Cncer de Pulmo com Mutao do EGFR Luiz










- Slides: 37

Câncer de Pulmão com Mutação do EGFR Luiz Henrique Araujo, MD, Ph. D

Disclosures According to Brazilian regulatory policy 1595/2000 from Conselho Federal de Medicina and to RDC 96/2008 from ANVISA, I declare: Clinical Research: as medical investigator, I participate of sponsored trials of: LILLY, BOHERINGER, MSD, BRISTOL, ROCHE, PFIZER, ASTRAZENECA, NOVARTIS, MERCK Speaker: MSD, BRISTOL, ASTRAZENECA, BOHERINGER, MERCK, ROCHE Consulting as Advisory Board member: MSD, ROCHE, AZ I have no stock exchange actions in any of these pharmaceutical companies. My pre-requisites for participating in these activities are the autonomy of scientific thought, the independence of opinions and freedom of expression, aspects that AZ respects.

Osimertinib vs So. C EGFR-TKI as first-line treatment in patients with EGFRm advanced NSCLC (FLAURA): plasma ct. DNA analysis Jhanelle E Gray 1, Isamu Okamoto 2, Virote Sriuranpong 3, Johan Vansteenkiste 4, Fumio Imamura 5, Jong Seok Lee 6, Yong-Kek Pang 7, Manuel Cobo 8, Kazuo Kasahara 9, Rachel Hodge 10, Brian Lentrichia 11, Simon Dearden 10, Suresh S Ramalingam 12 1 Department of Thoracic Oncology, H. Lee Moffitt Cancer Center & Research Institute, Tampa, FL, USA; 2 Research Institute for Diseases of the Chest, Graduate School of Medical Sciences, Kyushu University Hospital, Fukuoka, Japan ; 3 Division of Medical Oncology, Department of Medicine, Faculty of Medicine, Chulalongkorn University and the King Chulalongkorn Memorial Hospital, Bangkok, Thailand ; 4 Respiratory Oncology Unit, University Hospital KU Leuven, Belgium; 5 Department of Thoracic Oncology, Osaka International Cancer Institute, Osaka, Japan ; 6 Department of Internal Medicine, Seoul National University College of Medicine, Seoul, Republic of Korea; National University Bundang Hospital, Seongnam, Republic of Korea; 7 Division of Respiratory Medicine, Department of Medicine, Faculty of Medicine, University Malaya Medical Centre, Lembah Pantai, Kuala Lumpur; 8 Medical Oncology Section, Hospital Universitario Málaga Regional, IBIMA, Málaga, Spain ; 9 Department of Respiratory Medicine, Kanazawa University Hospital, Kanazawa, Japan; 10 Astra. Zeneca, Cambridge, UK; 11 Astra. Zeneca, Waltham, MA, USA; 12 Emory University, Winship Cancer Institute, Atlanta, GA, USA Presented by Jhanelle E Gray

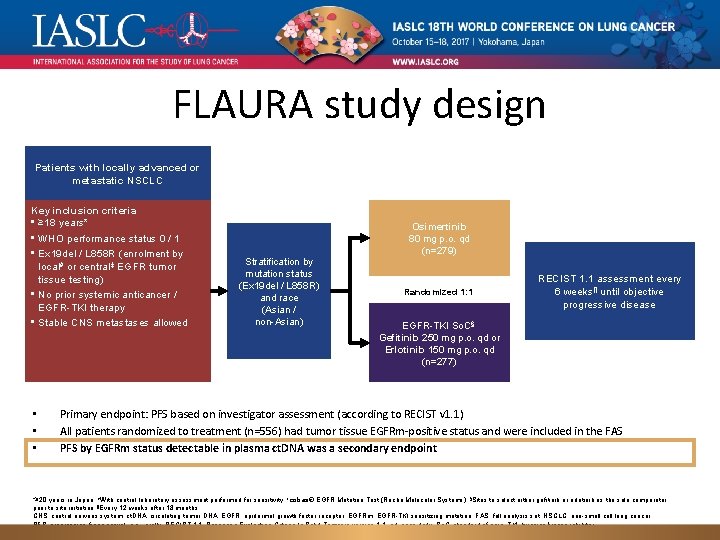
FLAURA study design Patients with locally advanced or metastatic NSCLC Key inclusion criteria • ≥ 18 years* • WHO performance status 0 / 1 • Ex 19 del / L 858 R (enrolment by local# or central‡ EGFR tumor tissue testing) • No prior systemic anticancer / EGFR-TKI therapy • Stable CNS metastases allowed • • • Stratification by mutation status (Ex 19 del / L 858 R) and race (Asian / non-Asian) Osimertinib 80 mg p. o. qd (n=279) Randomized 1: 1 RECIST 1. 1 assessment every 6 weeks¶ until objective progressive disease EGFR-TKI So. C§; Gefitinib 250 mg p. o. qd or Erlotinib 150 mg p. o. qd (n=277) Primary endpoint: PFS based on investigator assessment (according to RECIST v 1. 1) All patients randomized to treatment (n=556) had tumor tissue EGFRm-positive status and were included in the FAS PFS by EGFRm status detectable in plasma ct. DNA was a secondary endpoint *≥ 20 years in Japan; #With central laboratory assessment performed for sensitivity; ‡cobas® EGFR Mutation Test (Roche Molecular Systems); §Sites to select either gefitinib or erlotinib as the sole comparator prior to site initiation; ¶Every 12 weeks after 18 months. CNS, central nervous system; ct. DNA, circulating tumor DNA; EGFR, epidermal growth factor receptor; EGFRm, EGFR-TKI sensitizing mutation; FAS, full analysis set; NSCLC, non-small cell lung cancer; PFS, progression-free survival; p. o. , orally; RECIST 1. 1, Response Evaluation Criteria In Solid Tumours version 1. 1; qd, once daily; So. C, standard of care; TKI, tyrosine kinase inhibitor; WHO, World Health Organization.

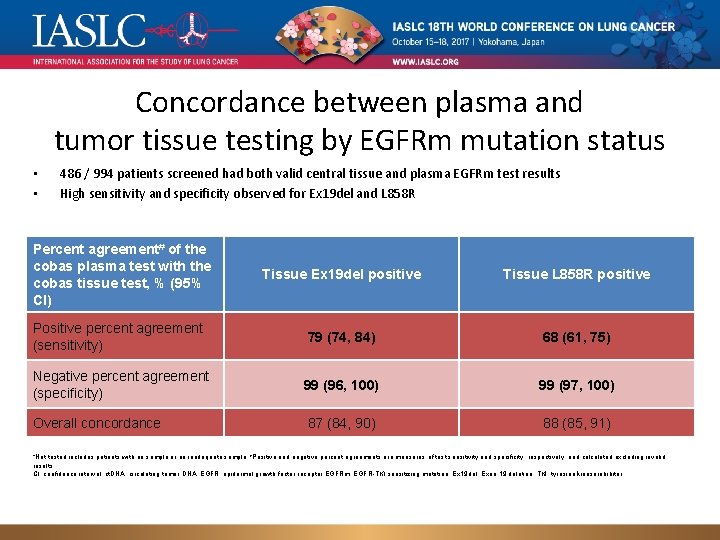
Concordance between plasma and tumor tissue testing by EGFRm mutation status • • 486 / 994 patients screened had both valid central tissue and plasma EGFRm test results High sensitivity and specificity observed for Ex 19 del and L 858 R Percent agreement# of the cobas plasma test with the cobas tissue test, % (95% CI) Tissue Ex 19 del positive Tissue L 858 R positive Positive percent agreement (sensitivity) 79 (74, 84) 68 (61, 75) Negative percent agreement (specificity) 99 (96, 100) 99 (97, 100) Overall concordance 87 (84, 90) 88 (85, 91) *Not tested includes patients with no sample or an inadequate sample; #Positive and negative percent agreements are measures of test sensitivity and specificity, respectively, and calculated excluding invalid results. CI, confidence interval; ct. DNA, circulating tumor DNA; EGFR, epidermal growth factor receptor EGFRm, EGFR-TKI sensitizing mutation; Ex 19 del, Exon 19 deletion; TKI, tyrosine kinase inhibitor.

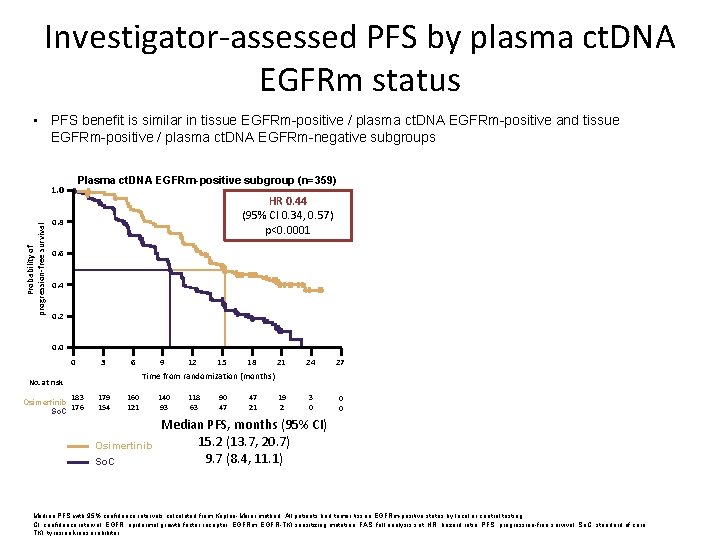
Investigator-assessed PFS by plasma ct. DNA EGFRm status • PFS benefit is similar in tissue EGFRm-positive / plasma ct. DNA EGFRm-positive and tissue EGFRm-positive / plasma ct. DNA EGFRm-negative subgroups 1. 0 HR 0. 44 (95% CI 0. 34, 0. 57) p<0. 0001 0. 8 Probability of progression-free survival Plasma ct. DNA EGFRm-negative subgroup (n=124) Plasma ct. DNA EGFRm-positive subgroup (n=359) 1. 0 0. 6 0. 4 0. 2 HR 0. 48 (95% CI 0. 28, 0. 80) p=0. 0047 0. 8 0. 6 0. 4 0. 2 0. 0 0 3 6 No. at risk 183 Osimertinib 176 So. C 179 154 9 12 15 18 21 Time from randomization (months) 160 121 Osimertinib So. C 140 93 118 63 90 47 47 21 19 2 24 0 27 3 6 No. at risk 3 0 Median PFS, months (95% CI) 15. 2 (13. 7, 20. 7) 9. 7 (8. 4, 11. 1) 0 0 60 Osimertinib 64 So. C 9 12 15 18 21 24 27 6 7 1 1 0 0 Time from randomization (months) 55 57 51 52 Osimertinib So. C 50 43 44 36 40 27 19 15 Median PFS, months (95% CI) 23. 5 (17. 8, 24. 3) 15. 0 (9. 7, 18. 3) Longer in both arms Median PFS with 95% confidence intervals calculated from Kaplan-Meier method. All patients had tumor tissue EGFRm-positive status by local or central testing. CI, confidence interval; EGFR, epidermal growth factor receptor; EGFRm, EGFR-TKI sensitizing mutation; FAS, full analysis set; HR, hazard ratio; PFS, progression-free survival; So. C, standard of care; TKI, tyrosine kinase inhibitor.

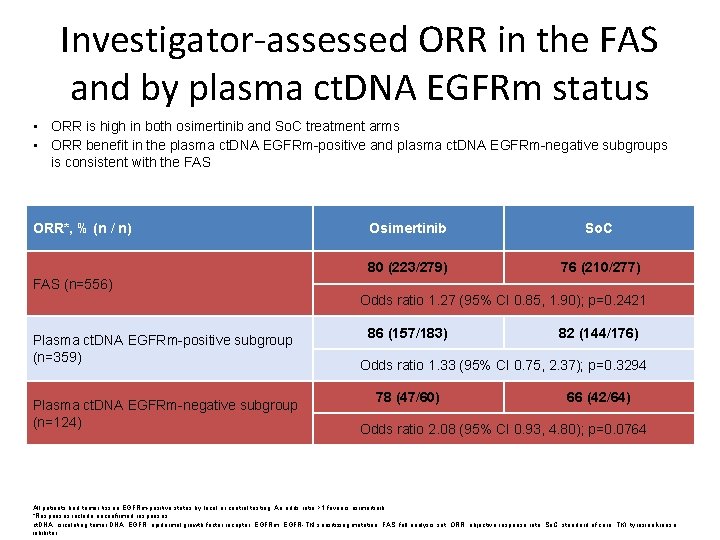
Investigator-assessed ORR in the FAS and by plasma ct. DNA EGFRm status • ORR is high in both osimertinib and So. C treatment arms • ORR benefit in the plasma ct. DNA EGFRm-positive and plasma ct. DNA EGFRm-negative subgroups is consistent with the FAS ORR*, % (n / n) Osimertinib So. C 80 (223/279) 76 (210/277) FAS (n=556) Odds ratio 1. 27 (95% CI 0. 85, 1. 90); p=0. 2421 Plasma ct. DNA EGFRm-positive subgroup (n=359) Plasma ct. DNA EGFRm-negative subgroup (n=124) 86 (157/183) 82 (144/176) Odds ratio 1. 33 (95% CI 0. 75, 2. 37); p=0. 3294 78 (47/60) 66 (42/64) Odds ratio 2. 08 (95% CI 0. 93, 4. 80); p=0. 0764 All patients had tumor tissue EGFRm-positive status by local or central testing. An odds ratio >1 favours osimertinib. *Responses include unconfirmed responses. ct. DNA, circulating tumor DNA; EGFR, epidermal growth factor receptor; EGFRm, EGFR-TKI sensitizing mutation; FAS. full analysis set; ORR, objective response rate; So. C, standard of care; TKI, tyrosine kinase inhibitor.

Summary • In the FLAURA screened population, high concordance was observed between central tissue (cobas ® EGFR Mutation Test) and plasma (cobas® EGFR Mutation Test v 2) testing for EGFRm, consistent with AURA 3 concordance data 1 • In the FLAURA overall FAS, all patients had tumor tissue EGFRm-positive status by local or central testing, and osimertinib resulted in significant improvement in PFS over So. C – HR 0. 46 (95% CI 0. 37, 0. 57); p<0. 0001 • In the tissue EGFRm positive / plasma ct. DNA EGFRm-positive subgroup, osimertinib resulted in significant improvement in PFS over So. C, consistent with the overall FAS – HR 0. 44 (95% CI 0. 34, 0. 57); p<0. 0001 • In the tissue EGFRm positive / plasma ct. DNA EGFRm-negative subgroup, median PFS for both treatment arms was prolonged – Previous studies have shown low levels of ct. DNA shedding to correlate with tumor burden 2, 3 and lack of detectable ct. DNA early in EGFR-TKI therapy to be associated with better clinical prognosis 4 • Where feasible, tumor biopsy tissue testing is recommended for patients with plasma ct. DNA EGFRm-negative status • These results support the clinical utility of plasma ct. DNA EGFRm testing for selecting patients eligible for first-line osimertinib treatment 1. Wu et al. J Thorac Oncol 2016; 12(S 1): MA 08. 03; 2. Diaz et al. J Clin Oncol 2014; 32: 579– 86; 3. Diehl et al. Nat Med 2008; 14: 985– 90; 4. Mok et al. Clin Cancer Res 2015; 21: 3196– 203. CI, confidence interval; ct. DNA, circulating tumor DNA; EGFR, epidermal growth factor receptor; EGFRm, EGFR-TKI sensitizing mutation; FAS, full analysis set; HR, hazard ratio; PFS, progression-free survival; So. C, standard of care; TKI, tyrosine kinase inhibitor.

Detection of EGFR mutations from plasma ct. DNA in the osimertinib Phase III trial (AURA 3): comparison of three plasma assays Myung-Ju Ahn 1, Ji-Youn Han 2, Chun-Ming Tsai 3, Angelo Delmonte 4, Te-Chun Hsia 5, Janessa Laskin 6, Sang-We Kim 7, Yong He 8, Toyoaki Hida 9, Makoto Maemondo 10, Terufumi Kato 11, Suzanne Jenkins 12, Aleksandra Markovets 13, Kenneth S Thress 13, Tony Mok 14 1 Section of Hematology-Oncology, Department of Medicine, Samsung Medical Center, Sungkyunkwan University School of Medicine, Seoul, Republic of Korea; 2 Center for Lung Cancer, National Cancer Center, Goyang, Republic of Korea; 3 Department of Oncology, Taipei-Veterans General Hospital, Taipei, Taiwan; 4 Oncology Unit, Istituto Romagnolo per lo Studio e la Cura dei Tumori, Meldola, Italy; 5 Department of Respiratory Therapy, China Medical University, Taichung City, Taiwan; 6 Medical Oncology, BC Cancer Agency, British Columbia Cancer Agency, Vancouver, BC, Canada; 7 Department of Oncology, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Republic of Korea; 8 Department of Respiratory Disease, Daping Hospital, Third Military Medical University, Chongqing, China; 9 Department of Thoracic Oncology, Aichi Cancer Center, Nagoya, Japan; 10 Division of Pulmonary Medicine, Allergy, and Rheumatology, Department of Internal Medicine, Iwate Medical University School of Medicine, Iwate, Japan; 11 Department of Thoracic Oncology, Kanagawa Cancer Center, Yokohama, Japan; 12 Astra. Zeneca, Personalised Healthcare and Biomarkers, Cambridge, UK; 13 Astra. Zeneca, Oncology IMED, Waltham, MA, USA; 14 State Key Laboratory of South China, Hong Kong Cancer Institute, Department of Clinical Oncology, Chinese University of Hong Kong, Shatin, Hong Kong, China Presented by Myung-Ju Ahn

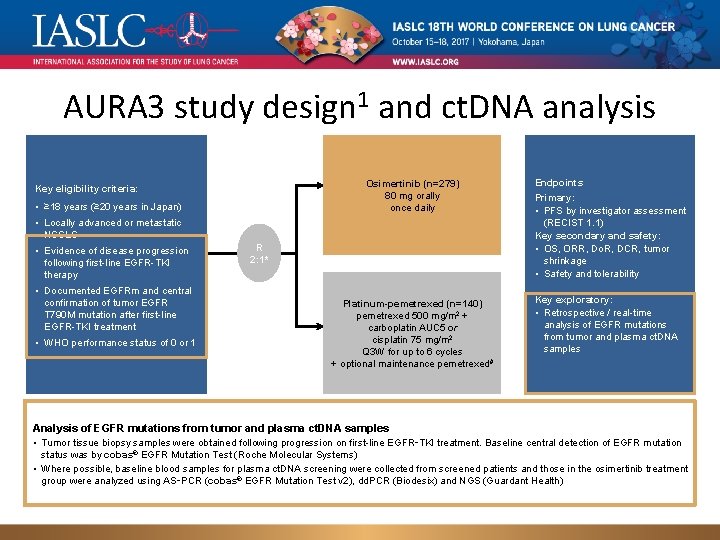
AURA 3 study design 1 and ct. DNA analysis Osimertinib (n=279) 80 mg orally once daily Key eligibility criteria: • ≥ 18 years (≥ 20 years in Japan) • Locally advanced or metastatic NSCLC • Evidence of disease progression following first-line EGFR-TKI therapy • Documented EGFRm and central confirmation of tumor EGFR T 790 M mutation after first-line EGFR-TKI treatment • WHO performance status of 0 or 1 R 2: 1* Platinum-pemetrexed (n=140) pemetrexed 500 mg/m 2 + carboplatin AUC 5 or cisplatin 75 mg/m 2 Q 3 W for up to 6 cycles + optional maintenance pemetrexed# Endpoints Primary: • PFS by investigator assessment (RECIST 1. 1) Key secondary and safety: • OS, ORR, Do. R, DCR, tumor shrinkage • Safety and tolerability Key exploratory: • Retrospective / real-time analysis of EGFR mutations from tumor and plasma ct. DNA samples Analysis of EGFR mutations from tumor and plasma ct. DNA samples • Tumor tissue biopsy samples were obtained following progression on first-line EGFR‑TKI treatment. Baseline central detection of EGFR mutation status was by cobas® EGFR Mutation Test (Roche Molecular Systems) • Where possible, baseline blood samples for plasma ct. DNA screening were collected from screened patients and those in the osimertinib treatment group were analyzed using AS‑PCR (cobas® EGFR Mutation Test v 2), dd. PCR (Biodesix) and NGS (Guardant Health)

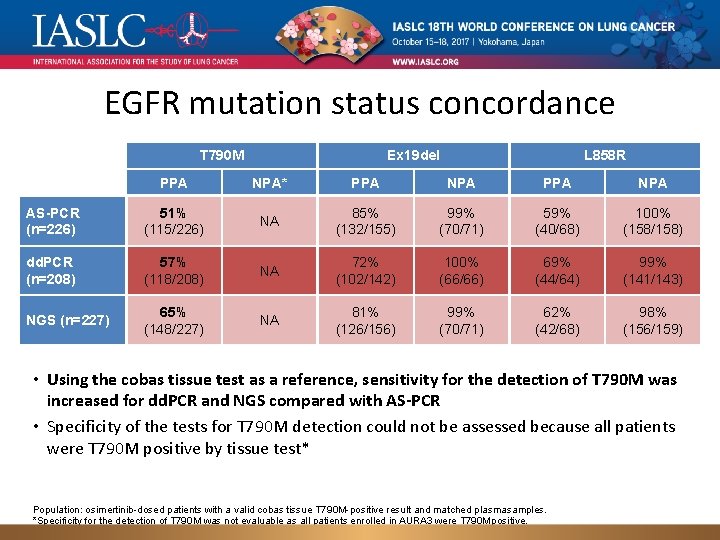
EGFR mutation status concordance T 790 M Ex 19 del L 858 R PPA NPA* PPA NPA AS-PCR (n=226) 51% (115/226) NA 85% (132/155) 99% (70/71) 59% (40/68) 100% (158/158) dd. PCR (n=208) 57% (118/208) NA 72% (102/142) 100% (66/66) 69% (44/64) 99% (141/143) NGS (n=227) 65% (148/227) NA 81% (126/156) 99% (70/71) 62% (42/68) 98% (156/159) • Using the cobas tissue test as a reference, sensitivity for the detection of T 790 M was increased for dd. PCR and NGS compared with AS-PCR • Specificity of the tests for T 790 M detection could not be assessed because all patients were T 790 M positive by tissue test* Population: osimertinib-dosed patients with a valid cobas tissue T 790 M-positive result and matched plasma samples. *Specificity for the detection of T 790 M was not evaluable as all patients enrolled in AURA 3 were T 790 M positive. NA, not applicable; NPA, negative percent agreement (specificity); PPA, positive percent agreement (sensitivity).

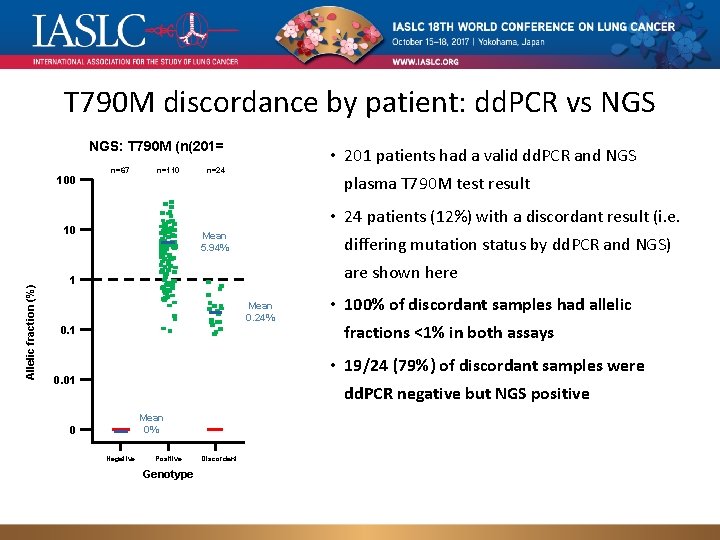
T 790 M discordance by patient: dd. PCR vs NGS: T 790 M (n(201= 100 n=67 n=110 plasma T 790 M test result • 24 patients (12%) with a discordant result (i. e. 10 Allelic fraction (%) • 201 patients had a valid dd. PCR and NGS n=24 Mean 5. 94% differing mutation status by dd. PCR and NGS) are shown here 1 Mean 0. 24% 0. 1 • 100% of discordant samples had allelic fractions <1% in both assays • 19/24 (79%) of discordant samples were 0. 01 dd. PCR negative but NGS positive Mean 0% 0 Negative Positive Discordant Genotype Green = NGS positive result, red = NGS negative result; population: osimertinib-dosed patients with a valid cobas tissue T 790 M-positive result and matched plasma samples.

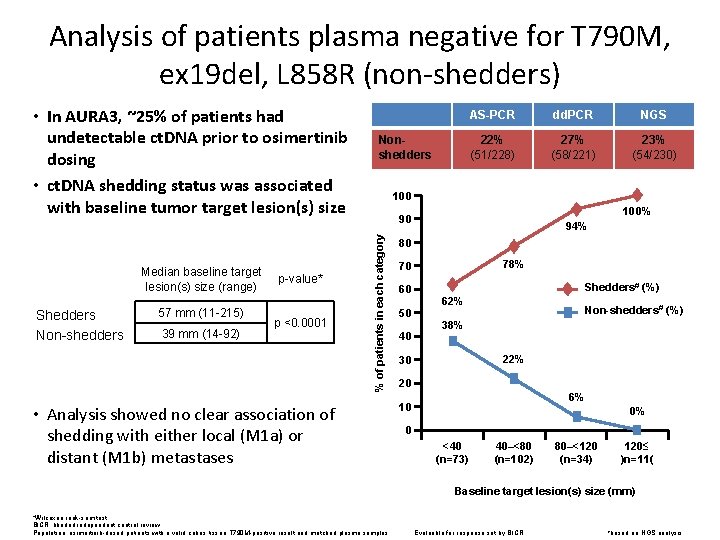
Analysis of patients plasma negative for T 790 M, ex 19 del, L 858 R (non-shedders) Median baseline target lesion(s) size (range) Shedders Non-shedders 57 mm (11 -215) 39 mm (14 -92) p-value* p <0. 0001 Nonshedders AS-PCR dd. PCR NGS 22% (51/228) 27% (58/221) 23% (54/230) 100% 90 % of patients in each category • In AURA 3, ~25% of patients had undetectable ct. DNA prior to osimertinib dosing • ct. DNA shedding status was associated with baseline tumor target lesion(s) size • Analysis showed no clear association of shedding with either local (M 1 a) or distant (M 1 b) metastases 94% 80 78% 70 60 50 40 Shedders# (%) 62% Non-shedders# (%) 38% 22% 30 20 6% 10 0% 0 <40 (n=73) 40–<80 (n=102) 80–<120 (n=34) 120≤ )n=11( Baseline target lesion(s) size (mm) *Wilcoxon rank-sum test. BICR, blinded independent central review. Population: osimertinib-dosed patients with a valid cobas tissue T 790 M-positive result and matched plasma samples. Evaluable for response set by BICR #based on NGS analysis

Conclusions • Using the cobas tissue test as a reference, sensitivity for the detection of T 790 M was increased for dd. PCR and NGS compared with AS-PCR • Robust associations were observed between quantitative dd. PCR and NGS assays when determining AFs for T 790 M, ex 19 del and L 858 R mutations, despite the different assay platforms • All discordant samples had AFs ≤ 1% in both dd. PCR and NGS assays • Optimizing DNA input helped increase sensitivity of dd. PCR assays without decreasing specificity • Approximately 25% of AURA 3 patients did not appear to shed ct. DNA prior to osimertinib dosing, as evidenced by the absence of three EGFR mutations by AS-PCR, dd. PCR and NGS • The larger the target lesion size at baseline, the greater the chance the patient will have detectable shed ct. DNA in the plasma. Further studies are needed to determine how other biological factors correlate with tumor ct. DNA shedding • Patients who have progressed on a prior EGFR-TKI and with a plasma T 790 M-negative result should undergo a biopsy to determine T 790 M status

Characterizing the genomic landscape of EGFR C 797 S in lung cancer using ct. DNA next-generation sequencing Zofia Piotrowska 1, Rebecca J. Nagy 2, Stephen Fairclough 2, Richard B. Lanman 2, Nicolas Marcoux 1, Scott Gettinger 3, Taofeek K. Owonikoko 4, Suresh S. Ramalingam 4, Lecia V. Sequist 1 1 - Massachusetts General Hospital/Harvard Medical School, Boston, USA 2 - Guardant Health, Redwood City, CA, USA 3 - Yale University School of Medicine, New Haven, CT, USA 4 - Emory University School of Medicine, Atlanta, GA, USA Genomic landscape of EGFR C 797 S in lung cancer ct. DNA, Presented by Zofia Piotrowska 1/12

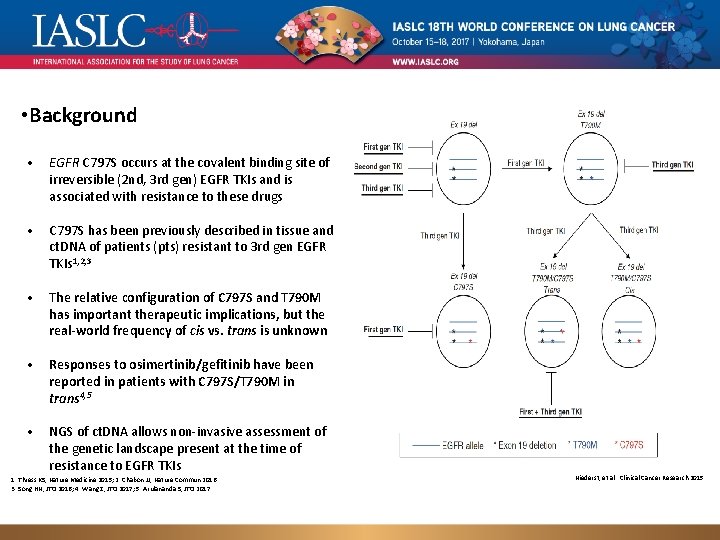
• Background • EGFR C 797 S occurs at the covalent binding site of irreversible (2 nd, 3 rd gen) EGFR TKIs and is associated with resistance to these drugs • C 797 S has been previously described in tissue and ct. DNA of patients (pts) resistant to 3 rd gen EGFR TKIs 1, 2, 3 • The relative configuration of C 797 S and T 790 M has important therapeutic implications, but the real-world frequency of cis vs. trans is unknown • Responses to osimertinib/gefitinib have been reported in patients with C 797 S/T 790 M in trans 4, 5 • NGS of ct. DNA allows non-invasive assessment of the genetic landscape present at the time of resistance to EGFR TKIs 1. Thress KS, Nature Medicine 2015; 2. Chabon JJ, Nature Commun 2016. 3. Song HN, JTO 2016; 4. Wang Z, JTO 2017; 5. Arulananda S, JTO 2017. Genomic landscape of EGFR C 797 S in lung cancer ct. DNA, Presented by Zofia Piotrowska Niederst, et al. Clinical Cancer Research 2015 3/12

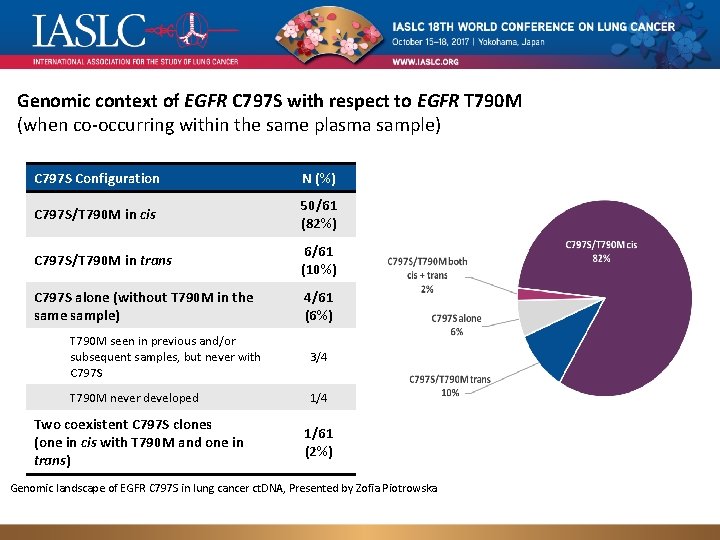
Genomic context of EGFR C 797 S with respect to EGFR T 790 M (when co-occurring within the same plasma sample) C 797 S Configuration N (%) C 797 S/T 790 M in cis 50/61 (82%) C 797 S/T 790 M in trans 6/61 (10%) C 797 S alone (without T 790 M in the sample) 4/61 (6%) T 790 M seen in previous and/or subsequent samples, but never with C 797 S 3/4 T 790 M never developed 1/4 Two coexistent C 797 S clones (one in cis with T 790 M and one in trans) 1/61 (2%) Genomic landscape of EGFR C 797 S in lung cancer ct. DNA, Presented by Zofia Piotrowska

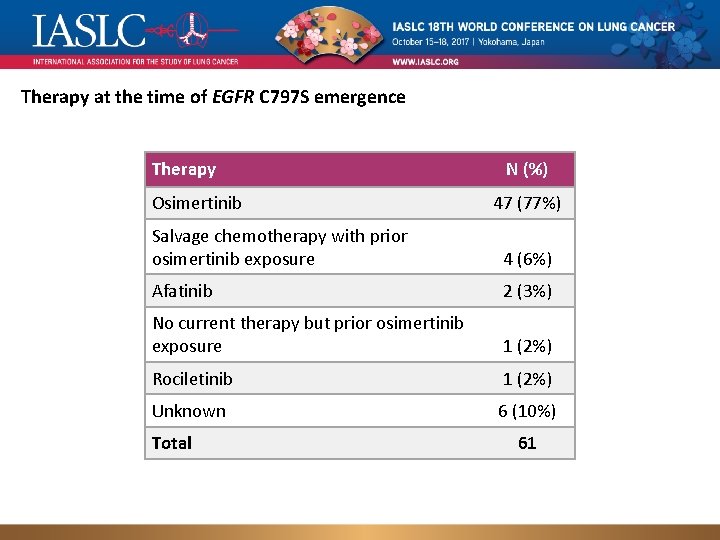
Therapy at the time of EGFR C 797 S emergence Therapy Osimertinib N (%) 47 (77%) Salvage chemotherapy with prior osimertinib exposure 4 (6%) Afatinib 2 (3%) No current therapy but prior osimertinib exposure 1 (2%) Rociletinib 1 (2%) Unknown 6 (10%) Total Genomic landscape of EGFR C 797 S in lung cancer ct. DNA, Presented by Zofia Piotrowska 61 8/12

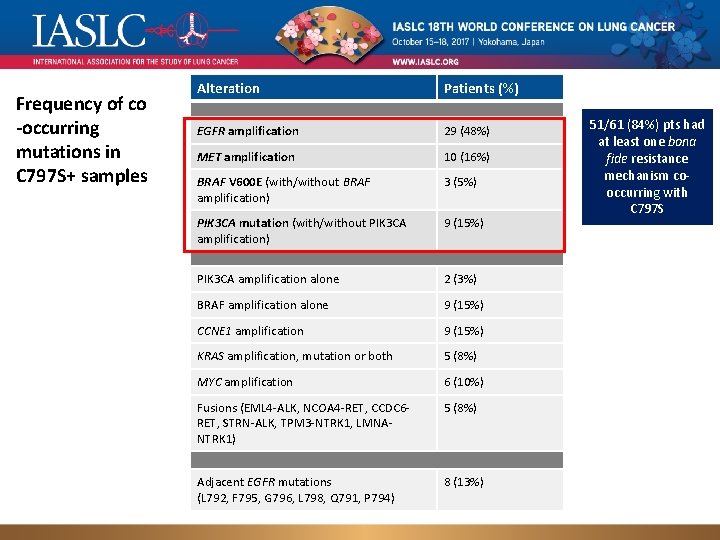
Frequency of co -occurring mutations in C 797 S+ samples Alteration Patients (%) EGFR amplification 29 (48%) MET amplification 10 (16%) BRAF V 600 E (with/without BRAF amplification) 3 (5%) PIK 3 CA mutation (with/without PIK 3 CA amplification) 9 (15%) PIK 3 CA amplification alone 2 (3%) BRAF amplification alone 9 (15%) CCNE 1 amplification 9 (15%) KRAS amplification, mutation or both 5 (8%) MYC amplification 6 (10%) Fusions (EML 4 -ALK, NCOA 4 -RET, CCDC 6 RET, STRN-ALK, TPM 3 -NTRK 1, LMNANTRK 1) 5 (8%) Adjacent EGFR mutations 8 (13%) (L 792, F 795, G 796, Presented L 798, Q 791, P 794) Genomic landscape of EGFR C 797 S in lung cancer ct. DNA, by Zofia Piotrowska 51/61 (84%) pts had at least one bona fide resistance mechanism cooccurring with C 797 S 9/12

Osimertinib resistance mediated by loss of EGFR T 790 M is associated with early resistance and competing resistance mechanisms Geoffrey R. Oxnard, Yuebi Hu, Kathryn F. Mileham, Philip Tracy, Nora Feeney, Lynette M. Sholl, Cloud P. Paweletz, Kenneth S. Thress, Pasi A. Jänne Dana-Farber Cancer Institute Levine Cancer Institute Astra-Zeneca

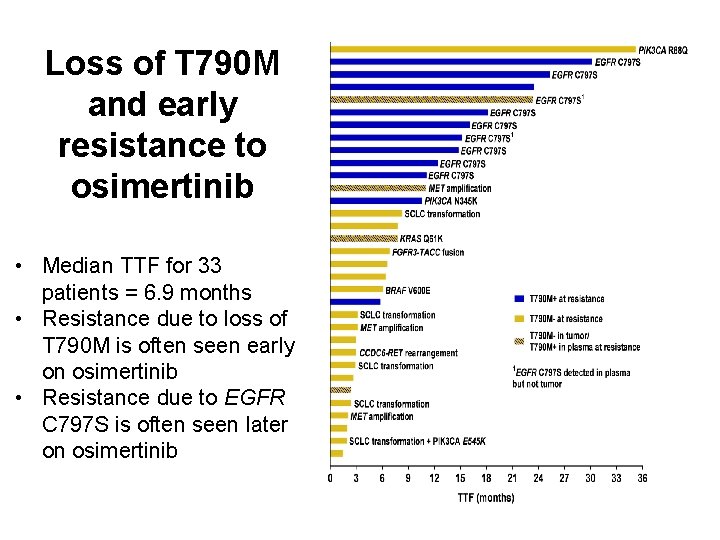
Loss of T 790 M and early resistance to osimertinib • Median TTF for 33 patients = 6. 9 months • Resistance due to loss of T 790 M is often seen early on osimertinib • Resistance due to EGFR C 797 S is often seen later on osimertinib

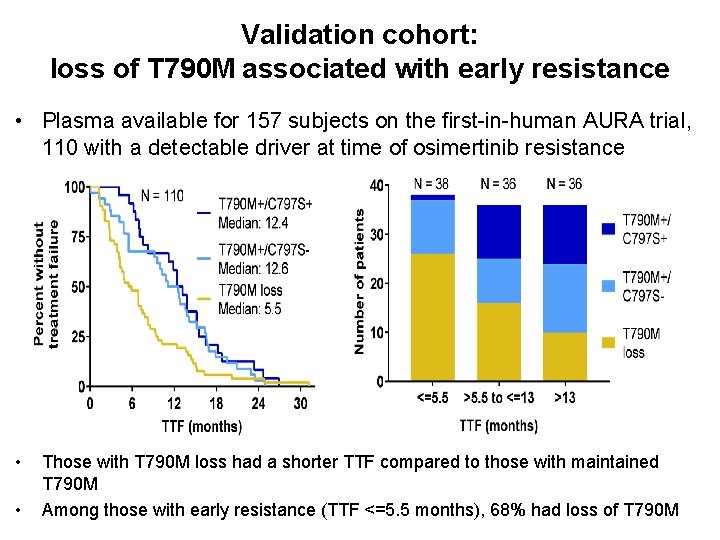
Validation cohort: loss of T 790 M associated with early resistance • Plasma available for 157 subjects on the first-in-human AURA trial, 110 with a detectable driver at time of osimertinib resistance • • Those with T 790 M loss had a shorter TTF compared to those with maintained T 790 M Among those with early resistance (TTF <=5. 5 months), 68% had loss of T 790 M

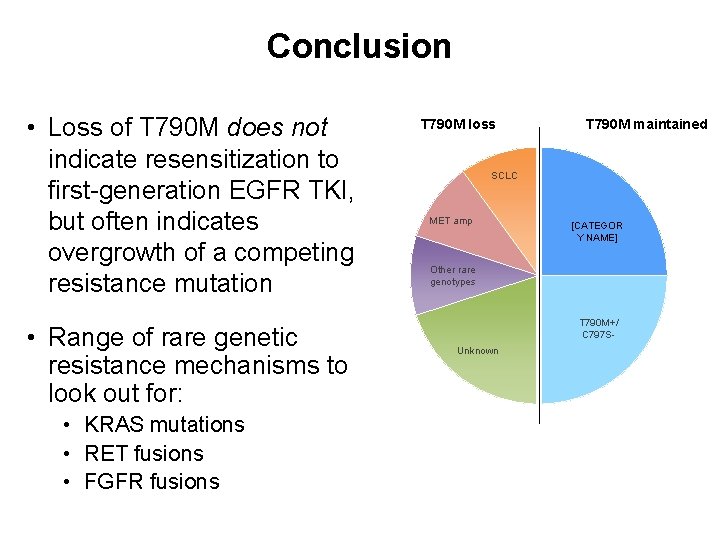
Conclusion • Loss of T 790 M does not indicate resensitization to first-generation EGFR TKI, but often indicates overgrowth of a competing resistance mutation • Range of rare genetic resistance mechanisms to look out for: • KRAS mutations • RET fusions • FGFR fusions T 790 M loss T 790 M maintained SCLC MET amp [CATEGOR Y NAME] Other rare genotypes T 790 M+/ C 797 SUnknown

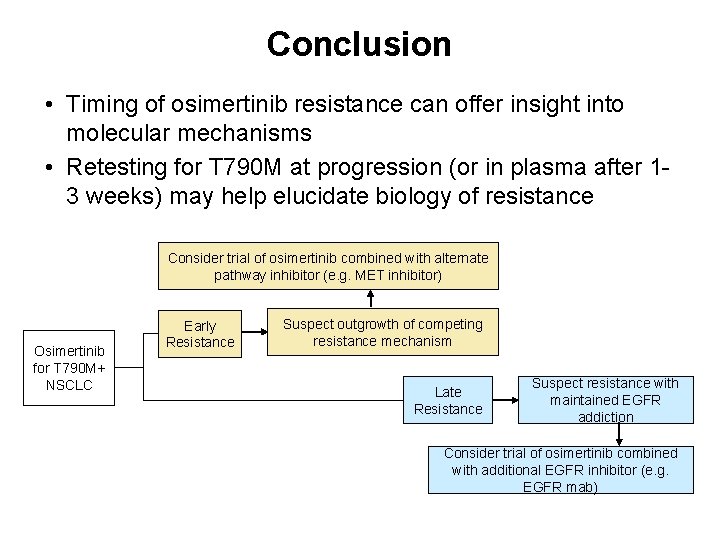
Conclusion • Timing of osimertinib resistance can offer insight into molecular mechanisms • Retesting for T 790 M at progression (or in plasma after 13 weeks) may help elucidate biology of resistance Consider trial of osimertinib combined with alternate pathway inhibitor (e. g. MET inhibitor) Osimertinib for T 790 M+ NSCLC Early Resistance Suspect outgrowth of competing resistance mechanism Late Resistance Suspect resistance with maintained EGFR addiction Consider trial of osimertinib combined with additional EGFR inhibitor (e. g. EGFR mab)

The Preclinical and Clinical Activity of Poziotinib, a Potent, Selective Inhibitor of EGFR Exon 20 Mutant NSCLC Yasir Y. Elamin, Jacqulyne P. Robichaux, Vincent K Lam, Anne Tsao, C. Lu, G. Blumenschein, J. Kurie, Julie R Brahmer, S. Li, T. Chen, A. Estrada-Bernal, A. Truini, M. Nilsson, A. T. Le, Z. Tan, S. Zhang, Robert C. Doebele, K. Politi, Z. Yang, S. Liu, Kwok-Kin Wong, and John V. Heymach University of Texas MD Anderson Cancer Center, Houston, Tx USA

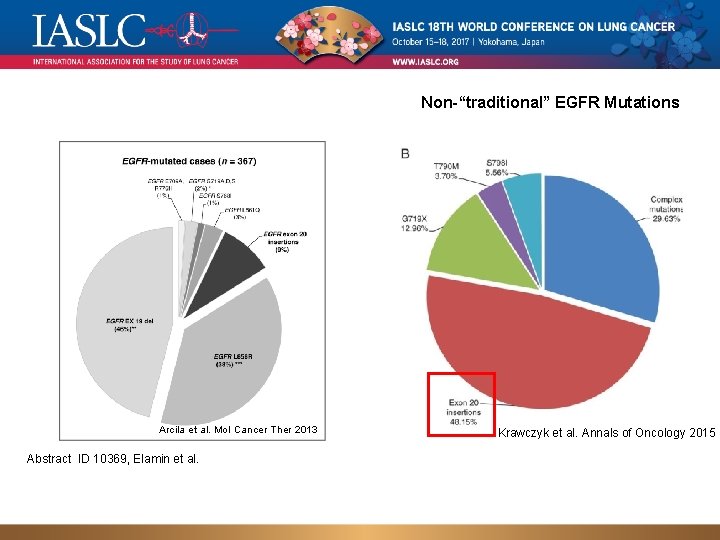
Non-“traditional” EGFR Mutations Arcila et al. Mol Cancer Ther 2013 Abstract ID 10369, Elamin et al. Krawczyk et al. Annals of Oncology 2015

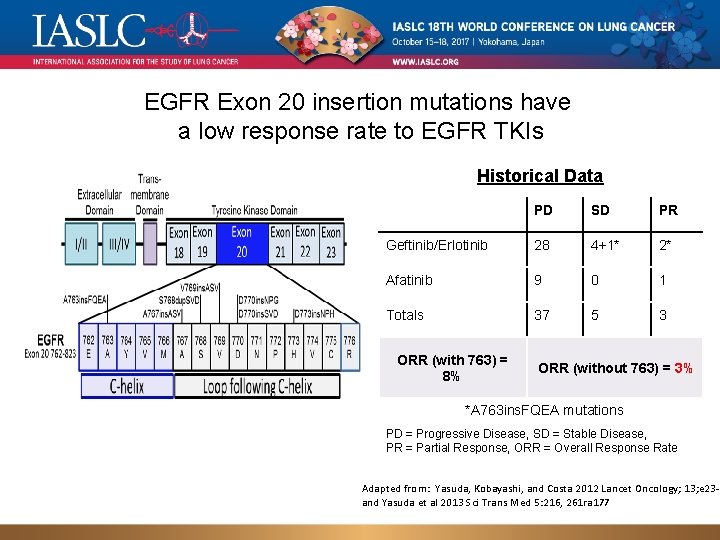
EGFR Exon 20 insertion mutations have a low response rate to EGFR TKIs Historical Data PD SD PR Geftinib/Erlotinib 28 4+1* 2* Afatinib 9 0 1 Totals 37 5 3 ORR (with 763) = 8% ORR (without 763) = 3% *A 763 ins. FQEA mutations PD = Progressive Disease, SD = Stable Disease, PR = Partial Response, ORR = Overall Response Rate Adapted from: Yasuda, Kobayashi, and Costa 2012 Lancet Oncology; 13; e 23 -3 and Yasuda et al 2013 Sci Trans Med 5: 216, 261 ra 177

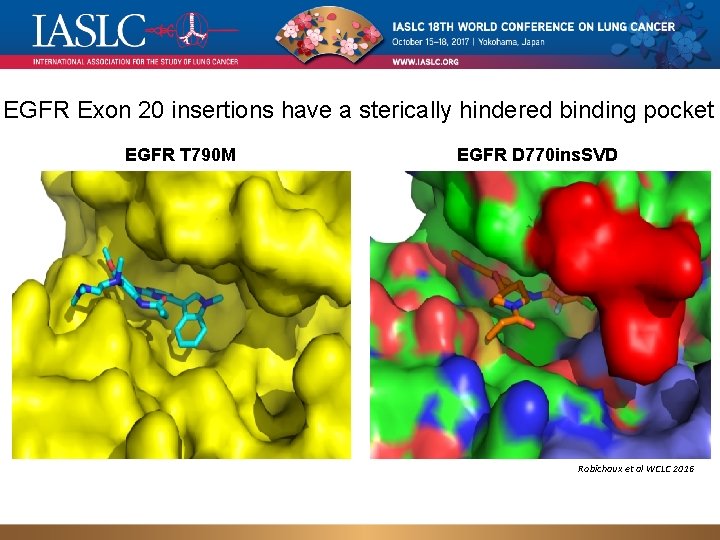
EGFR Exon 20 insertions have a sterically hindered binding pocket EGFR T 790 M EGFR D 770 ins. SVD Robichaux et al WCLC 2016

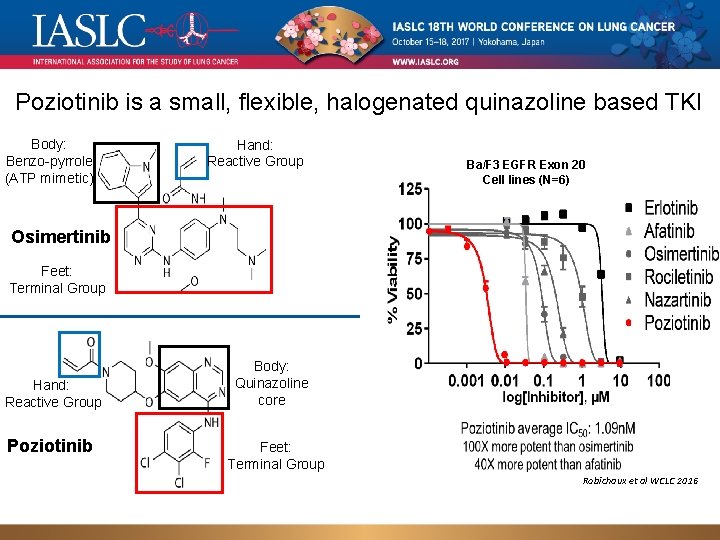
Poziotinib is a small, flexible, halogenated quinazoline based TKI Body: Benzo-pyrrole (ATP mimetic) Hand: Reactive Group Ba/F 3 EGFR Exon 20 Cell lines (N=6) Osimertinib Feet: Terminal Group Hand: Reactive Group Poziotinib Body: Quinazoline core Feet: Terminal Group Robichaux et al WCLC 2016

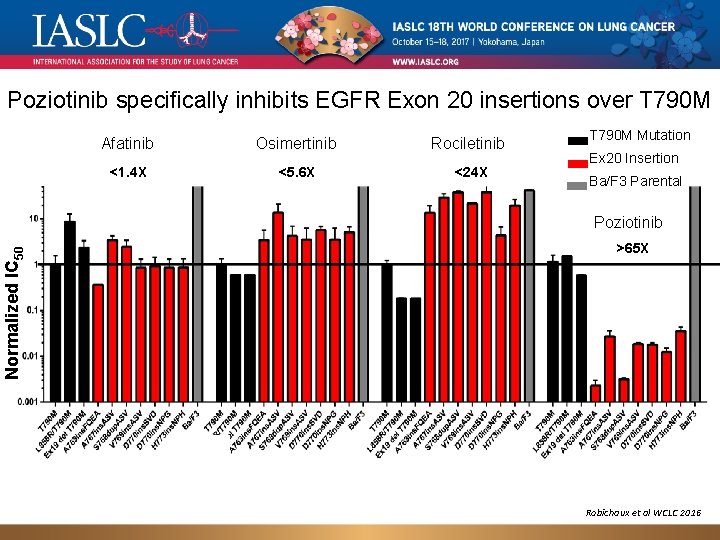
Poziotinib specifically inhibits EGFR Exon 20 insertions over T 790 M Afatinib Osimertinib Rociletinib <1. 4 X <5. 6 X <24 X T 790 M Mutation Ex 20 Insertion Ba/F 3 Parental Normalized IC 50 Poziotinib >65 X Robichaux et al WCLC 2016

Phase II study of Poziotinib for EGFR exon 20 mutations: preliminary efficacy results Design: Open-label, single center trial at MDACC -Cohort 1: EGFR exon 20 mutant NSCLC (planned N=30; 25 enrolled as of 10/2017) -Cohort 2: Her 2 exon 20 (N=30) Primary objective: Assess objective response rate (ORR, RECIST 1. 1) Secondary objectives: PFS, OS, disease control rate, duration of response, safety and toxicity Eligibility: 1. EGFR or HER 2 exon 20 mutation 2. One or more prior systemic therapy. 3. Brain metastases permitted if asymptomatic and stable, without escalating steroids or anticonvulsants. Abstract ID 10369, Elamin et al.

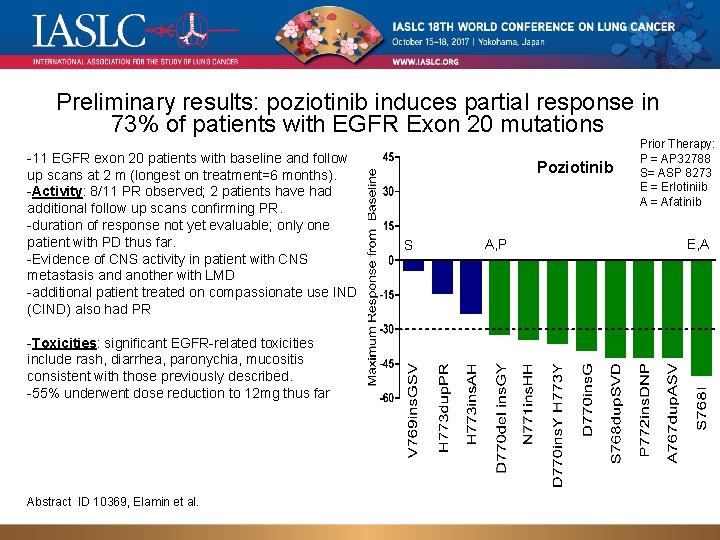
Preliminary results: poziotinib induces partial response in 73% of patients with EGFR Exon 20 mutations -11 EGFR exon 20 patients with baseline and follow up scans at 2 m (longest on treatment=6 months). -Activity: 8/11 PR observed; 2 patients have had additional follow up scans confirming PR. -duration of response not yet evaluable; only one patient with PD thus far. -Evidence of CNS activity in patient with CNS metastasis and another with LMD -additional patient treated on compassionate use IND (CIND) also had PR -Toxicities: significant EGFR-related toxicities include rash, diarrhea, paronychia, mucositis consistent with those previously described. -55% underwent dose reduction to 12 mg thus far Abstract ID 10369, Elamin et al. Poziotinib S A, P Prior Therapy: P = AP 32788 S= ASP 8273 E = Erlotiniib A = Afatinib E, A

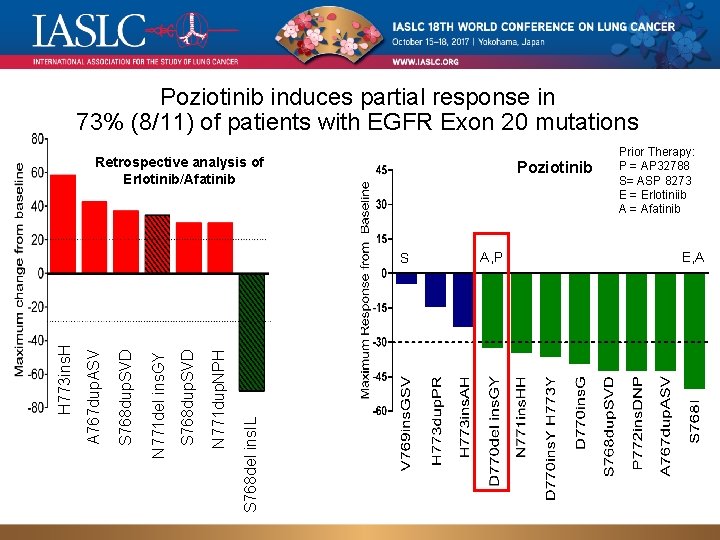
Poziotinib induces partial response in 73% (8/11) of patients with EGFR Exon 20 mutations Retrospective analysis of Erlotinib/Afatinib Poziotinib S 768 del ins. IL N 771 dup. NPH S 768 dup. SVD N 771 del ins. GY S 768 dup. SVD A 767 dup. ASV H 773 ins. H S A, P Prior Therapy: P = AP 32788 S= ASP 8273 E = Erlotiniib A = Afatinib E, A

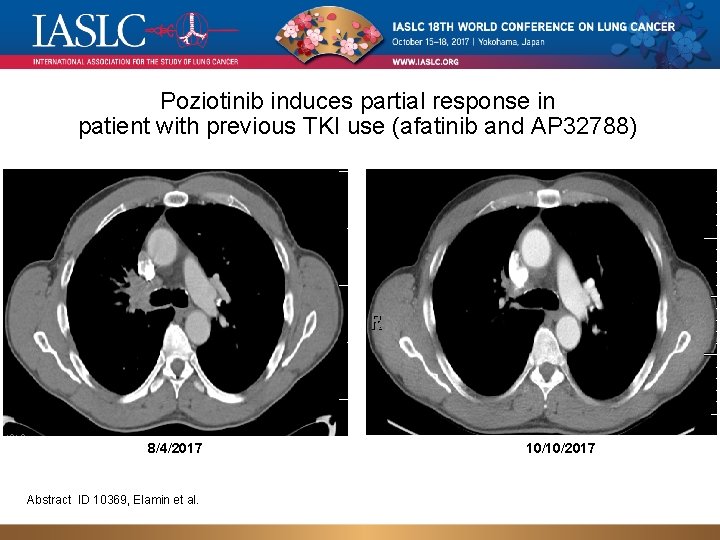
Poziotinib induces partial response in patient with previous TKI use (afatinib and AP 32788) 8/4/2017 Abstract ID 10369, Elamin et al. 10/10/2017

Conclusions 1. EGFR exon 20 insertions are resistant to most 1 st, 2 nd, and 3 rd-gen TKIs due a sterically hindered binding pocket. In retrospective studies EGFR exon 20 mutant patients have a median PFS<2 m and response rates are low (<20%) 2. Poziotinib specifically inhibits exon 20 insertions over T 790 M due to compound size and flexibility. It is >40 x more potent in vitro than clinically available TKIs. It reduces ≥ 80% of tumor burden in both EGFR PDX and GEM models. 3. Poziotinib demonstrates evidence of significant antitumor activity in EGFR exon 20 mutant NSCLC patients, with preliminary data showing an ORR of 73%. 4. Further study will be needed to confirm the high levels of activity, determine the duration of response and to investigate potential mechanisms of resistance. 5. An international, multicenter study has recently started enrollment. Contact John Heymach jheymach@mdanderson. org for information. Abstract ID 10369, Elamin et al.

Conclusions • In the FLAURA screened population, high concordance was observed between tissue and plasma testing for EGFRm • Using the cobas tissue test as a reference, sensitivity for the detection of T 790 M was increased for dd. PCR and NGS compared with AS-PCR • 10% of patients may progress on 3 rd generation EGFR TKI with T 790 M/C 797 S trans configuration, and might respond to combination • Loss of T 79 M is associated with early progression, poor outcome and overgrowth of a competing resistance mutation • Poziotinib demonstrates evidence of significant antitumor activity in EGFR exon 20 mutant NSCLC patients, with preliminary data showing an ORR of 73% 36

Obrigado Luiz Henrique Araujo, MD, Ph. D luiz. lima@inca. gov. br luizaraujo@institutocoi. org