USES OF STANDARD ELECTRODE POTENTIALS THERE ARE THREE3








- Slides: 26

USES OF STANDARD ELECTRODE POTENTIALS THERE ARE THREE(3) USES OF STANDARD ELECTRODE POTENTIAL: v. TO MEASURE THE RELATIVE STRENGTHS OF OXIDIZING AND REDUCING AGENTS. v TO CALCULATE THE STANDARD CELL POTENTIALS. v TO PREDICT POSSIBLE REACTIONS.

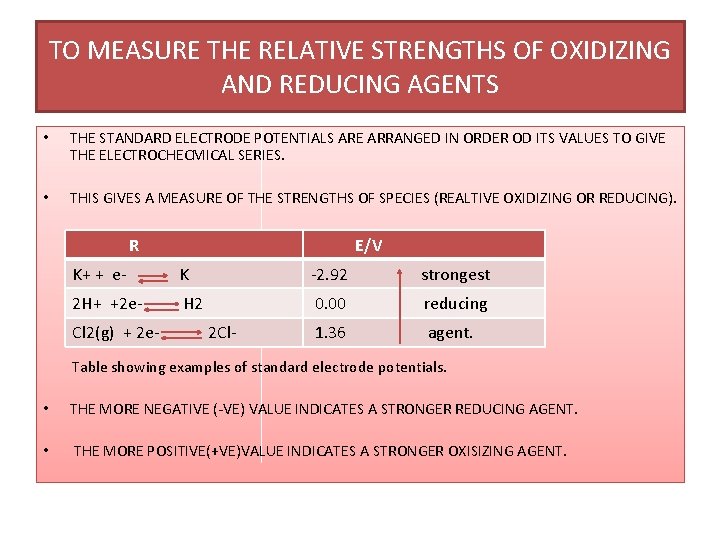
TO MEASURE THE RELATIVE STRENGTHS OF OXIDIZING AND REDUCING AGENTS • THE STANDARD ELECTRODE POTENTIALS ARE ARRANGED IN ORDER OD ITS VALUES TO GIVE THE ELECTROCHECMICAL SERIES. • THIS GIVES A MEASURE OF THE STRENGTHS OF SPECIES (REALTIVE OXIDIZING OR REDUCING). R E/V K+ + e- K -2. 92 strongest 2 H+ +2 e- H 2 0. 00 reducing 1. 36 agent. Cl 2(g) + 2 e- 2 Cl- Table showing examples of standard electrode potentials. • THE MORE NEGATIVE (-VE) VALUE INDICATES A STRONGER REDUCING AGENT. • THE MORE POSITIVE(+VE)VALUE INDICATES A STRONGER OXISIZING AGENT.

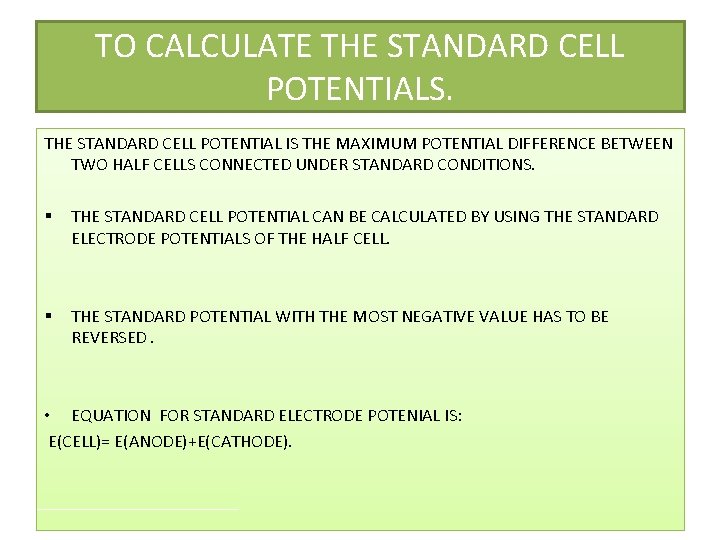
TO CALCULATE THE STANDARD CELL POTENTIALS. THE STANDARD CELL POTENTIAL IS THE MAXIMUM POTENTIAL DIFFERENCE BETWEEN TWO HALF CELLS CONNECTED UNDER STANDARD CONDITIONS. § THE STANDARD CELL POTENTIAL CAN BE CALCULATED BY USING THE STANDARD ELECTRODE POTENTIALS OF THE HALF CELL. § THE STANDARD POTENTIAL WITH THE MOST NEGATIVE VALUE HAS TO BE REVERSED. • EQUATION FOR STANDARD ELECTRODE POTENIAL IS: E(CELL)= E(ANODE)+E(CATHODE).

QUESTION § CALCULATE THE E (cell) VALUE IF THE STANDARD ELECTRODE POTENTIAL OF CHLORINE IS 1. 36 V AND POTASSIUM IS 2. 92 V. ANSWER

ANSWER. • SINCE POTASSIUM HAS THE MORE NEGATIVE ELECTRODE POTENTIAL THE SIGN IS REVERSED. E(CELL)=E(ANODE)+E(CATHODE) E(CELL)=+2. 92 V + 1. 36 V E(CELL)=+4. 28 V.

TO PREDICT POSSIBLE REACTIONS. • A REACTION WILL ONLY OCCUR IF: • THE E. M. F OF THE REACTION IS POSITIVE , THE REACTION IS SAID TO BE FEASIBLE. • THE REACTION WILL NOT OCCUR IF: • THE EMF OF THE REACTION IS NEGATIVE.

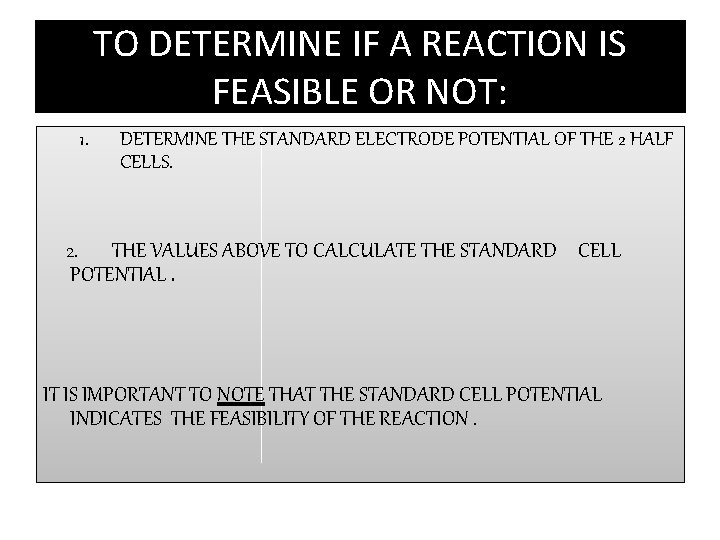
TO DETERMINE IF A REACTION IS FEASIBLE OR NOT: 1. DETERMINE THE STANDARD ELECTRODE POTENTIAL OF THE 2 HALF CELLS. 2. THE VALUES ABOVE TO CALCULATE THE STANDARD CELL POTENTIAL. IT IS IMPORTANT TO NOTE THAT THE STANDARD CELL POTENTIAL INDICATES THE FEASIBILITY OF THE REACTION.

EFFECT OF CONCENTRATION ON ELECTRODE POTENTIAL. • THE ELECTRODE POTENTIAL IS MEASURED UNDER STANDARD CONDITIONS, i. e MAINTAINED CONCENTRATION , TEMPERATURE AND PRESSURE. • WHEN THERE IS A CHANGE IN THESE VALUES IT AFFECTS THE ELECTRODE POTENTIAL, TEMPERATURE AND PRESSURE AND BE KEPT CONSTANT BUT CONCENTRATION CANNOT.

USING LE CHARTERLIE’S PRINCIPLE. • WHEN A REACTION OCCURS THE AMOUNT OF A SUBSTANCE USED IS NOT THE AMOUNT PRODUCED, HENCE THERE IS A CONCENTRATION CHANGE. • IF THERE IS A DECREASE IN THE CONC IN THE CATHODE , TAKE IN TO CONSIDERATION LE CHARTERLIE’S PRINCIPLE.

LE CHARTERLIE’S PRINCIPLE STATES THAT IF A SYSTEM IN EQUILIBRIUM IS DISTURBED BY CHANGES IN THE DETERMING FACTOR , e. g. CONCENTRATION, THE SYSTEM WILL TEND TO SHIFT ITS EQULIBRIUM POSITION SO AS TO COUNTERACT THE EFFECT OF THE DISTURBANCE.

ENERGY STORAGE DEVICES • ENERGY STORAGE DEVICES CONVERT CHEMICAL ENERGY INTO ELECTRICAL, TWO SUCH EXAMPLES ARE BATTERIES AND CELLS. • A BATTERY IS MANY CELLS JOINED IN SERIES OR PARALLEL. • PRIMARY CELLS ARE BATTERIES THAT PRODUCE EMF FROM IRREVERSIBLE CHEMICAL REACTIONS. • SECONDARY CELLS ARE BATTERIES WHICH PRODUCE EMF FROM REVERSIBLE CHEMICAL REACTIONS.

THERE ARE FIVE(5) TYPES OF CELLS /BATTERIES: 1. THE DANIEll CELL 2. THE DRY LECLANCHE CELL. 3. ALKALINE CELL. 4. LEAD –ACID ACCUMULATOR. 5. FEUL CELL.

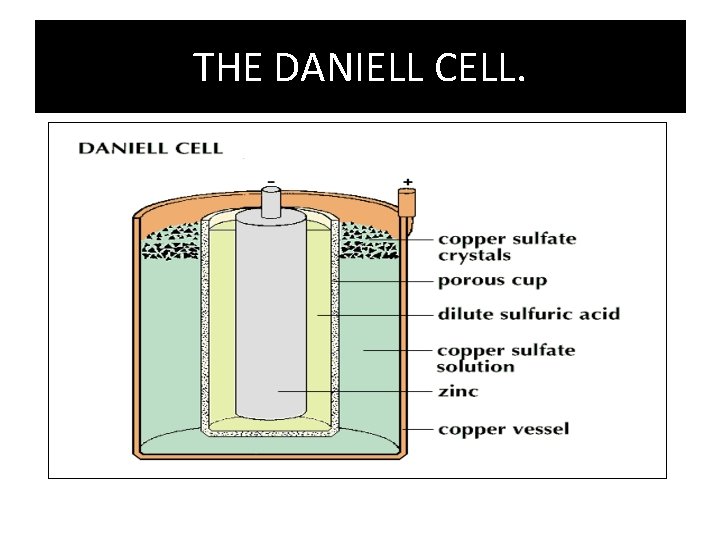
THE DANIELL CELL. • THIS IS THE FIRST CELL TO BE USED. • IT WAS INVENTED BY JOHN FREDERIC DANIELL. • IT CONSIST OF Cu POT FILLED WITH Cu. SO 4 • A POROUS POT IS IMMERSED IN THE COPPER POT ABOVE. • THE POROUS POT COINTAINED A ZINC ANODE WHICH TO THE CENTRE • THE EMF UNDER IS 1. 10 VOLTS.

THE DANIELL CELL.

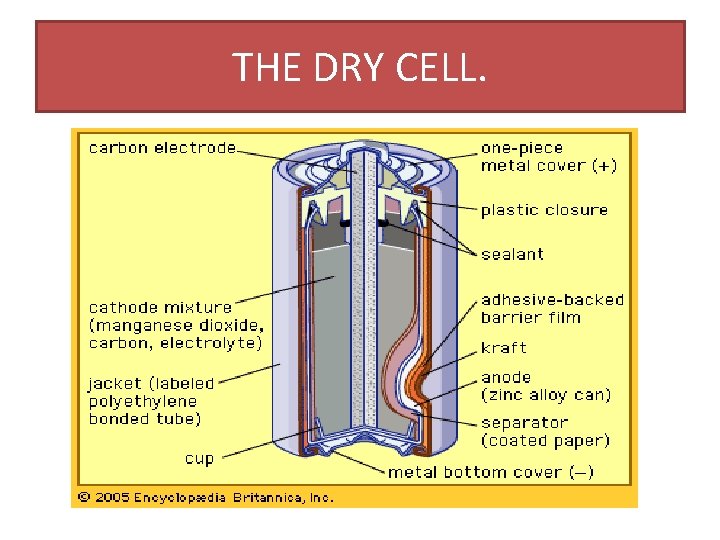
THE DRY LECLANCHE CELL. • THE WAS INVENTED IN 1866 BY GEORGES LECLANCHE. • POROUS POT CONSIST OF A CARBON ROD DIPPED INTO A MANGANESE AND CARBON POWDER PASTE. • THE POROUS POT IS SURROUNDED BY ANOTHER PASTE OF ZINC CHLORIDE AND AMMONIUM CHLORIDE DISSOLVED IN WATER. • THE ABOVE IS ENCLOSED IN A ZINC CASE WHICH ACTS AS THE ANODE.

THE DRY CELL.

REACTIONS THAT OCCUR WHEN THE DRY CELL IS OPERATING. • WHEN THE CELL IS OPERATING : 1. THE OUTER ZINC CASE OXIDISES , THIS IS SEEN IN THE FOLLOWING EQUATION Zn(s) Zn 2+ (aq) + 2 e. 2. AT THE CATHODE, THE EQUATION BELOW SHOWS THE REACTION. 2 Mn. O 2(s) +2 H+(aq) + 2 e Mn 2 O(s) +H 20. • THE ELECTROLYTE OF THE CELL LEAK OUT AS THE ZINC CONTAINER GETS THINNER. • DUE TO THE AMMONIUM CHLORIDE IN THE CELL THE ZINC CASING WARES AWAY EVEN WHEN NOT IN USE. • THE CELL HAS AN EMF OF 1. 5 VOLTS AND CAN LAST UP TO 1. 5 YEARS.

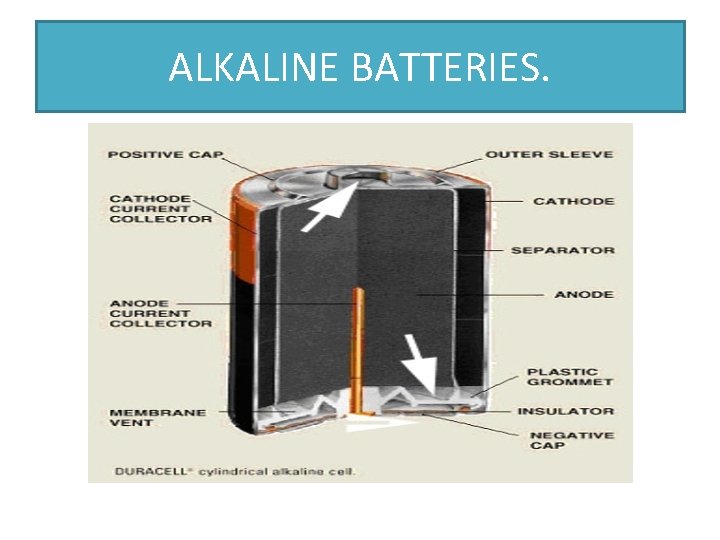
ALKALINE BATTERY • THESE ARE SIMILAR TO LECLANCHE DRY CELLS. THE DRY CELL USES POTASSIUM HYDROXIDE INSTEAD OF AMMONIUM CHLORIDE AS THE ELECTROLYTE. • THE ANODE IS MADE UP OF ZINC POWDER WHICH INCREASES THE RATE OF REACTION. • THE CATHODE IS MADE UP OF MANGANESE DIOXIDE. • THE HALF EQUATIONS FOR THE REACTION ARE AS FOLLOWS: Zn(s) + 2 OH-(aq) 2 Mn. O 2(s) +H 2 O+2 e Zn(OH)2(aq) + 2 e AND Mn 2 O 3(S) + 2 OH-(aq) NOTE: BATTERIES SHOULD BE REMOVED FROM DEVICES BEFORE STORAGE.

ALKALINE BATTERIES.

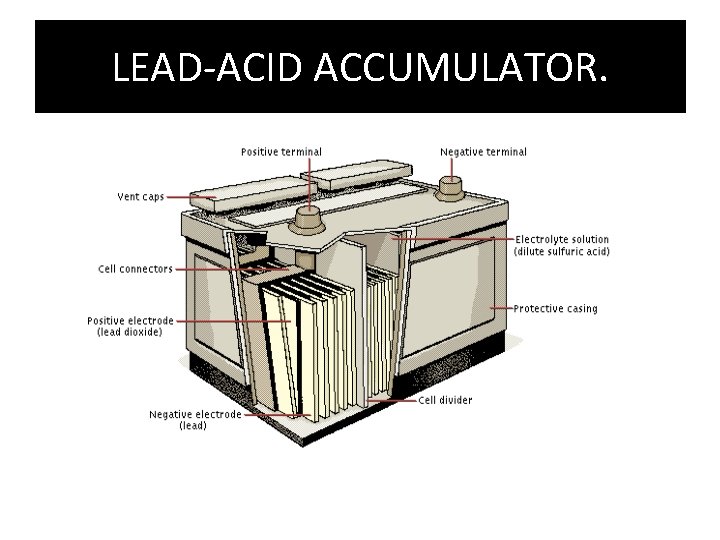
THE LEAD ACID ACCUMULATOR. • IT WAS INVENTED IN 1859 BY GASTON PLANTE. • THEY ARE THE OLDEST TYPE OF RECHARGEABLE BATTERIES. • THIS CONSIST OR 2 -6 CELLS (CONNECTED IN SERIES), GENERATING 6/12 VOLTS. • THE ABOVE PROVIDES ENOUGH ELECTRICITY FOR STARTING AND IN ENGINE IN A VEHICLE • THE CELL CONSIST OF A LEAD ANODE AND A LEAD (IV) OXIDE IN CONCENTRATED H 2 SO 4.

LEAD-ACID ACCUMULATOR.

REACTIONS THAT OCCUR WHEN THE LEAD-ACID ACCUMULATOR IS OPERATING. • WHEN THE CELL IS OPERATING: 1. THE ANODE IS OXIDISED FROM Pb TO Pb 2+ IONS. THE EQUATION FOR THIS REACTION IS : PB(s) Pb 2+ (aq) + 2 e. 2. AT THE CATHODE THE Pb. O 2 REACTS WITH THE H+ IONS IN THE H 2 SO 4 TO FORM Pb 2+ IONS. THE EQUATION FOR THSI REACTION IS : Pb. O 2(s) +4 H+(aq) +2 e PB 2+(aq) +2 H 2 O(l). 3. THE Pb 2+ IONS THAT IS FORMED REACTS WITH THE SO 4 IONS IN THE ACID AND LEAD SULPHATE IS FORMED. THE EQUATION FOR THIS REACTION IS: Pb 2+(aq) +SO 4(aq) Pb. SO 4(s). 4. THE ALTERNATOR RECHARGES THE BATTERY BY PASSING AND ELECTRIC CURRENT THUS RESTORING THE BATTERY TO ITS ORIGINAL CONDITION. NOTE: IF THERE IS A BUILD UP OF Pb. SO 4 WHICH BECOMES COARSER AND INERT THEN THIS PREVENTS THE BATTERY FROM RETURNING TO ITS ORIGINAL CONDITION BY RECHARGING.

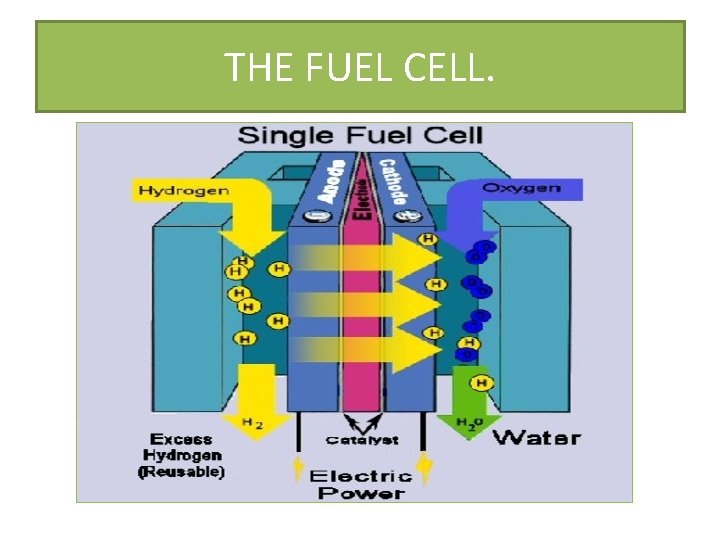
THE FUEL CELL. • THIS IS A DEVICE THAT CONVERTS THE CHEMICAL ENERGY FROM A FUEL INTO ELECTRICITY THROUGH A CHEMICAL REACTION. • IT IS A PRIMARY CELL , IT IS DIFFERENT IN THE SENSE THAT THE ELECTRICITY SOURCE CAN BE REPLACED CONSTANTLY. • THE ELECTRODES ARE RELATIVELY INERT AND ONLY CATALYZE THE CELL REACTIONS. • PRESENTLY THE FUEL CELL IN USE IS THE HYDROGEN-OXYGEN FUEL CELL. • THE H-O FUEL CELL IS HIGHLY EFFICIENT AND POLLUTION FREE. • THEY ARE USED IN SPACE CRAFTS FOR HEAT, ELECTRICITY AND TO PROVIDE DRINKING WATER FOR ASTRONAUTS.

THE FUEL CELL. IT CONSISTS OF WARM POTASSIUM HYDROXIDE SOLUTION WHICH IS HELD BETWEEN POROUS CARBON ELECTRODES. THE ELECTRODES ARE COATED WITH A CATALYST E. G PLATINUM OR NICKEL THIS INCREASES THE RATE OF REACTION.

THE FUEL CELL.

REACTIONS OCCURING WHEN THE CELL IS OPERATING. 1. HYDROGEN ENTERS INTO THE –VE COMPARTMENT OF THE CELL AND DIFFUSES THROUGH THE CARBON ANODE. 2. THE CATALYST SEPARATES THE GAS INTO IONS AND ELECTRONS. 3. THE EQUATION FOR THE REACTION AT THE ANODE IS: H 2(g) +2 OH(aq) 2 H 2 O(l) +2 e. 4. OXYGEN ENTERS INTO THE +VE COMPARTMENT OF THE CELL AND DIFFUSES INTO THE POTASSIUM HYDROXIDE. 5. OXYGEN , WATER AND ELECTRONS COMBINE CATALYTICALLY TO FORM HYDROXIDE IONS , THIS CAN BE SEEN IN THE EQUATION BELOW. O 2(g) +2 H 2 O(l) +4 e 4 OH(aq). THE OVERALL EQUATION FOR THE HYDROGEN OXYGEN CELL IS : 2 H 2(g) +O 2(g) 2 H 2 O(l).