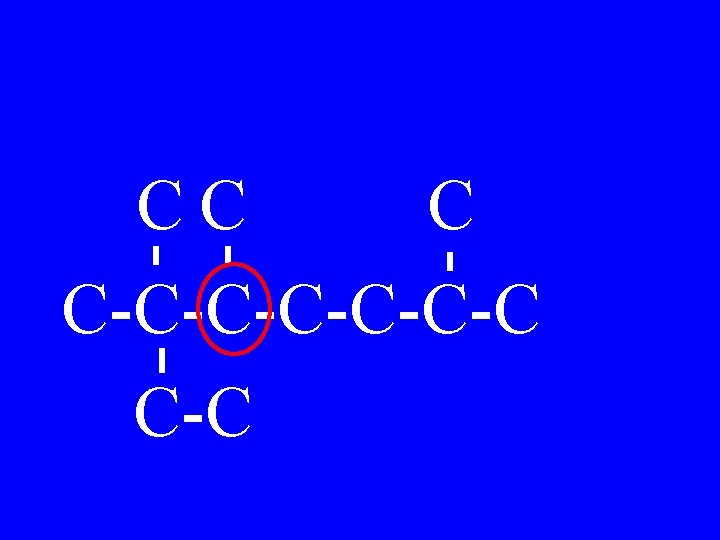
Drill Name the Following CC C CCCC CC
















- Slides: 29

Drill: Name the Following CC C C-C-C-C C-C

Stereochemistry

Stereochemistry Any chemistry which involves orientation in space

Use Models

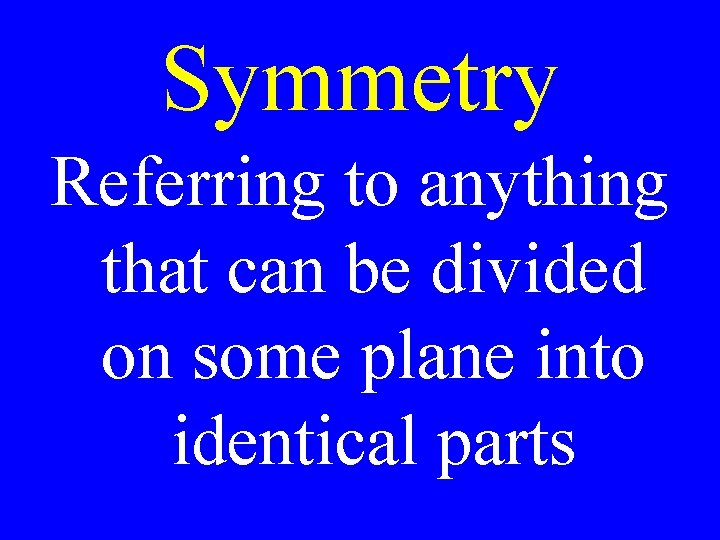
Symmetry Referring to anything that can be divided on some plane into identical parts

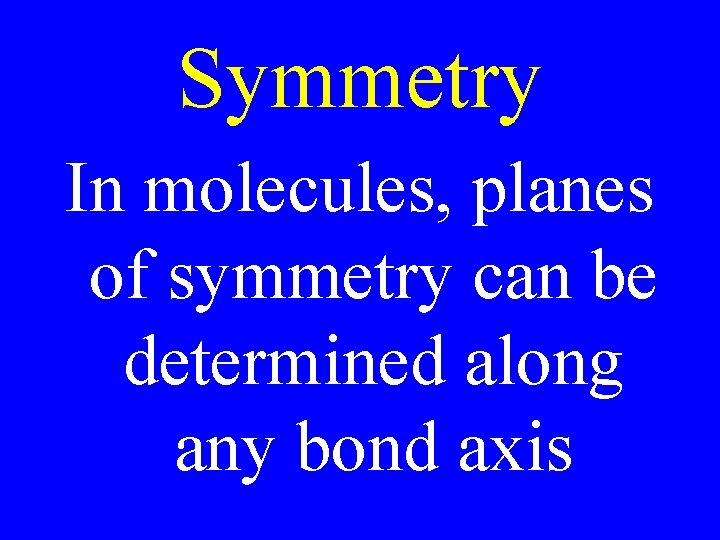
Symmetry In molecules, planes of symmetry can be determined along any bond axis

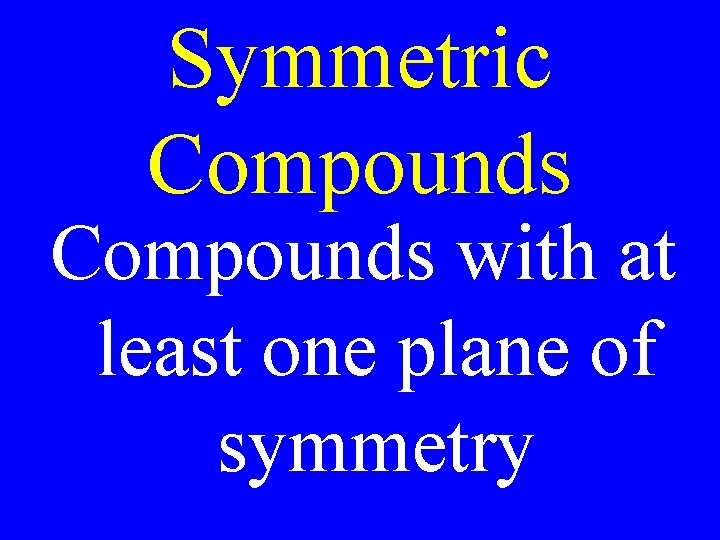
Symmetric Compounds with at least one plane of symmetry

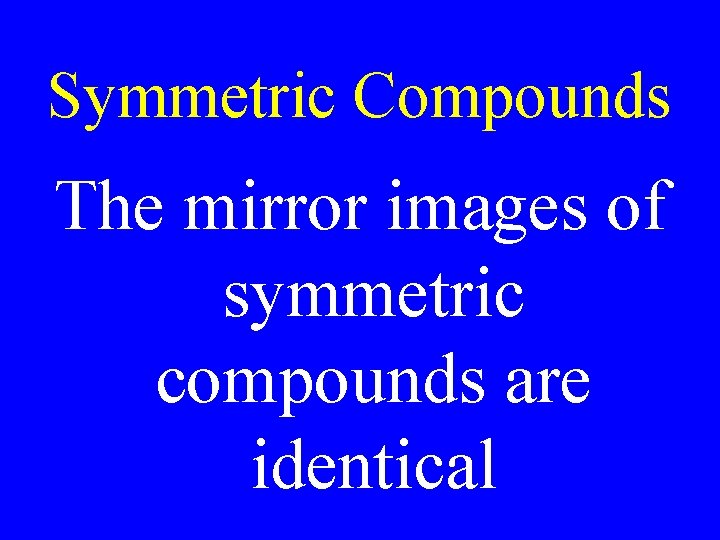
Symmetric Compounds The mirror images of symmetric compounds are identical

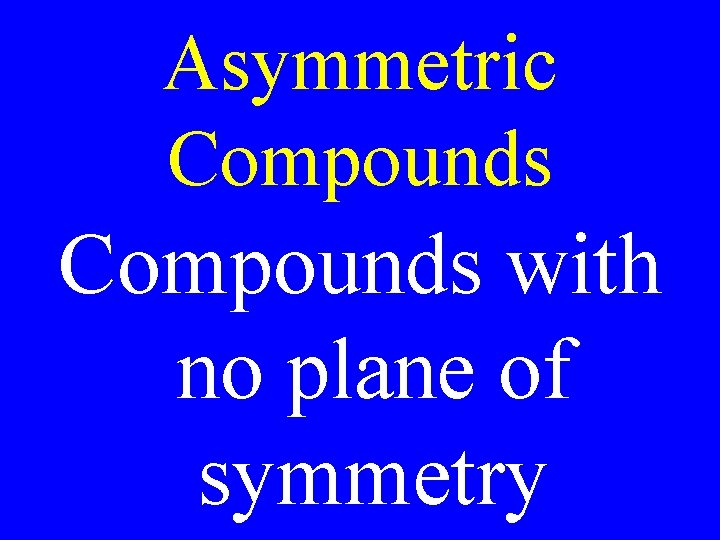
Asymmetric Compounds with no plane of symmetry

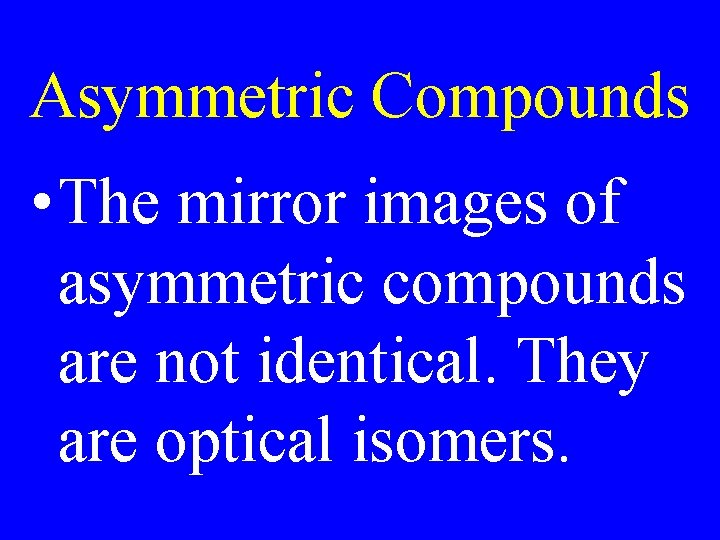
Asymmetric Compounds • The mirror images of asymmetric compounds are not identical. They are optical isomers.

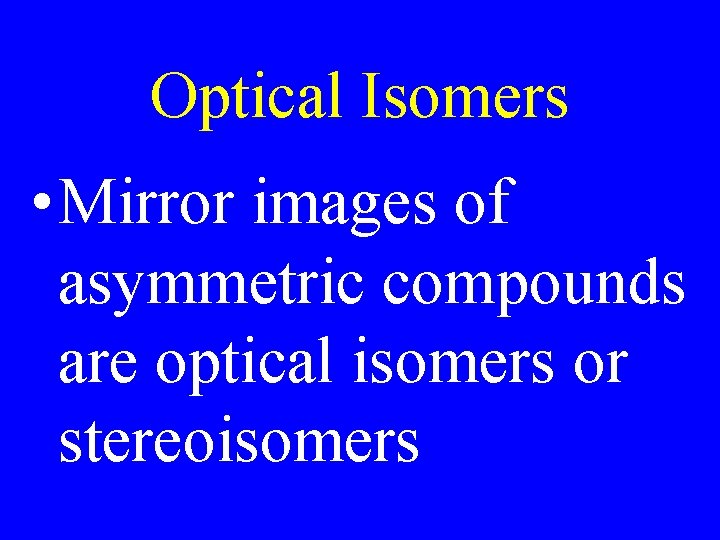
Optical Isomers • Mirror images of asymmetric compounds are optical isomers or stereoisomers

Stereoisomerism of C • A carbon center must be bound to 4 different things to be asymmetric

Stereoisomerism of C • Any carbon center bound to 3 or less things has at least one plane of symmetry

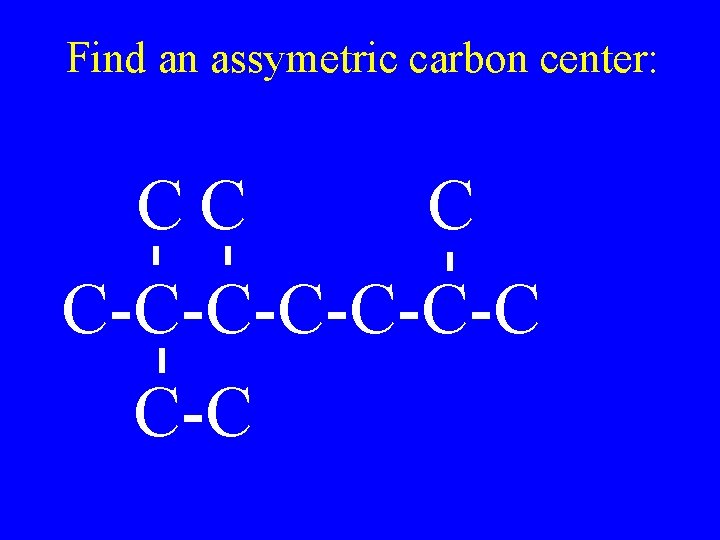
Find an assymetric carbon center: CC C C-C-C-C C-C

CC C C-C-C-C C-C

Stereoisomerism of C • Use modeling

Stereoisomerism of Carbon Chains • The constituents on two adjacent carbons spend more time in the staggered position

Stereoisomerism of Carbon Chains • Use modeling to show aligned & staggered positions

Polarity of Hydrocarbons • Hydrocarbons are non-polar

Perfectly Symmetrical Compounds All perfectly symmetrical compounds are non-polar

Less than perfectly Symmetrical Compounds • Compounds with any plane of asymmetry can be polar if the bonds are polar

Diffusion • Movement down an energy gradient

Thermochemistry • Compounds will eventually move to the lowest possible energy state (the most stable state)

Thermochemistry of Organic Compounds • Stability is sometimes determined by how the compound interferes with its surroundings

Thermochemistry of Organic Compounds • To increase stability, organic compounds tend to curl up

Thermochemistry of Organic Compounds • Ring structures are more stable than their corresponding straight chain structures

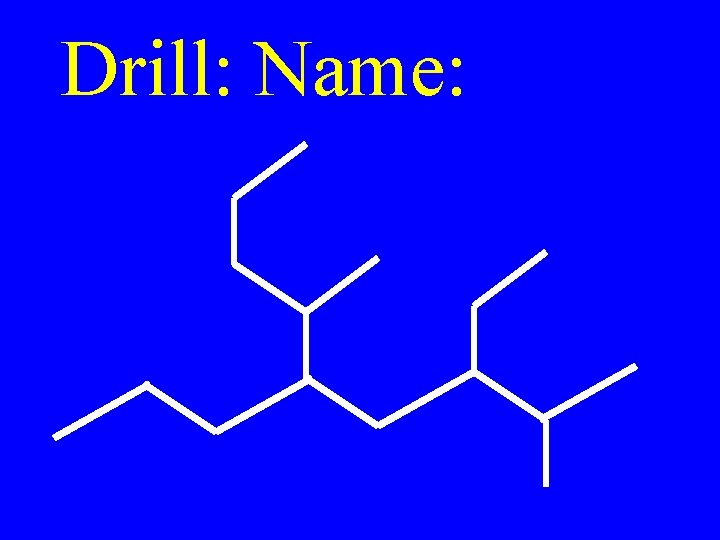
Drill: Name:

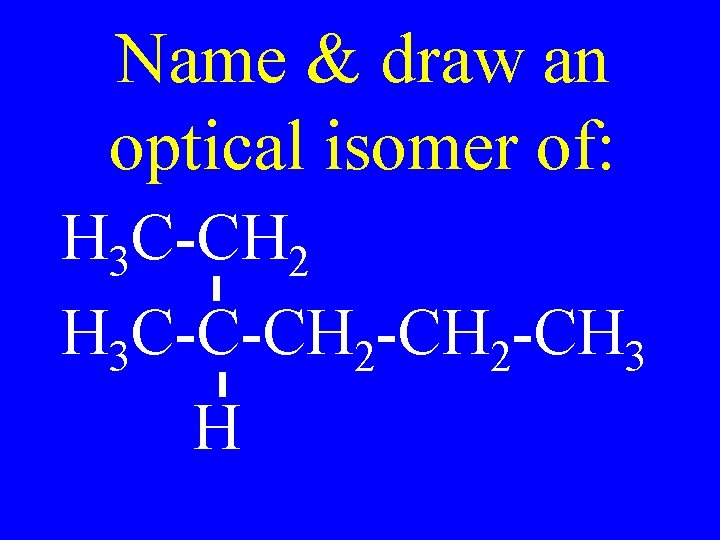
Name & draw an optical isomer of: H 3 C-CH 2 H 3 C-C-CH 2 -CH 3 H

Draw & Name at least 5 isomers of C 6 H 14