Electrode Potentials In a redox reaction electrons are
- Slides: 9

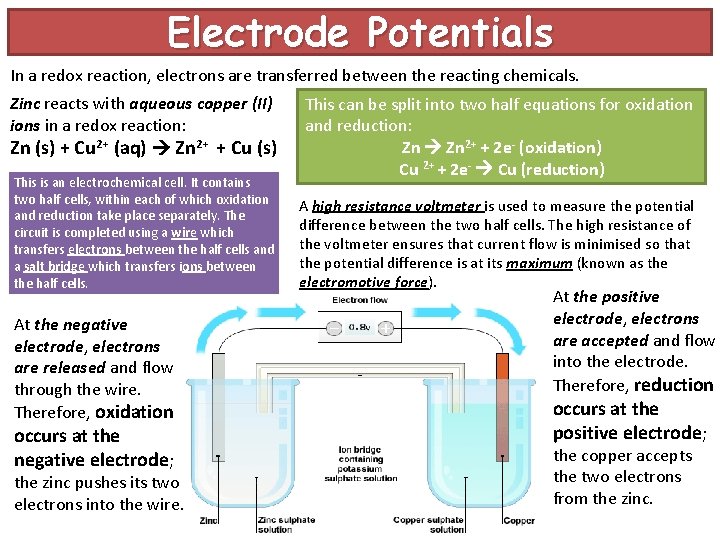
Electrode Potentials In a redox reaction, electrons are transferred between the reacting chemicals. Zinc reacts with aqueous copper (II) ions in a redox reaction: Zn (s) + Cu 2+ (aq) Zn 2+ + Cu (s) This is an electrochemical cell It contains two half cells, within each of which oxidation and reduction take place separately. The circuit is completed using a wire which transfers electrons between the half cells and a salt bridge which transfers ions between the half cells. At the negative electrode, electrons are released and flow through the wire. Therefore, oxidation occurs at the negative electrode; the zinc pushes its two electrons into the wire. This can be split into two half equations for oxidation and reduction: Zn 2+ + 2 e- (oxidation) Cu 2+ + 2 e- Cu (reduction) A high resistance voltmeter is used to measure the potential difference between the two half cells. The high resistance of the voltmeter ensures that current flow is minimised so that the potential difference is at its maximum (known as the electromotive force). At the positive electrode, electrons are accepted and flow into the electrode. Therefore, reduction occurs at the positive electrode; the copper accepts the two electrons from the zinc.

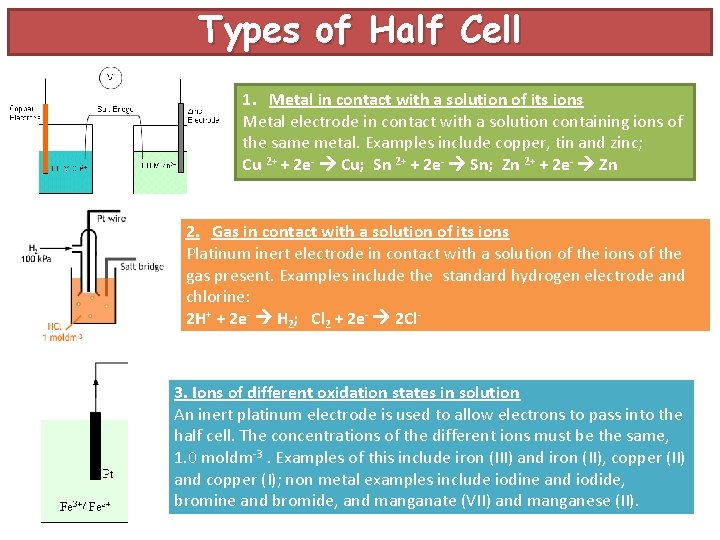
Types of Half Cell 1. Metal in contact with a solution of its ions Metal electrode in contact with a solution containing ions of the same metal. Examples include copper, tin and zinc; Cu 2+ + 2 e- Cu; Sn 2+ + 2 e- Sn; Zn 2+ + 2 e- Zn 2. Gas in contact with a solution of its ions Platinum inert electrode in contact with a solution of the ions of the gas present. Examples include the standard hydrogen electrode and chlorine: 2 H+ + 2 e- H 2; Cl 2 + 2 e- 2 Cl- 3. Ions of different oxidation states in solution An inert platinum electrode is used to allow electrons to pass into the half cell. The concentrations of the different ions must be the same, 1. 0 moldm-3. Examples of this include iron (III) and iron (II), copper (II) and copper (I); non metal examples include iodine and iodide, bromine and bromide, and manganate (VII) and manganese (II).

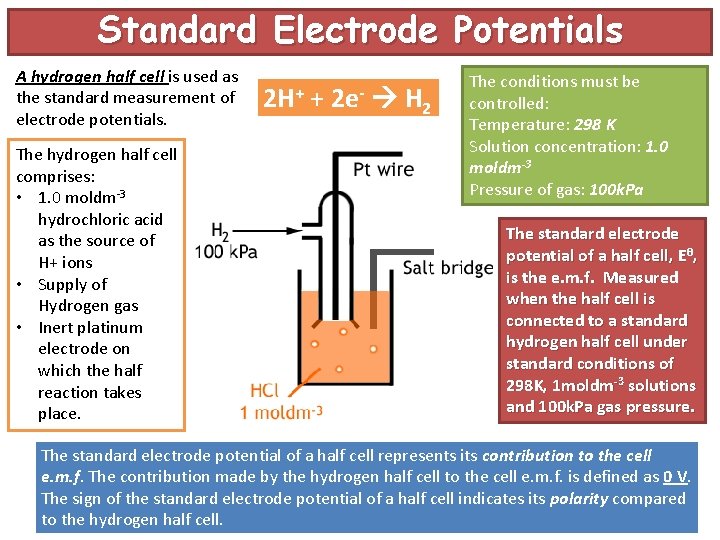
Standard Electrode Potentials A hydrogen half cell is used as the standard measurement of electrode potentials. The hydrogen half cell comprises: • 1. 0 moldm-3 hydrochloric acid as the source of H+ ions • Supply of Hydrogen gas • Inert platinum electrode on which the half reaction takes place. 2 H+ + 2 e- H 2 The conditions must be controlled: Temperature: 298 K Solution concentration: 1. 0 moldm-3 Pressure of gas: 100 k. Pa The standard electrode potential of a half cell, Eθ, is the e. m. f. Measured when the half cell is connected to a standard hydrogen half cell under standard conditions of 298 K, 1 moldm-3 solutions and 100 k. Pa gas pressure. The standard electrode potential of a half cell represents its contribution to the cell e. m. f. The contribution made by the hydrogen half cell to the cell e. m. f. is defined as 0 V. The sign of the standard electrode potential of a half cell indicates its polarity compared to the hydrogen half cell.

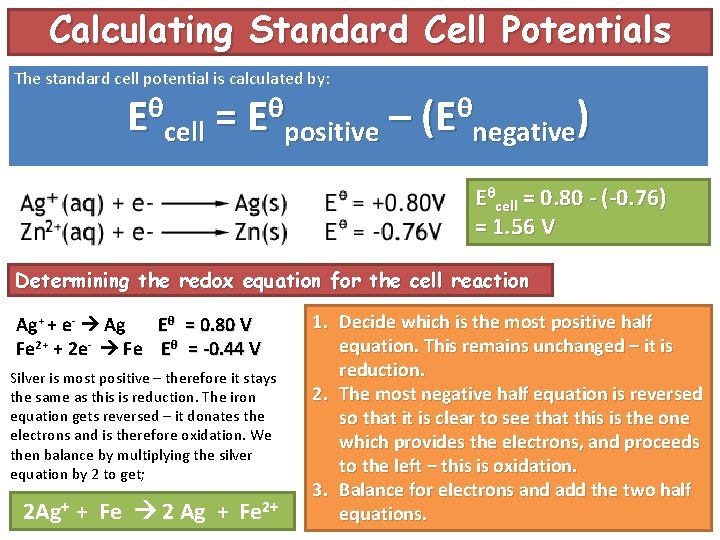
Calculating Standard Cell Potentials The standard cell potential is calculated by: Eθcell = Eθpositive – (Eθnegative) Eθcell = 0. 80 - (-0. 76) = 1. 56 V Determining the redox equation for the cell reaction Ag+ + e- Ag Eθ = 0. 80 V Fe 2+ + 2 e- Fe Eθ = -0. 44 V Silver is most positive – therefore it stays the same as this is reduction. The iron equation gets reversed – it donates the electrons and is therefore oxidation. We then balance by multiplying the silver equation by 2 to get; 2 Ag+ + Fe 2 Ag + Fe 2+ 1. Decide which is the most positive half equation. This remains unchanged – it is reduction. 2. The most negative half equation is reversed so that it is clear to see that this is the one which provides the electrons, and proceeds to the left – this is oxidation. 3. Balance for electrons and add the two half equations.

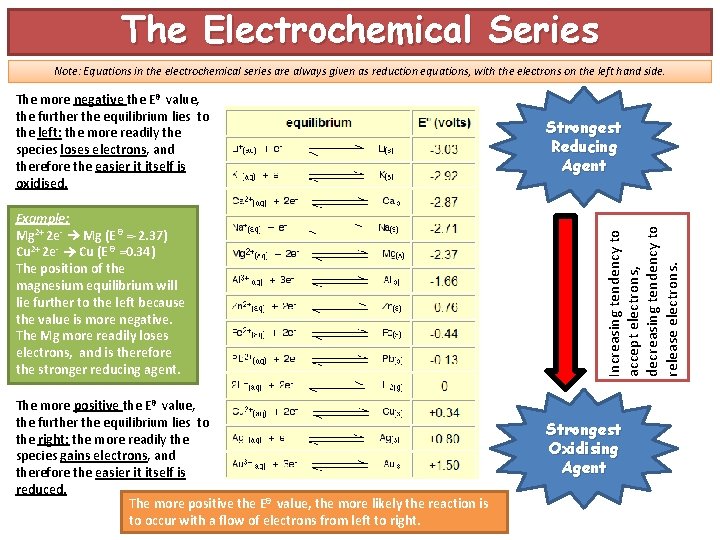
The Electrochemical Series Note: Equations in the electrochemical series are always given as reduction equations, with the electrons on the left hand side. Example: Mg 2+ 2 e- Mg (E θ =-2. 37) Cu 2+ 2 e- Cu (E θ =0. 34) The position of the magnesium equilibrium will lie further to the left because the value is more negative. The Mg more readily loses electrons, and is therefore the stronger reducing agent. The more positive the Eθ value, the further the equilibrium lies to the right: the more readily the species gains electrons, and therefore the easier it itself is reduced. The more positive the Eθ value, the more likely the reaction is to occur with a flow of electrons from left to right. Strongest Reducing Agent Increasing tendency to accept electrons, decreasing tendency to release electrons. The more negative the Eθ value, the further the equilibrium lies to the left: the more readily the species loses electrons, and therefore the easier it itself is oxidised. Strongest Oxidising Agent

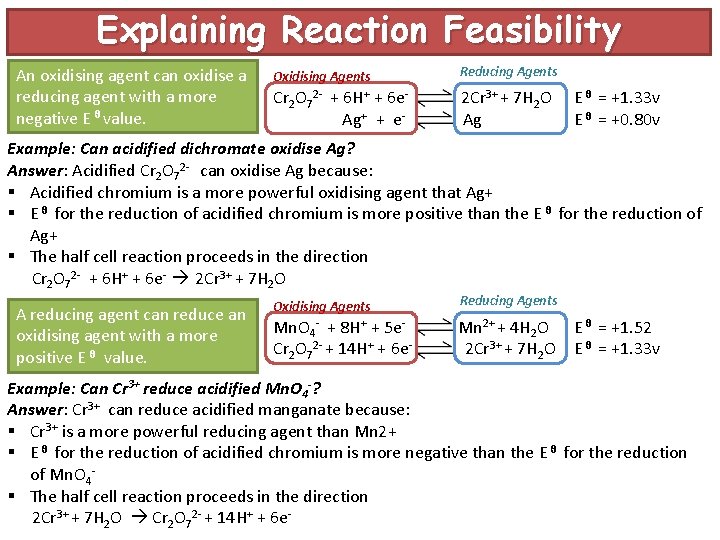
Explaining Reaction Feasibility An oxidising agent can oxidise a reducing agent with a more negative E θ value. Oxidising Agents Reducing Agents Cr 2 O 72 - + 6 H+ + 6 e. Ag+ + e- 2 Cr 3+ + 7 H 2 O Ag E θ = +1. 33 v E θ = +0. 80 v Example: Can acidified dichromate oxidise Ag? Answer: Acidified Cr 2 O 72 - can oxidise Ag because: § Acidified chromium is a more powerful oxidising agent that Ag+ § E θ for the reduction of acidified chromium is more positive than the E θ for the reduction of Ag+ § The half cell reaction proceeds in the direction Cr 2 O 72 - + 6 H+ + 6 e- 2 Cr 3+ + 7 H 2 O A reducing agent can reduce an oxidising agent with a more positive E θ value. Oxidising Agents Reducing Agents Mn. O 4 - + 8 H+ + 5 e. Cr 2 O 72 - + 14 H+ + 6 e- Mn 2+ + 4 H 2 O 2 Cr 3+ + 7 H 2 O E θ = +1. 52 E θ = +1. 33 v Example: Can Cr 3+ reduce acidified Mn. O 4 -? Answer: Cr 3+ can reduce acidified manganate because: § Cr 3+ is a more powerful reducing agent than Mn 2+ § E θ for the reduction of acidified chromium is more negative than the E θ for the reduction of Mn. O 4§ The half cell reaction proceeds in the direction 2 Cr 3+ + 7 H 2 O Cr 2 O 72 - + 14 H+ + 6 e-

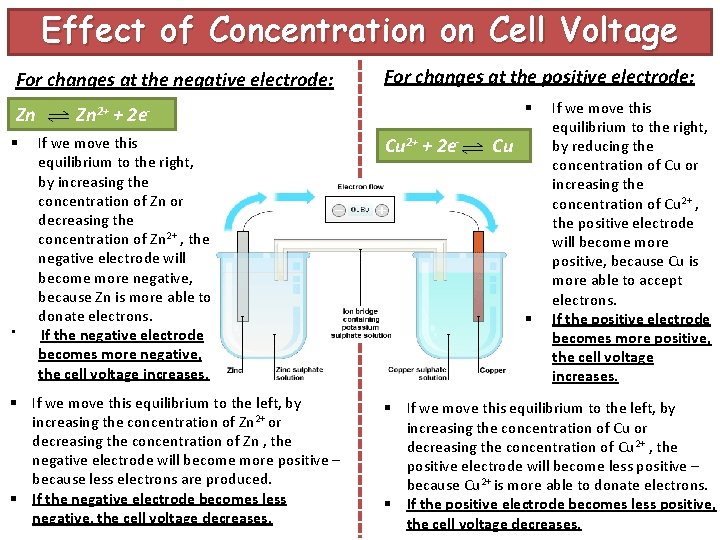
Effect of Concentration on Cell Voltage For changes at the negative electrode: Zn § § For changes at the positive electrode: § Zn 2+ + 2 e. If we move this equilibrium to the right, by increasing the concentration of Zn or decreasing the concentration of Zn 2+ , the negative electrode will become more negative, because Zn is more able to donate electrons. If the negative electrode becomes more negative, the cell voltage increases. § If we move this equilibrium to the left, by increasing the concentration of Zn 2+ or decreasing the concentration of Zn , the negative electrode will become more positive – because less electrons are produced. § If the negative electrode becomes less negative, the cell voltage decreases. Cu 2+ + 2 e- Cu § If we move this equilibrium to the right, by reducing the concentration of Cu or increasing the concentration of Cu 2+ , the positive electrode will become more positive, because Cu is more able to accept electrons. If the positive electrode becomes more positive, the cell voltage increases. § If we move this equilibrium to the left, by increasing the concentration of Cu or decreasing the concentration of Cu 2+ , the positive electrode will become less positive – because Cu 2+ is more able to donate electrons. § If the positive electrode becomes less positive, the cell voltage decreases.

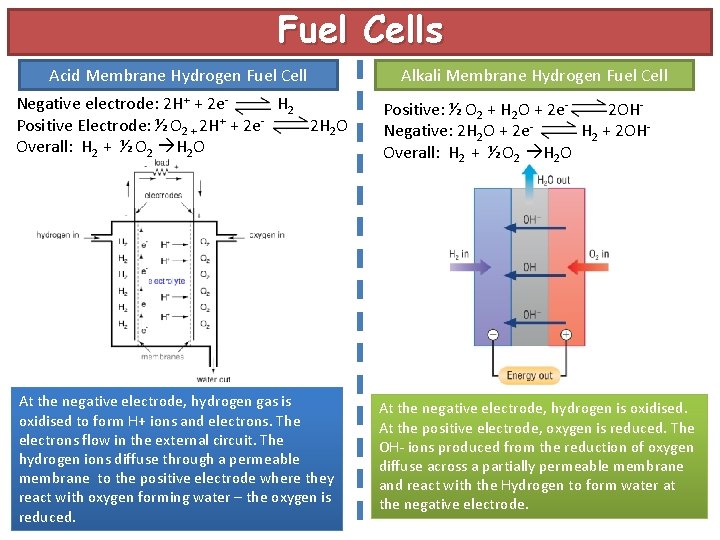
Fuel Cells Acid Membrane Hydrogen Fuel Cell Alkali Membrane Hydrogen Fuel Cell Negative electrode: 2 H+ + 2 e. H 2 Positive Electrode: ½ O 2 + 2 H+ + 2 e 2 H 2 O Overall: H 2 + ½ O 2 H 2 O Positive: ½ O 2 + H 2 O + 2 e 2 OHNegative: 2 H 2 O + 2 e. H 2 + 2 OHOverall: H 2 + ½ O 2 H 2 O At the negative electrode, hydrogen gas is oxidised to form H+ ions and electrons. The electrons flow in the external circuit. The hydrogen ions diffuse through a permeable membrane to the positive electrode where they react with oxygen forming water – the oxygen is reduced. At the negative electrode, hydrogen is oxidised. At the positive electrode, oxygen is reduced. The OH- ions produced from the reduction of oxygen diffuse across a partially permeable membrane and react with the Hydrogen to form water at the negative electrode.

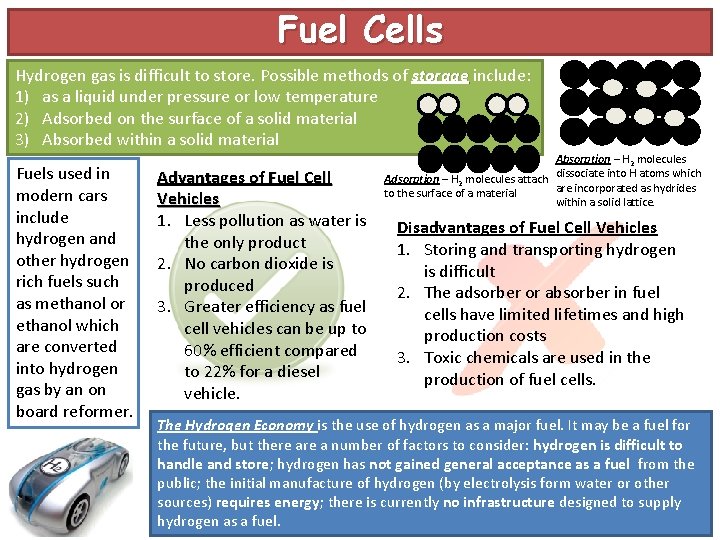
Fuel Cells Hydrogen gas is difficult to store. Possible methods of storage include: 1) as a liquid under pressure or low temperature 2) Adsorbed on the surface of a solid material 3) Absorbed within a solid material Fuels used in modern cars include hydrogen and other hydrogen rich fuels such as methanol or ethanol which are converted into hydrogen gas by an on board reformer. Advantages of Fuel Cell Vehicles 1. Less pollution as water is the only product 2. No carbon dioxide is produced 3. Greater efficiency as fuel cell vehicles can be up to 60% efficient compared to 22% for a diesel vehicle. Absorption – H 2 molecules Adsorption – H 2 molecules attach dissociate into H atoms which are incorporated as hydrides to the surface of a material within a solid lattice. Disadvantages of Fuel Cell Vehicles 1. Storing and transporting hydrogen is difficult 2. The adsorber or absorber in fuel cells have limited lifetimes and high production costs 3. Toxic chemicals are used in the production of fuel cells. The Hydrogen Economy is the use of hydrogen as a major fuel. It may be a fuel for the future, but there a number of factors to consider: hydrogen is difficult to handle and store; hydrogen has not gained general acceptance as a fuel from the public; the initial manufacture of hydrogen (by electrolysis form water or other sources) requires energy; there is currently no infrastructure designed to supply hydrogen as a fuel.