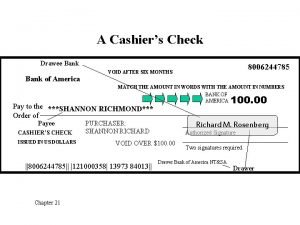
Drill Name the following Review Drill Check HW


























- Slides: 38

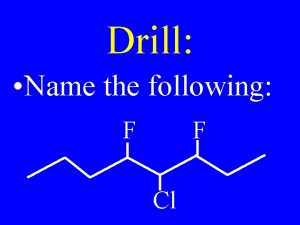
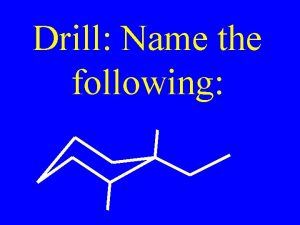
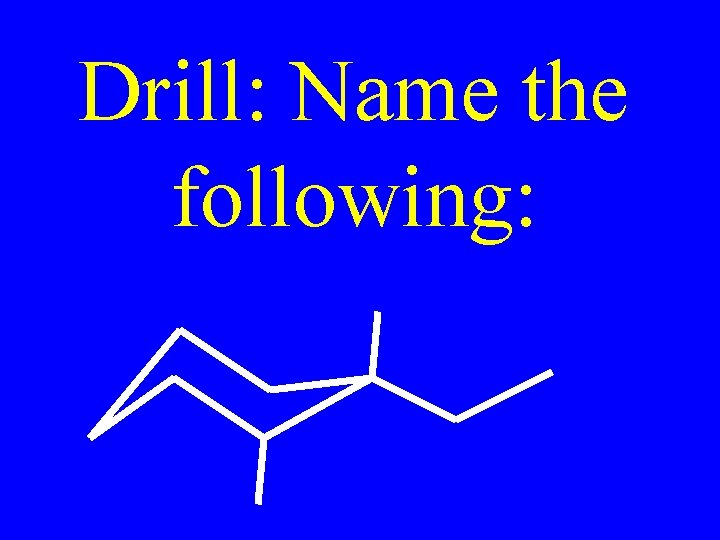
Drill: Name the following:

Review Drill & Check HW

Organic HW • Review PPs 5 – 7. • Complete Test 1 & turn it in tomorrow.

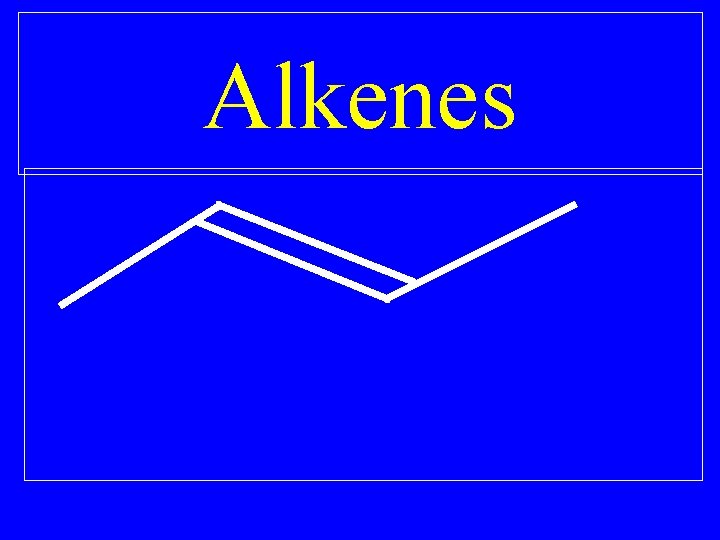
Alkenes

Alkenes • Hydrocarbons with at least one double bond

Alkenes • Each double bond reduces the number of hydrogens in the hydrocarbon by two

Alkenes • All alkenes are unsaturated hydrocarbons

Unsaturated Hydrocarbons • Contain less than the maximum number of hydrogens

Alkenes

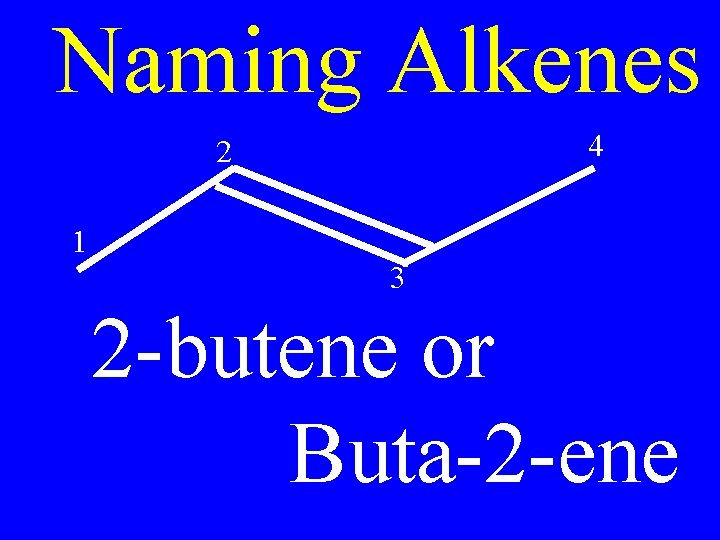
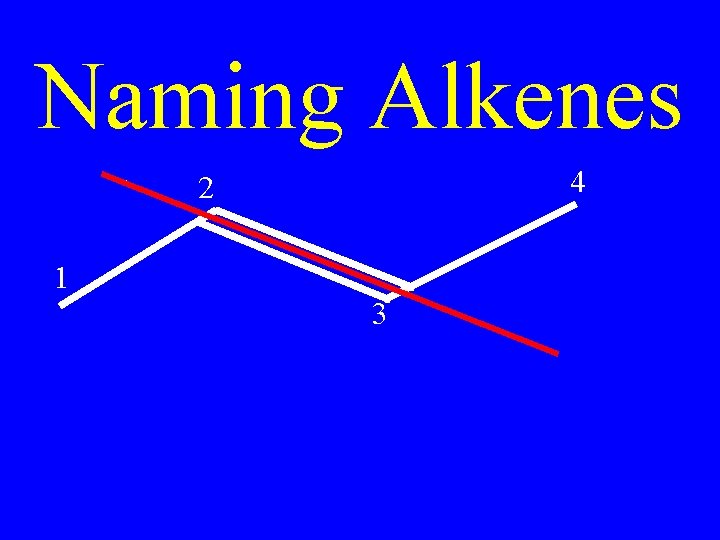
Naming Alkenes • Number the carbons on the chain; so that, the double bond has the lowest possible number

Naming Alkenes • The double bond will always fall between two numbered carbons

Naming Alkenes • Apply the lower of those two numbers to the double bond

Naming Alkenes 4 2 1 3 2 -butene or Buta-2 -ene

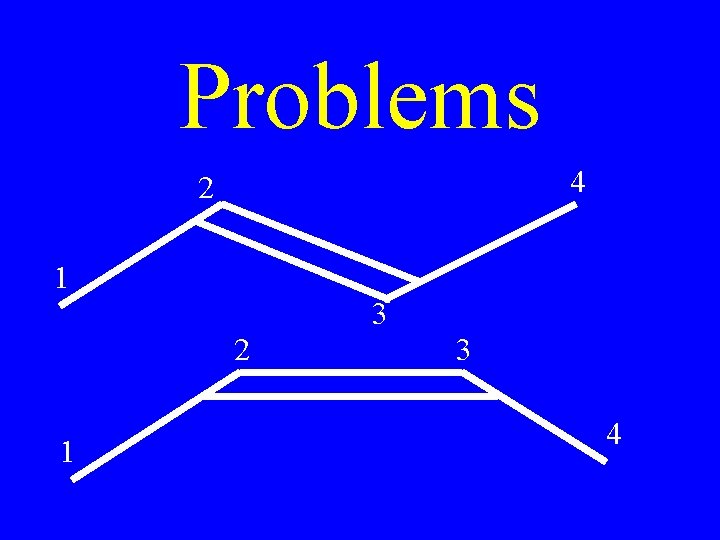
Problems 4 2 1 3 4

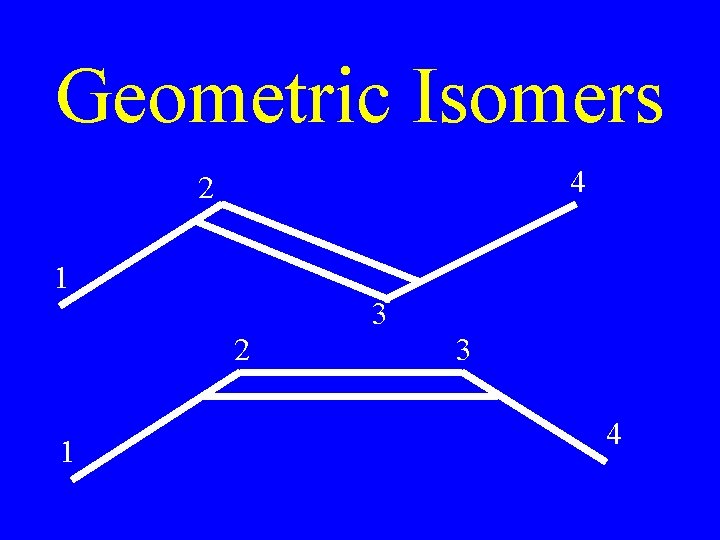
Geometric Isomers 4 2 1 3 4

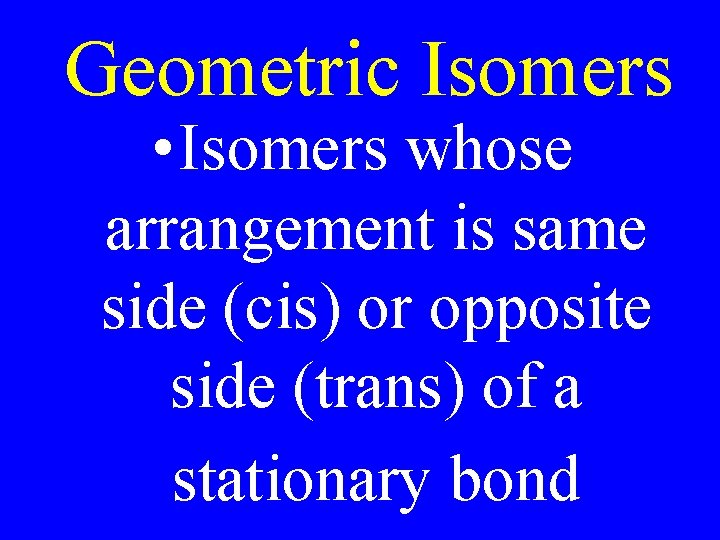
Geometric Isomers • Isomers whose arrangement is same side (cis) or opposite side (trans) of a stationary bond

Naming Alkenes • Draw a line through the double bond parallel to each of the bonds in the double bond

Naming Alkenes 4 2 1 3

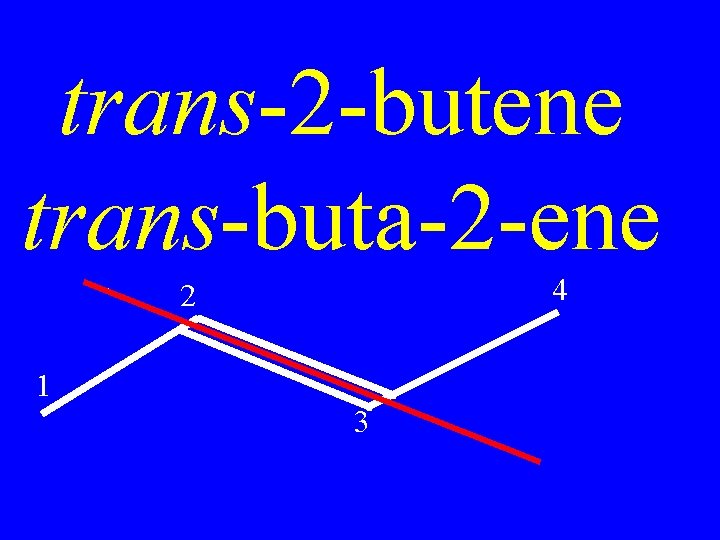
Naming Alkenes • If the two ends of the chain are on the same side of the line, it is a cis-double bond

Naming Alkenes • If the two ends of the chain are on opposite sides of the line, it is a trans-double bond

Naming Alkenes • Place cis or trans at the start of the name in the organic compound

trans-2 -butene trans-buta-2 -ene 4 2 1 3

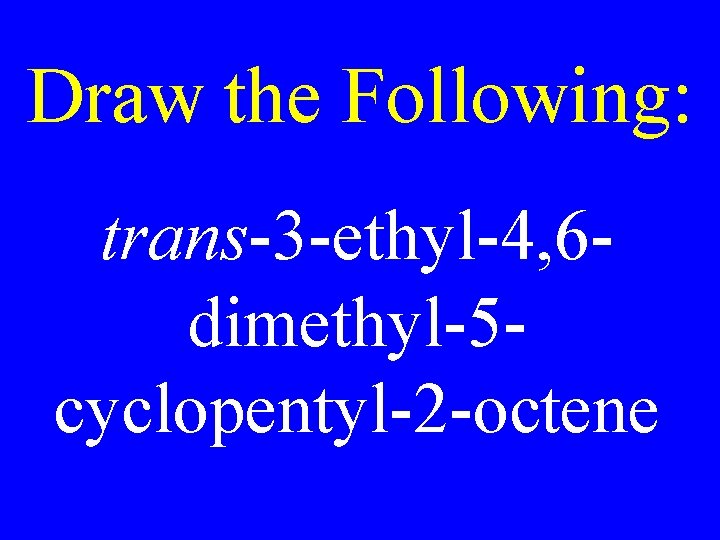
Draw the Following: trans-3 -ethyl-4, 6 dimethyl-5 cyclopentyl-2 -octene


Drill: Draw the condensed, skeletal, & stick structures for: cis-3 -ethyl-4, 6 -dimethyl-5 cyclopropyl-2 -octene

Organic Chm HW • Test 2 on Cycloalkanes & alkenes is on Friday

Review

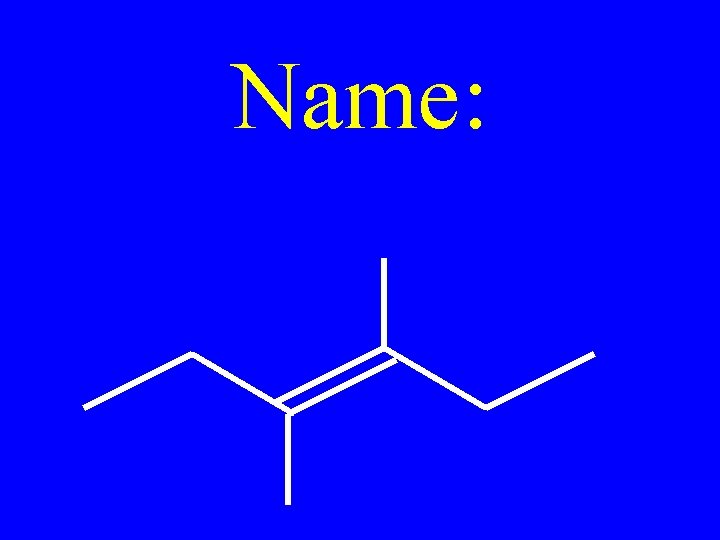
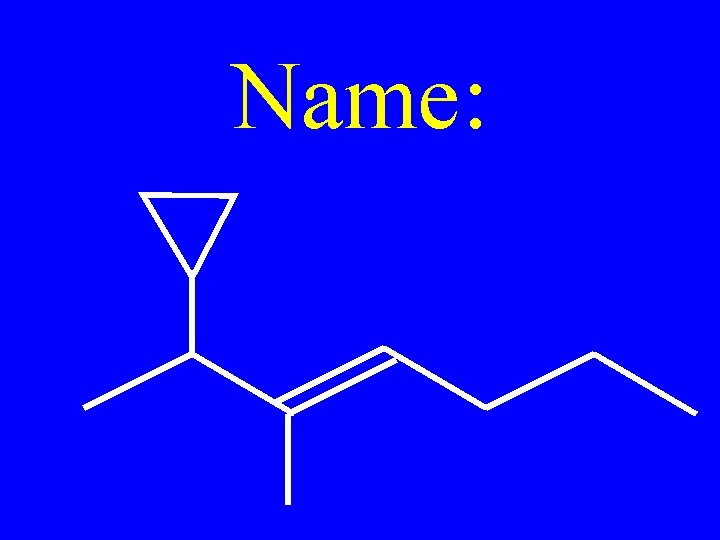
Name:

Name:

Name:





Draw: • trans-1 cyclobutyl-2 methyl-3 -hexene

Draw: cis-2 -methyl-1 octylcyclohexene

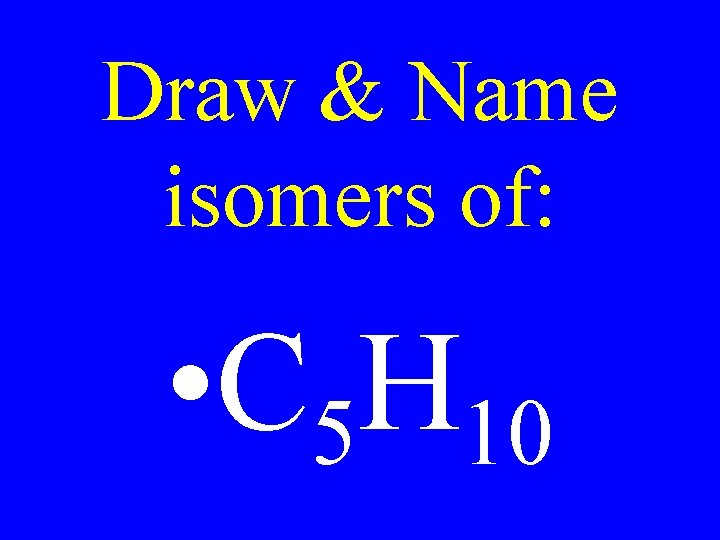
Draw & Name isomers of: • C 5 H 10

Naming Alkenes • Name the alkenes on the board
 Draw three noncollinear points j k and l
Draw three noncollinear points j k and l Behavior check in check out sheet
Behavior check in check out sheet Behavior check in check out sheet
Behavior check in check out sheet Check in check out behavior intervention
Check in check out behavior intervention Check in check out system for students
Check in check out system for students Jobbank
Jobbank Raster scan line in computer graphics
Raster scan line in computer graphics Check in check out behavior intervention
Check in check out behavior intervention Check-in/check-out intervention template
Check-in/check-out intervention template 1.7.6 - quick check: frost quick check
1.7.6 - quick check: frost quick check Endorse a check to someone else
Endorse a check to someone else Check my progress vocabulary check
Check my progress vocabulary check In these words: chapter 17
In these words: chapter 17 Hát kết hợp bộ gõ cơ thể
Hát kết hợp bộ gõ cơ thể Frameset trong html5
Frameset trong html5 Bổ thể
Bổ thể Tỉ lệ cơ thể trẻ em
Tỉ lệ cơ thể trẻ em Gấu đi như thế nào
Gấu đi như thế nào Chụp phim tư thế worms-breton
Chụp phim tư thế worms-breton Hát lên người ơi alleluia
Hát lên người ơi alleluia Kể tên các môn thể thao
Kể tên các môn thể thao Thế nào là hệ số cao nhất
Thế nào là hệ số cao nhất Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Công thức tiính động năng
Công thức tiính động năng Trời xanh đây là của chúng ta thể thơ
Trời xanh đây là của chúng ta thể thơ Mật thư anh em như thể tay chân
Mật thư anh em như thể tay chân 101012 bằng
101012 bằng Phản ứng thế ankan
Phản ứng thế ankan Các châu lục và đại dương trên thế giới
Các châu lục và đại dương trên thế giới Thể thơ truyền thống
Thể thơ truyền thống Quá trình desamine hóa có thể tạo ra
Quá trình desamine hóa có thể tạo ra Một số thể thơ truyền thống
Một số thể thơ truyền thống Cái miệng xinh xinh thế chỉ nói điều hay thôi
Cái miệng xinh xinh thế chỉ nói điều hay thôi Vẽ hình chiếu vuông góc của vật thể sau
Vẽ hình chiếu vuông góc của vật thể sau Thế nào là sự mỏi cơ
Thế nào là sự mỏi cơ đặc điểm cơ thể của người tối cổ
đặc điểm cơ thể của người tối cổ Thế nào là giọng cùng tên
Thế nào là giọng cùng tên Vẽ hình chiếu đứng bằng cạnh của vật thể
Vẽ hình chiếu đứng bằng cạnh của vật thể Tia chieu sa te
Tia chieu sa te