Drill 1 2 3 4 Name the following












- Slides: 14

Drill: ¡ 1. 2. 3. 4. Name the following: Na. Cl Na. OH Na 2 SO 4 Na 3 PO 4

Objective: ¡ The student will be able to: l Determine a formula writing method in order to write formulas for binary and tertiary ionic compounds.

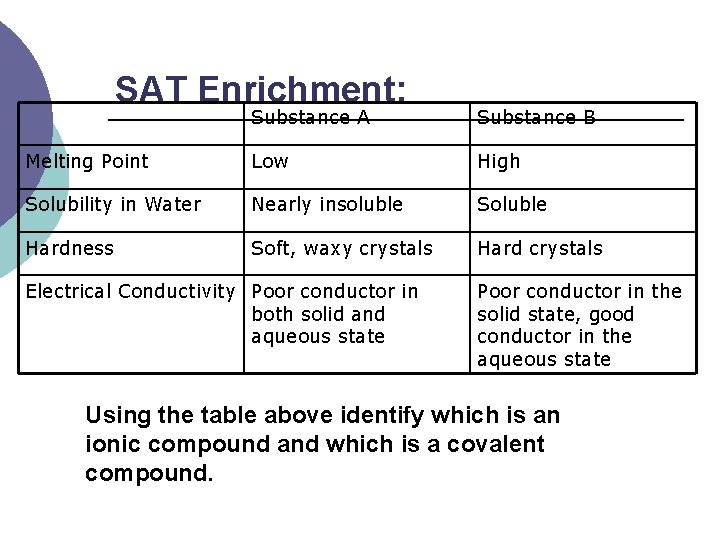
SAT Enrichment: Substance A Substance B Melting Point Low High Solubility in Water Nearly insoluble Soluble Hardness Soft, waxy crystals Hard crystals Electrical Conductivity Poor conductor in both solid and aqueous state Poor conductor in the solid state, good conductor in the aqueous state Using the table above identify which is an ionic compound and which is a covalent compound.

HSA: Algae leave the coral when the water is too warm. What kind of factor is temperature on this relationship? a. biotic b. abiotic c. parasitic d. commensalistic ¡

Engagement: ¡ Quiz Criss-Cross Method 10 min

Writing Formulas Ionic Compounds (Metals and Nonmetals)

Exploration ¡ Complete Writing formulas for ionic compounds (practice problems) worksheet (as a class). Any Questions? ?

Exploration Complete Writing Formulas for Binary Ionic Compounds worksheet. ¡ Think – Pair – Share ¡ l l Complete the worksheet Compare your worksheet with your partner beside you. Share answers with the whole table. Each table will review answers with the class.

Explanation ¡ ¡ ¡ Write a few monatomic ions and polyatomic ions on the board. Ask students to explain the differences and determine a definition for polyatomic ion.

Explanation ¡ Observe the following ions. l l l S-2 SO 3 SO 4 -2 -2 Sulfide Sulfite Sulfate Explain what the different endings mean in terms of the formulas for the ions above. ¡ Identify the ions that are monatomic and polyatomic. ¡

Explanation Complete Writing Formulas for Ionic Compounds with Polyatomic Ions. ¡ Think – Pair – Share ¡ l l Complete the worksheet Compare your worksheet with your partner beside you. Share answers with the whole table. Each table will review answers with the class.

Homework Assignment ¡ Writing Formulas for More Ionic Compounds with Polyatomic Ions

Summary Questions 1. How do ionic compounds form? 2. How are the charges of ions determined? 3. What is the difference b/w monatomic and polyatomic ions? 4. Design a way to remember how to determine the name of ionic compounds. 5. Identify a method that can be used to determine how to write the formula of an ionic compound.

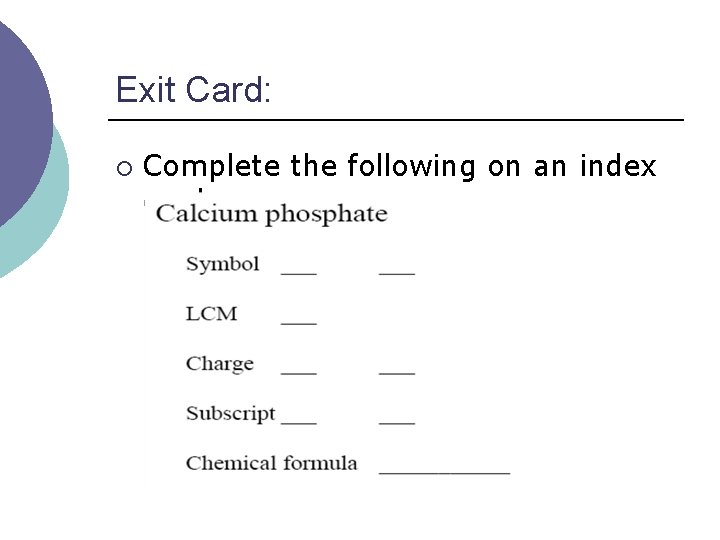
Exit Card: ¡ Complete the following on an index card.