STANDARD ELECTRODE POTENTIALS THE STANDARD HYDROGEN ELECTRODE In








- Slides: 15

STANDARD ELECTRODE POTENTIALS

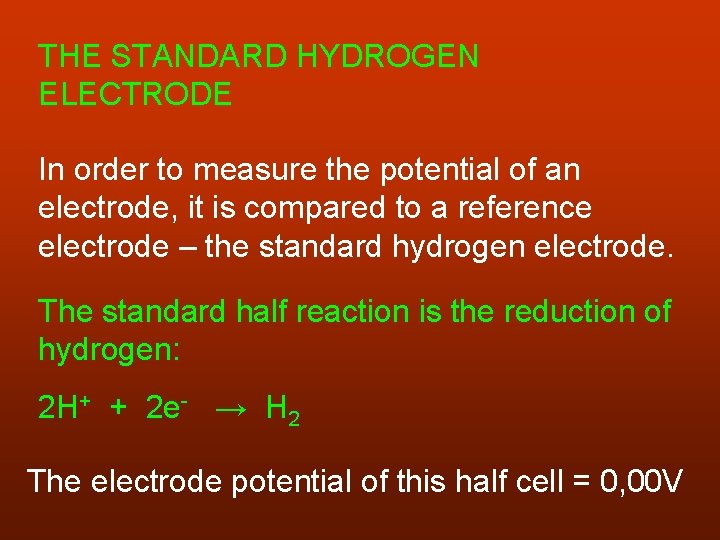
THE STANDARD HYDROGEN ELECTRODE In order to measure the potential of an electrode, it is compared to a reference electrode – the standard hydrogen electrode. The standard half reaction is the reduction of hydrogen: 2 H+ + 2 e- → H 2 The electrode potential of this half cell = 0, 00 V

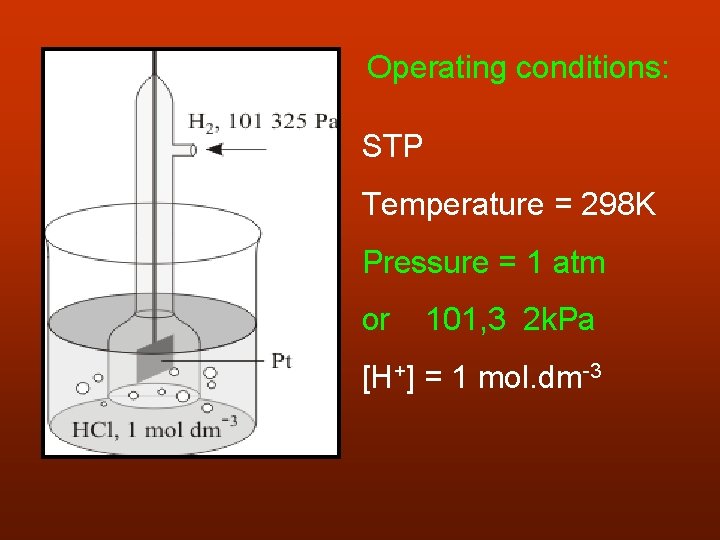
Operating conditions: STP Temperature = 298 K Pressure = 1 atm or 101, 3 2 k. Pa [H+] = 1 mol. dm-3

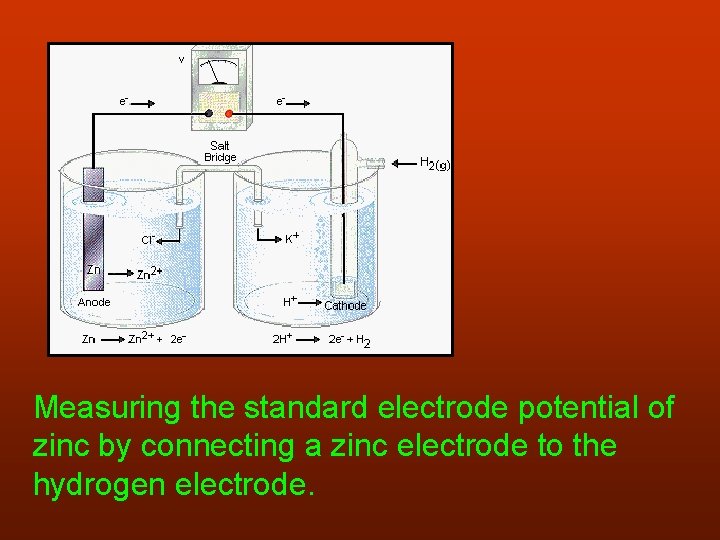
Measuring the standard electrode potential of zinc by connecting a zinc electrode to the hydrogen electrode.

The hydrogen electrode is the cathode. 2 H+(aq) + 2 e- → H 2(g) The zinc electrode is the anode. Zn(s) → Zn 2+(aq) + 2 e. The reading on the voltmeter is 0, 76 V and because electrons flow from the zinc to the hydrogen electrode Eo for the zinc half cell = - 0, 76 V

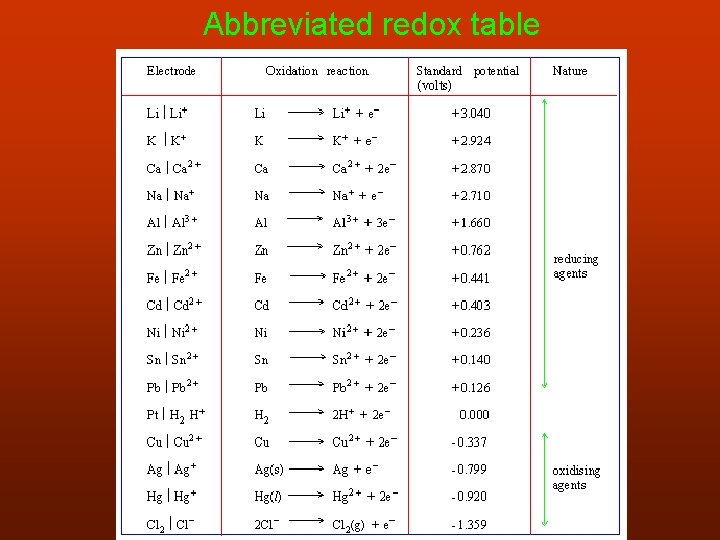
Abbreviated redox table

Full Redox potential table Any substance on the right will spontaneously react with something above it on the right of the table – and vice versa.

When using the table: • strong reducing agents are at the top of the table. • when combining half reactions, the one higher up the table will be the reducing agent. • when combining half reactions, the half reaction located higher up the table is written in reverse (from left to right) and the one lower down from right to left.

Using the table of standard electrode potentials: • determine the emf of a cell. • predicting whether a redox reaction will occur spontaneously; • balancing redox reactions.

Determining the emf of a cell: Eo cell = Eo reduction – Eo oxidation = Eo oxidising agent – Eo reducing agent = Eo cathode – Eo anode

For the cell: Zn / Zn 2+ // Cu 2+ / Cu The zinc electrode is the reducing agent and the anode as oxidation occurs at that electrode. Eo cell = Eo Cu – Eo Zn = 0, 34 – (-0, 76) = +1, 10 V

Predicting whether a redox reaction will occur spontaneously. • Write down the equation as you expect it to occur. • from the equation decide which half cell is the reducing/oxidising agent. • Determine Eocell based on this information. • If Eocell is a positive number, the reaction, as written, is non-spontaneous.

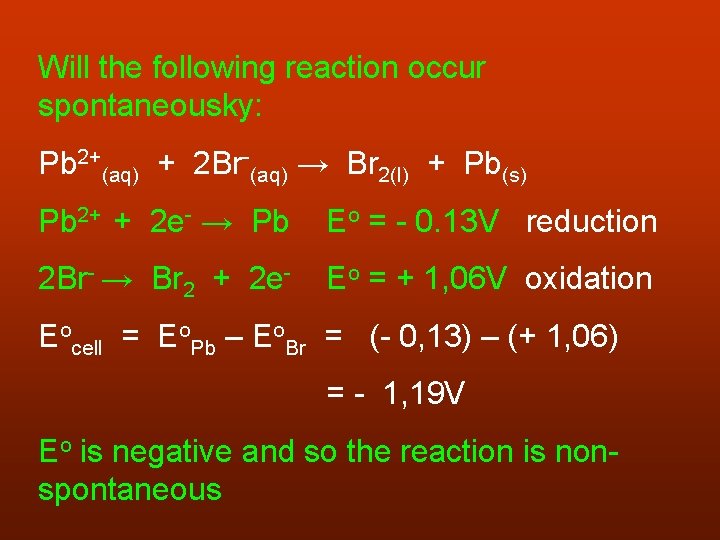
Will the following reaction occur spontaneousky: Pb 2+(aq) + 2 Br-(aq) → Br 2(l) + Pb(s) Pb 2+ + 2 e- → Pb Eo = - 0. 13 V reduction 2 Br- → Br 2 + 2 e- Eo = + 1, 06 V oxidation Eocell = Eo. Pb – Eo. Br = (- 0, 13) – (+ 1, 06) = - 1, 19 V Eo is negative and so the reaction is nonspontaneous

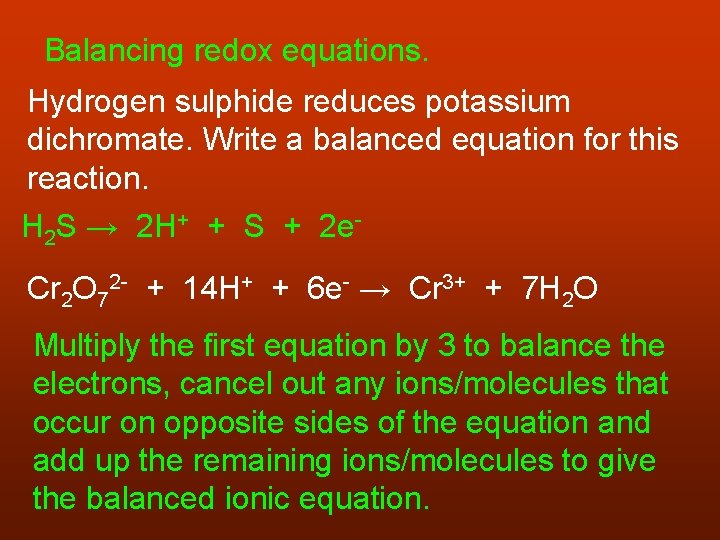
Balancing redox equations. Hydrogen sulphide reduces potassium dichromate. Write a balanced equation for this reaction. H 2 S → 2 H+ + S + 2 e. Cr 2 O 72 - + 14 H+ + 6 e- → Cr 3+ + 7 H 2 O Multiply the first equation by 3 to balance the electrons, cancel out any ions/molecules that occur on opposite sides of the equation and add up the remaining ions/molecules to give the balanced ionic equation.

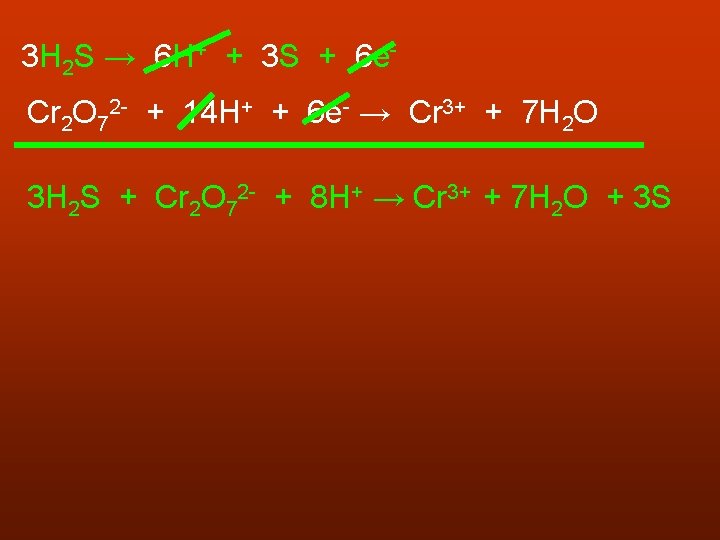
3 H 2 S → 6 H+ + 3 S + 6 e. Cr 2 O 72 - + 14 H+ + 6 e- → Cr 3+ + 7 H 2 O 3 H 2 S + Cr 2 O 72 - + 8 H+ → Cr 3+ + 7 H 2 O + 3 S