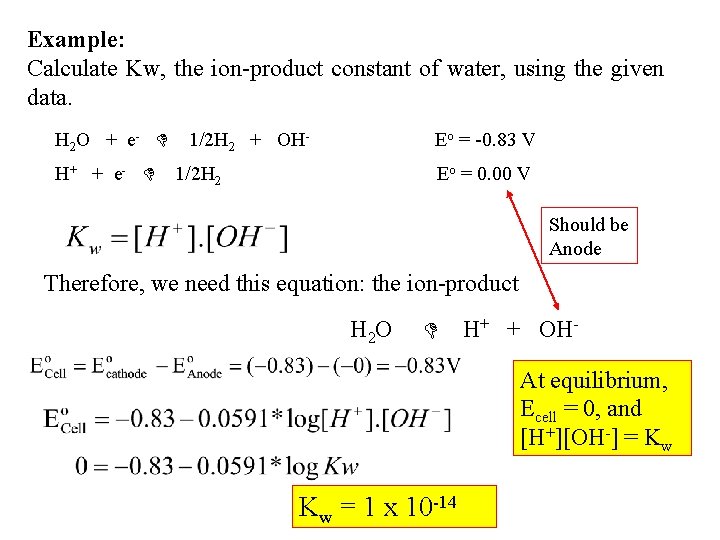
Example: Calculate Kw, the ion-product constant of water, using the given data. H 2 O + e - H+ + e - 1/2 H 2 + OH- Eo = -0. 83 V 1/2 H 2 Eo = 0. 00 V Should be Anode Therefore, we need this equation: the ion-product H 2 O H+ + OHAt equilibrium, Ecell = 0, and [H+][OH-] = Kw Kw = 1 x 10 -14