Section 10 Electrochemical Cells and Electrode Potentials Electrochemistry


- Slides: 14

Section 10 Electrochemical Cells and Electrode Potentials

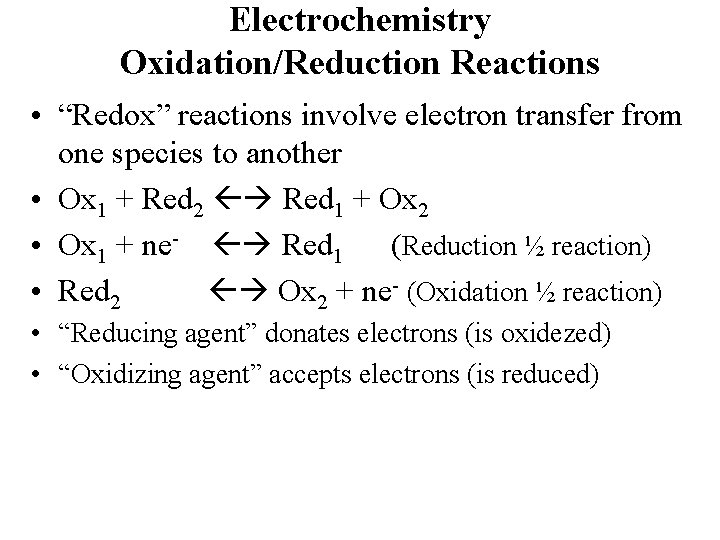
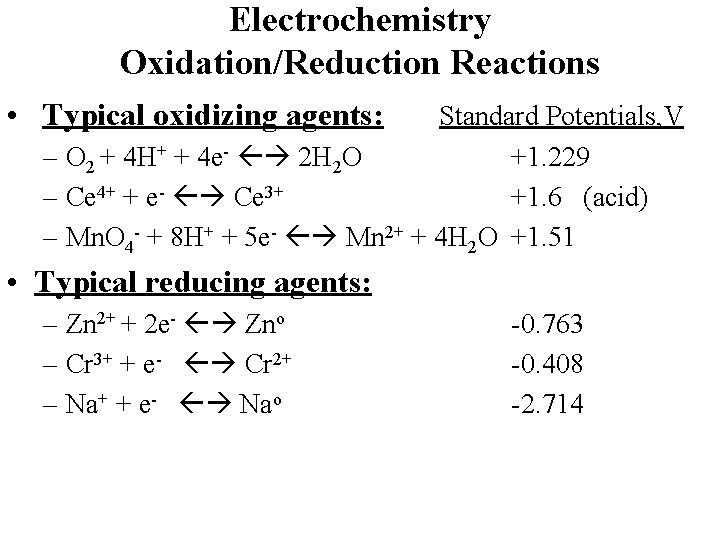
Electrochemistry Oxidation/Reduction Reactions • “Redox” reactions involve electron transfer from one species to another • Ox 1 + Red 2 Red 1 + Ox 2 • Ox 1 + ne- Red 1 (Reduction ½ reaction) • Red 2 Ox 2 + ne- (Oxidation ½ reaction) • “Reducing agent” donates electrons (is oxidezed) • “Oxidizing agent” accepts electrons (is reduced)

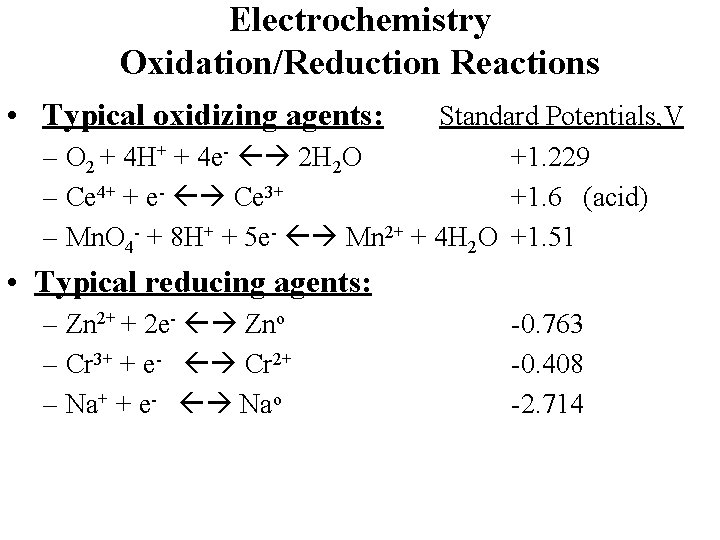
Electrochemistry Oxidation/Reduction Reactions • Typical oxidizing agents: Standard Potentials, V – O 2 + 4 H+ + 4 e- 2 H 2 O +1. 229 – Ce 4+ + e- Ce 3+ +1. 6 (acid) – Mn. O 4 - + 8 H+ + 5 e- Mn 2+ + 4 H 2 O +1. 51 • Typical reducing agents: – Zn 2+ + 2 e- Zno – Cr 3+ + e- Cr 2+ – Na+ + e- Nao -0. 763 -0. 408 -2. 714

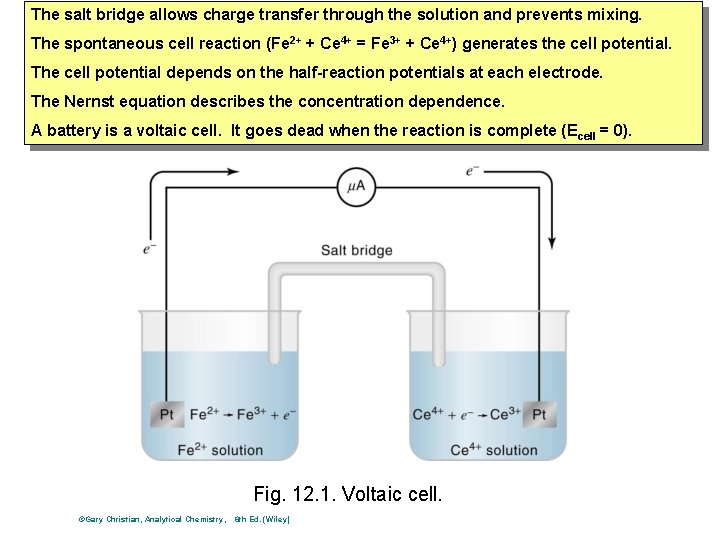
The salt bridge allows charge transfer through the solution and prevents mixing. The spontaneous cell reaction (Fe 2+ + Ce 4+ = Fe 3+ + Ce 4+) generates the cell potential. The cell potential depends on the half-reaction potentials at each electrode. The Nernst equation describes the concentration dependence. A battery is a voltaic cell. It goes dead when the reaction is complete (Ecell = 0). Fig. 12. 1. Voltaic cell. ©Gary Christian, Analytical Chemistry, 6 th Ed. (Wiley)

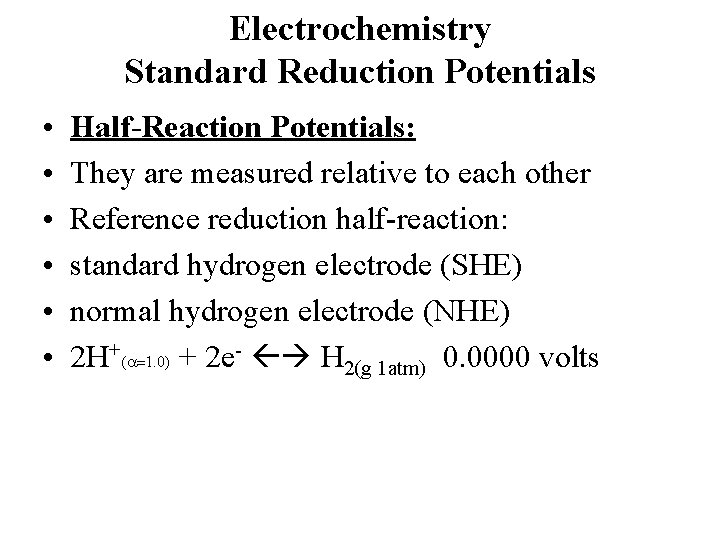
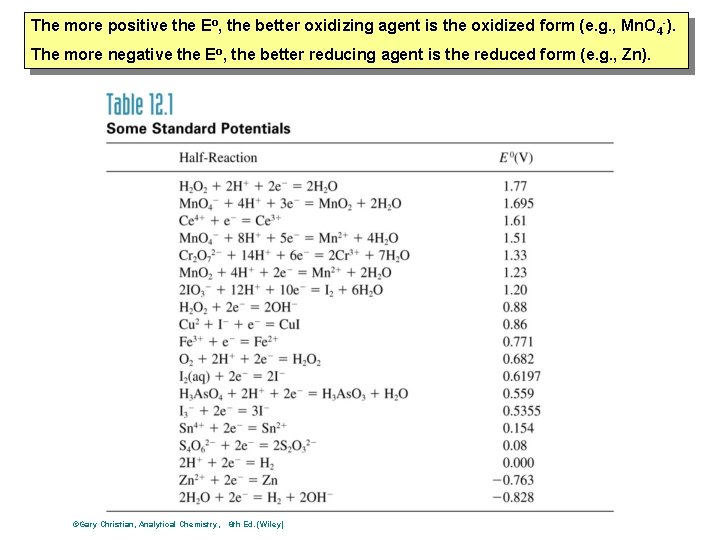
Electrochemistry Standard Reduction Potentials • • • Half-Reaction Potentials: They are measured relative to each other Reference reduction half-reaction: standard hydrogen electrode (SHE) normal hydrogen electrode (NHE) 2 H+(a=1. 0) + 2 e- H 2(g 1 atm) 0. 0000 volts

The more positive the Eo, the better oxidizing agent is the oxidized form (e. g. , Mn. O 4 -). The more negative the Eo, the better reducing agent is the reduced form (e. g. , Zn). ©Gary Christian, Analytical Chemistry, 6 th Ed. (Wiley)

Electrochemistry Reduction Potentials • General Conclusions: • 1. The more positive the electrode potential, the stronger an oxidizing agent the oxidized form is and the weaker a reducing agent the reduced form is • 2. The more negative the reduction potential, the weaker the oxidizing agent is the oxidized formis and the stronger the reducing agent the reduced form is.

Electrochemistry Oxidation/Reduction Reactions • Typical oxidizing agents: Standard Potentials, V – O 2 + 4 H+ + 4 e- 2 H 2 O +1. 229 – Ce 4+ + e- Ce 3+ +1. 6 (acid) – Mn. O 4 - + 8 H+ + 5 e- Mn 2+ + 4 H 2 O +1. 51 • Typical reducing agents: – Zn 2+ + 2 e- Zno – Cr 3+ + e- Cr 2+ – Na+ + e- Nao -0. 763 -0. 408 -2. 714

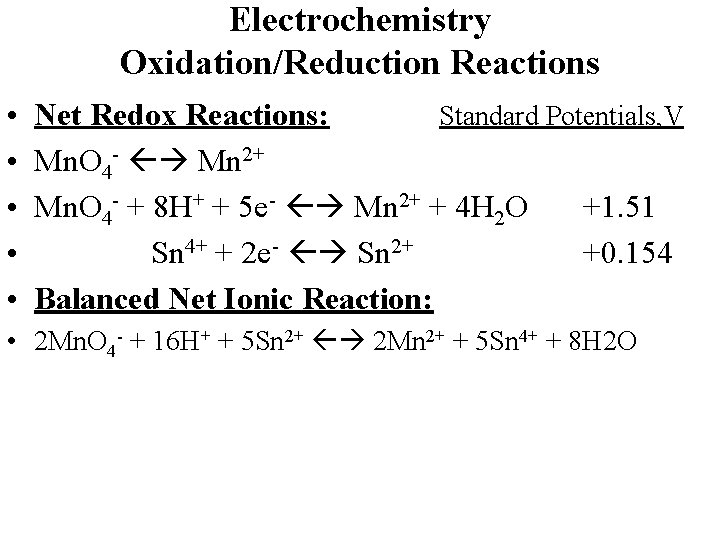
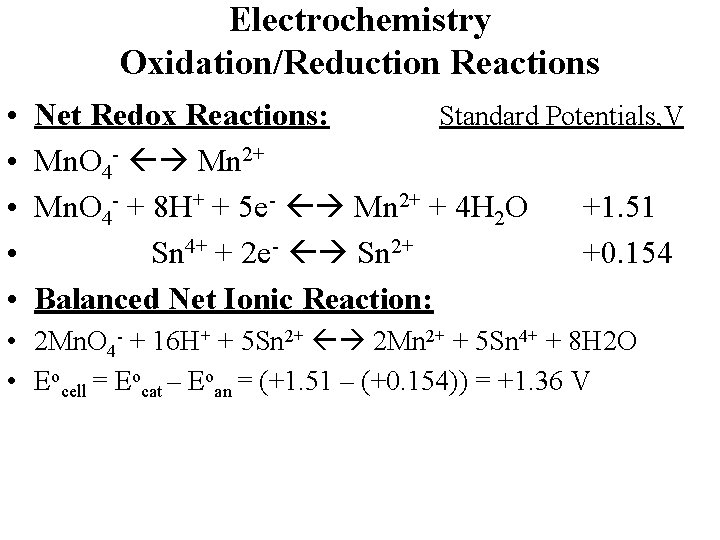
Electrochemistry Oxidation/Reduction Reactions • • • Net Redox Reactions: Standard Potentials, V Mn. O 4 - Mn 2+ Mn. O 4 - + 8 H+ + 5 e- Mn 2+ + 4 H 2 O +1. 51 Sn 4+ + 2 e- Sn 2+ +0. 154 Balanced Net Ionic Reaction: • 2 Mn. O 4 - + 16 H+ + 5 Sn 2+ 2 Mn 2+ + 5 Sn 4+ + 8 H 2 O

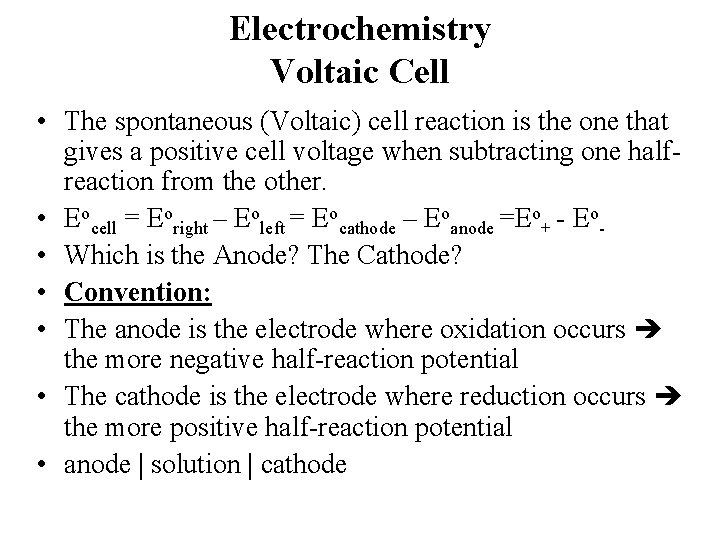
Electrochemistry Voltaic Cell • The spontaneous (Voltaic) cell reaction is the one that gives a positive cell voltage when subtracting one halfreaction from the other. • Eocell = Eoright – Eoleft = Eocathode – Eoanode =Eo+ - Eo • Which is the Anode? The Cathode? • Convention: • The anode is the electrode where oxidation occurs the more negative half-reaction potential • The cathode is the electrode where reduction occurs the more positive half-reaction potential • anode solution cathode

Electrochemistry Oxidation/Reduction Reactions • • • Net Redox Reactions: Standard Potentials, V Mn. O 4 - Mn 2+ Mn. O 4 - + 8 H+ + 5 e- Mn 2+ + 4 H 2 O +1. 51 Sn 4+ + 2 e- Sn 2+ +0. 154 Balanced Net Ionic Reaction: • 2 Mn. O 4 - + 16 H+ + 5 Sn 2+ 2 Mn 2+ + 5 Sn 4+ + 8 H 2 O • Eocell = Eocat – Eoan = (+1. 51 – (+0. 154)) = +1. 36 V

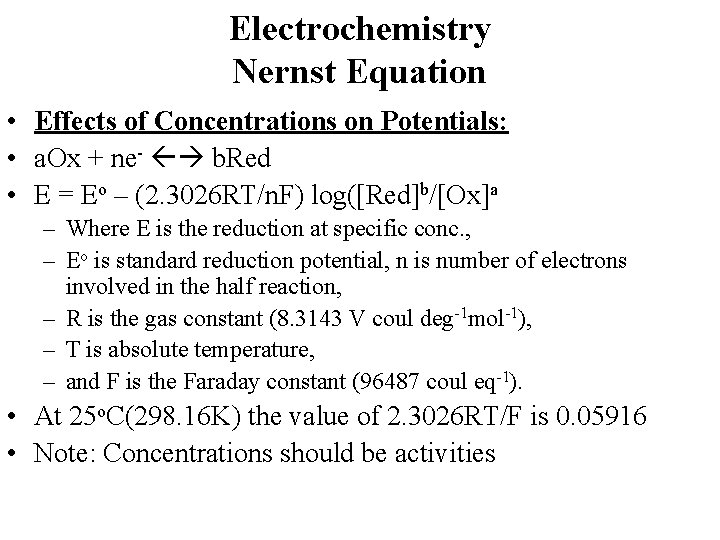
Electrochemistry Nernst Equation • Effects of Concentrations on Potentials: • a. Ox + ne- b. Red • E = Eo – (2. 3026 RT/n. F) log([Red]b/[Ox]a – Where E is the reduction at specific conc. , – Eo is standard reduction potential, n is number of electrons involved in the half reaction, – R is the gas constant (8. 3143 V coul deg-1 mol-1), – T is absolute temperature, – and F is the Faraday constant (96487 coul eq-1). • At 25 o. C(298. 16 K) the value of 2. 3026 RT/F is 0. 05916 • Note: Concentrations should be activities

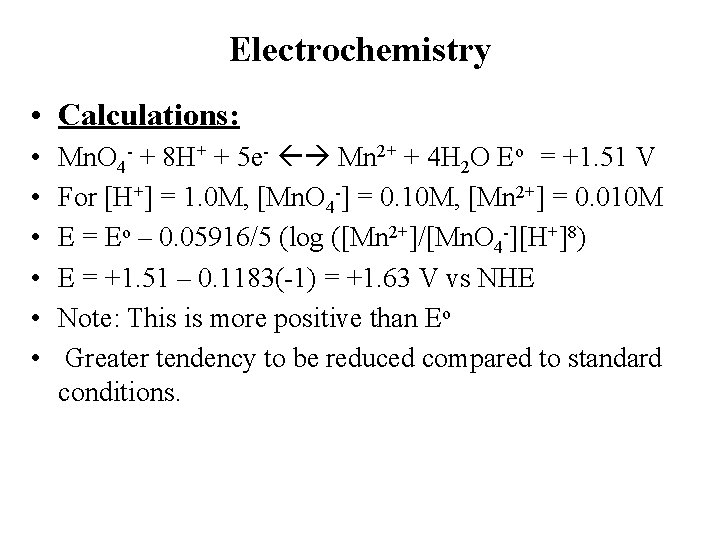
Electrochemistry • Calculations: • • • Mn. O 4 - + 8 H+ + 5 e- Mn 2+ + 4 H 2 O Eo = +1. 51 V For [H+] = 1. 0 M, [Mn. O 4 -] = 0. 10 M, [Mn 2+] = 0. 010 M E = Eo – 0. 05916/5 (log ([Mn 2+]/[Mn. O 4 -][H+]8) E = +1. 51 – 0. 1183(-1) = +1. 63 V vs NHE Note: This is more positive than Eo Greater tendency to be reduced compared to standard conditions.

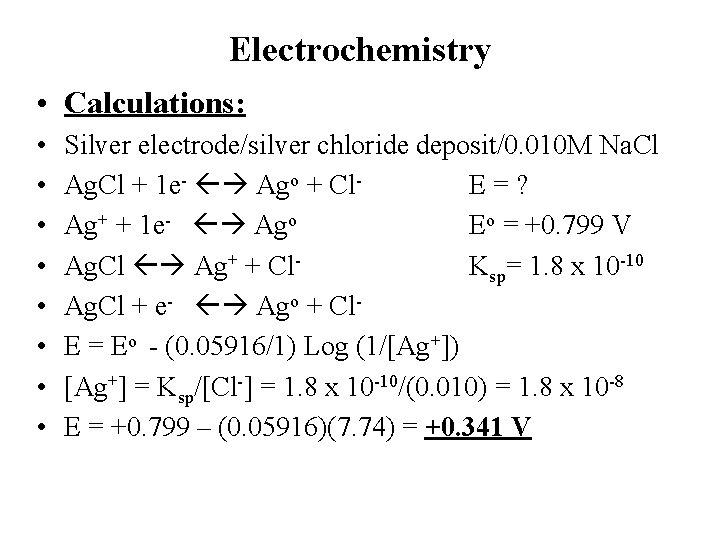
Electrochemistry • Calculations: • • Silver electrode/silver chloride deposit/0. 010 M Na. Cl Ag. Cl + 1 e- Ago + Cl. E=? Ag+ + 1 e- Ago Eo = +0. 799 V Ag. Cl Ag+ + Cl. Ksp= 1. 8 x 10 -10 Ag. Cl + e- Ago + Cl. E = Eo - (0. 05916/1) Log (1/[Ag+]) [Ag+] = Ksp/[Cl-] = 1. 8 x 10 -10/(0. 010) = 1. 8 x 10 -8 E = +0. 799 – (0. 05916)(7. 74) = +0. 341 V