Chemical Reactions METALS AND ACIDS gala03 Chemical Reactions













- Slides: 33

Chemical Reactions METALS AND ACIDS gala/03

Chemical Reactions METALS AND ACIDS Metals Magnesium Iron Sodium Calcium gala/03

Chemical Reactions METALS AND ACIDS Metals Acids Magnesium Hydrochloric acid Iron Sulphuric acid Sodium Nitric acid Calcium Ethanoic acid gala/03

Chemical Reactions METALS AND ACIDS Metals Acids Magnesium Hydrochloric acid Iron Sulphuric acid Sodium Nitric acid Calcium Ethanoic acid Metal + Acid Salt + Hydrogen gala/03

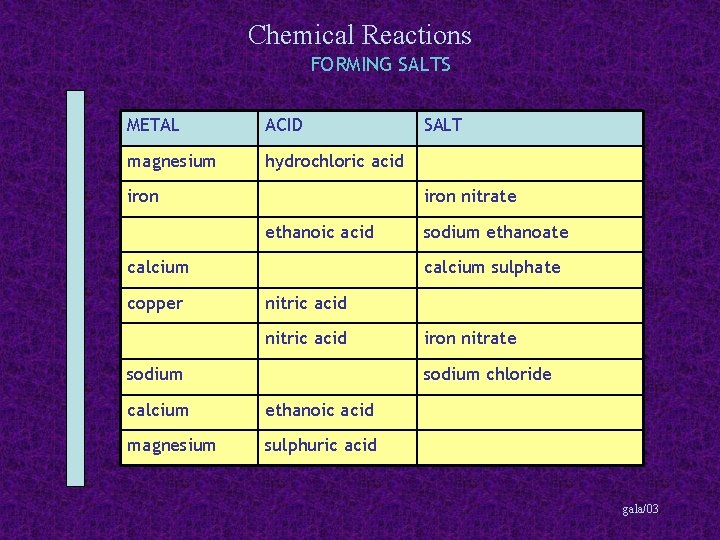
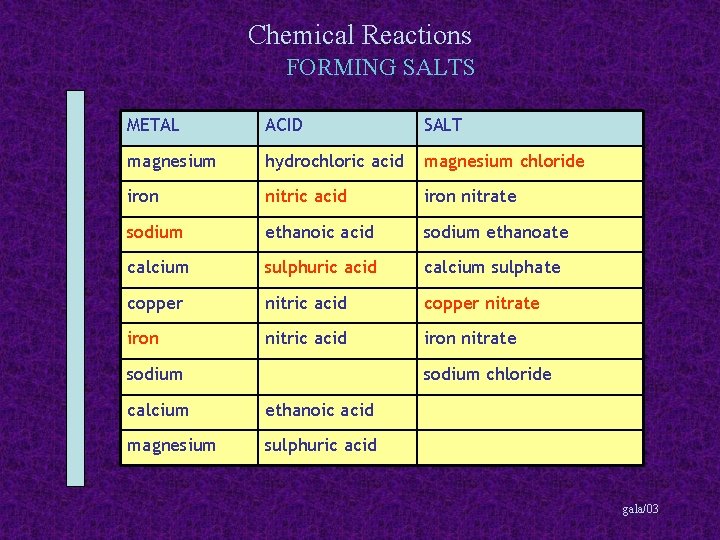
Chemical Reactions FORMING SALTS METAL ACID magnesium hydrochloric acid iron nitrate ethanoic acid calcium copper SALT sodium ethanoate calcium sulphate nitric acid sodium iron nitrate sodium chloride calcium ethanoic acid magnesium sulphuric acid gala/03

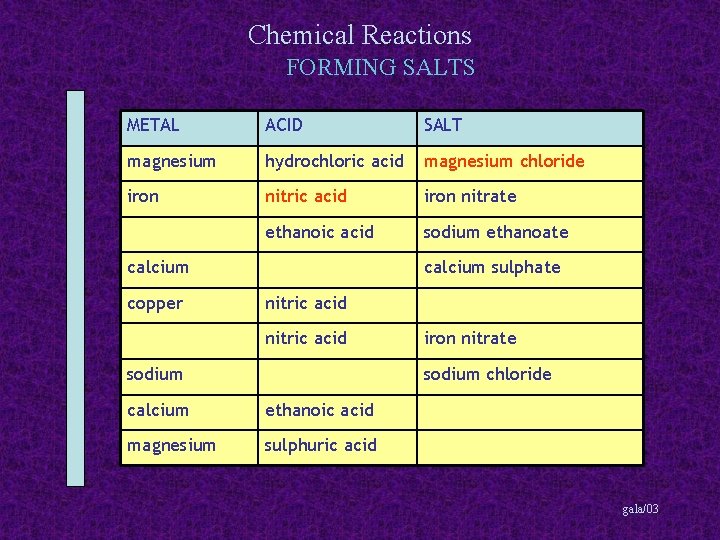
Chemical Reactions FORMING SALTS METAL ACID SALT magnesium hydrochloric acid magnesium chloride iron nitrate ethanoic acid calcium copper sodium ethanoate calcium sulphate nitric acid sodium iron nitrate sodium chloride calcium ethanoic acid magnesium sulphuric acid gala/03

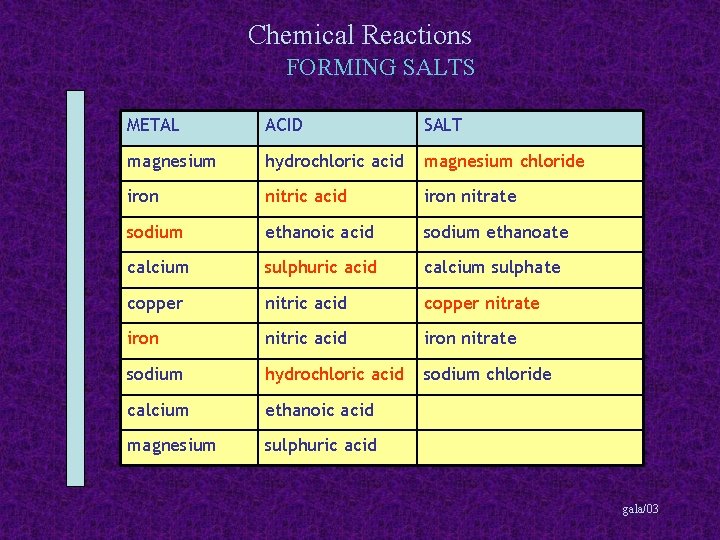
Chemical Reactions FORMING SALTS METAL ACID SALT magnesium hydrochloric acid magnesium chloride iron nitric acid iron nitrate ethanoic acid sodium ethanoate calcium copper calcium sulphate nitric acid sodium iron nitrate sodium chloride calcium ethanoic acid magnesium sulphuric acid gala/03

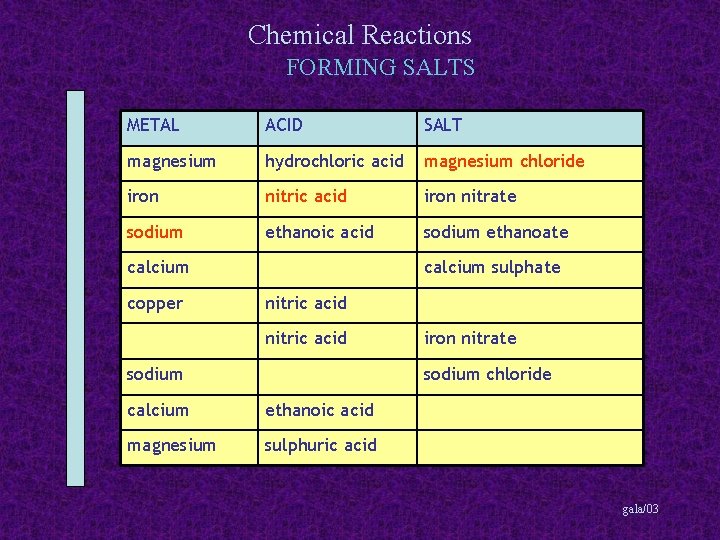
Chemical Reactions FORMING SALTS METAL ACID SALT magnesium hydrochloric acid magnesium chloride iron nitric acid iron nitrate sodium ethanoic acid sodium ethanoate calcium copper calcium sulphate nitric acid sodium iron nitrate sodium chloride calcium ethanoic acid magnesium sulphuric acid gala/03

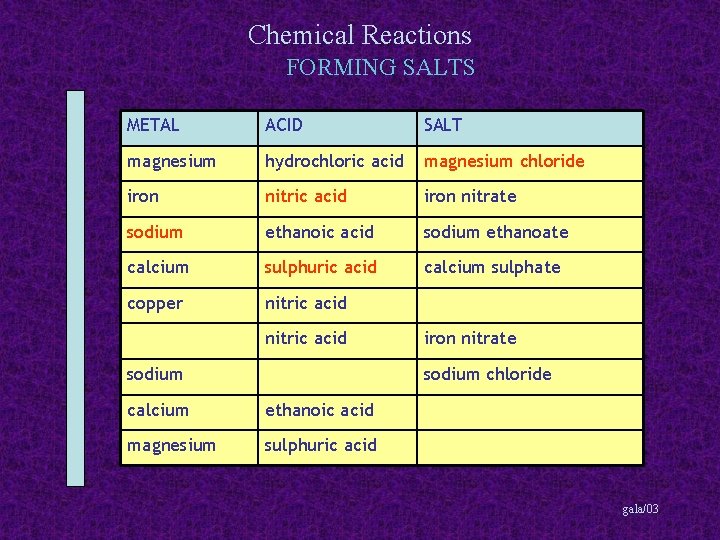
Chemical Reactions FORMING SALTS METAL ACID SALT magnesium hydrochloric acid magnesium chloride iron nitric acid iron nitrate sodium ethanoic acid sodium ethanoate calcium sulphuric acid calcium sulphate copper nitric acid sodium iron nitrate sodium chloride calcium ethanoic acid magnesium sulphuric acid gala/03

Chemical Reactions FORMING SALTS METAL ACID SALT magnesium hydrochloric acid magnesium chloride iron nitric acid iron nitrate sodium ethanoic acid sodium ethanoate calcium sulphuric acid calcium sulphate copper nitric acid No Reaction nitric acid iron nitrate sodium chloride calcium ethanoic acid magnesium sulphuric acid gala/03

Chemical Reactions FORMING SALTS METAL ACID SALT magnesium hydrochloric acid magnesium chloride iron nitric acid iron nitrate sodium ethanoic acid sodium ethanoate calcium sulphuric acid calcium sulphate copper nitric acid copper nitrate iron nitric acid iron nitrate sodium chloride calcium ethanoic acid magnesium sulphuric acid gala/03

Chemical Reactions FORMING SALTS METAL ACID SALT magnesium hydrochloric acid magnesium chloride iron nitric acid iron nitrate sodium ethanoic acid sodium ethanoate calcium sulphuric acid calcium sulphate copper nitric acid copper nitrate iron nitric acid iron nitrate sodium hydrochloric acid sodium chloride calcium ethanoic acid magnesium sulphuric acid gala/03

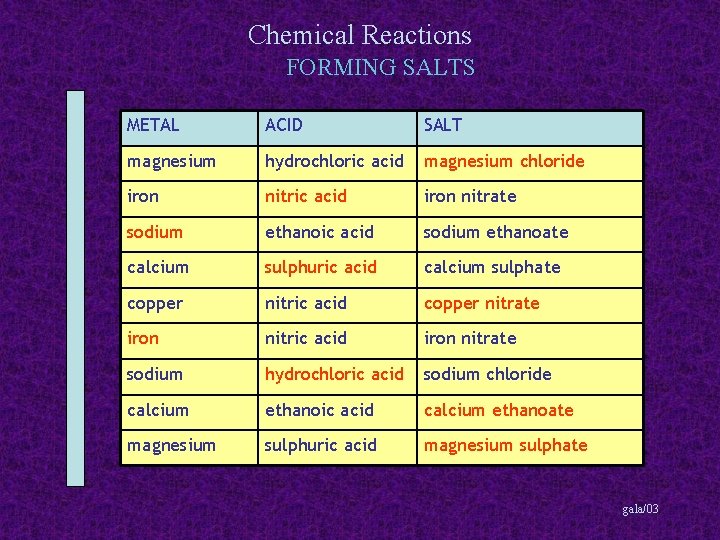
Chemical Reactions FORMING SALTS METAL ACID SALT magnesium hydrochloric acid magnesium chloride iron nitric acid iron nitrate sodium ethanoic acid sodium ethanoate calcium sulphuric acid calcium sulphate copper nitric acid copper nitrate iron nitric acid iron nitrate sodium hydrochloric acid sodium chloride calcium ethanoic acid calcium ethanoate magnesium sulphuric acid gala/03

Chemical Reactions FORMING SALTS METAL ACID SALT magnesium hydrochloric acid magnesium chloride iron nitric acid iron nitrate sodium ethanoic acid sodium ethanoate calcium sulphuric acid calcium sulphate copper nitric acid copper nitrate iron nitric acid iron nitrate sodium hydrochloric acid sodium chloride calcium ethanoic acid calcium ethanoate magnesium sulphuric acid magnesium sulphate gala/03

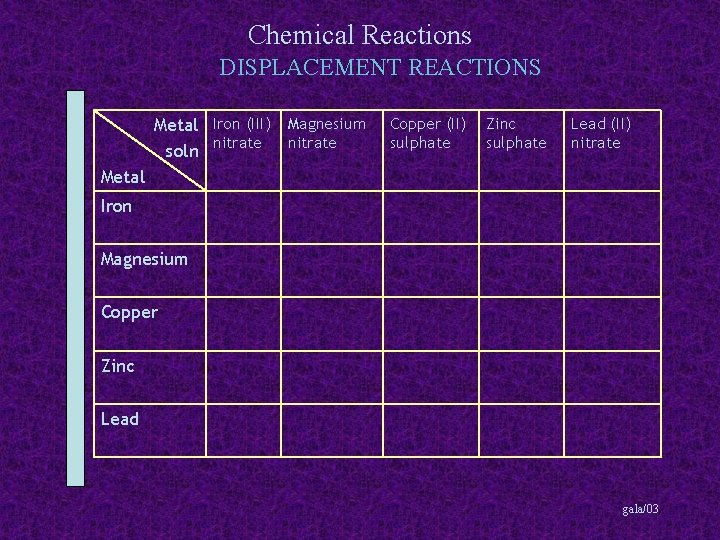
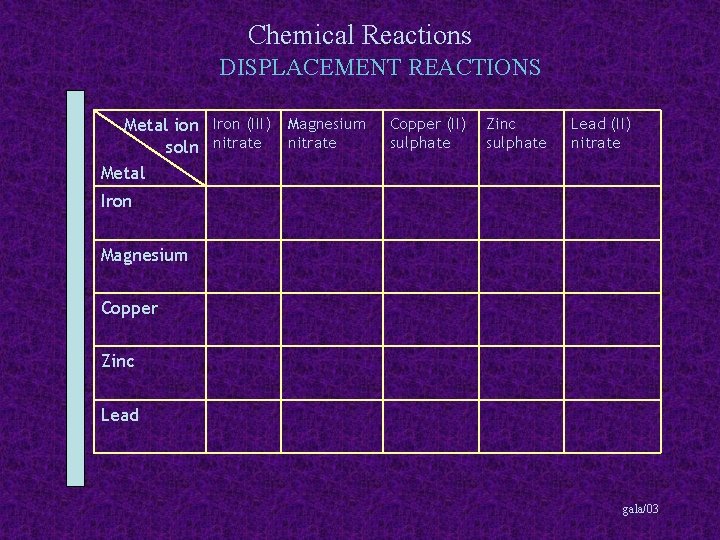
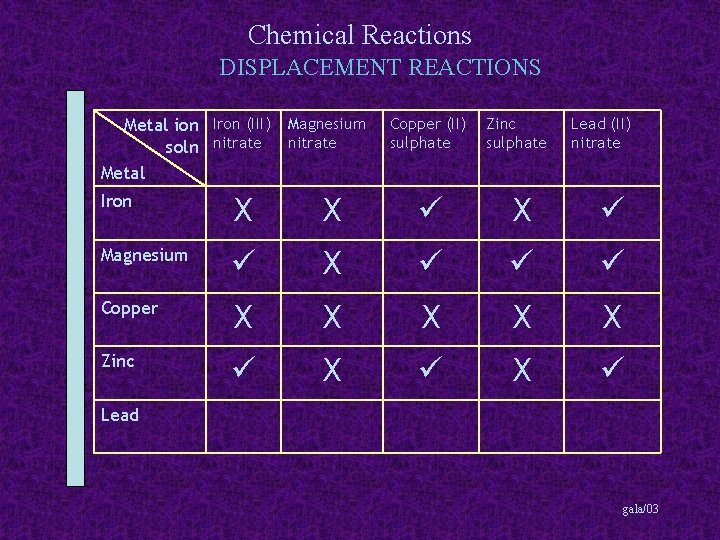
Chemical Reactions DISPLACEMENT REACTIONS Metal Iron (III) Magnesium soln nitrate Copper (II) sulphate Zinc sulphate Lead (II) nitrate Metal Iron Magnesium Copper Zinc Lead gala/03

Chemical Reactions DISPLACEMENT REACTIONS Instructions 1. Care! You should wear GOGGLES and LAB-COATS at all times during this practical 2. Obtain a dropping tile 3. Add a few drops of each of the solutions to individual ‘holes’ in the dropping tile. Make sure you know which solution is which 4. Choose a metal 5. Add one piece of that metal to each of the solutions 6. Observe – you will need to watch carefully for any evidence of a reaction taking place 7. If a reaction takes place, put a tick into the relevant box. If no reaction takes place, put a cross. 8. If a reaction takes place, try and describe what is happening 9. Wash all the reactions into a large beaker – do NOT put any solids into the sink! 10. Repeat for each metal using fresh solutions gala/03

Chemical Reactions DISPLACEMENT REACTIONS Metal ion Iron (III) Magnesium soln nitrate Metal Copper (II) sulphate Zinc sulphate Lead (II) nitrate Iron Magnesium Copper Zinc Lead gala/03

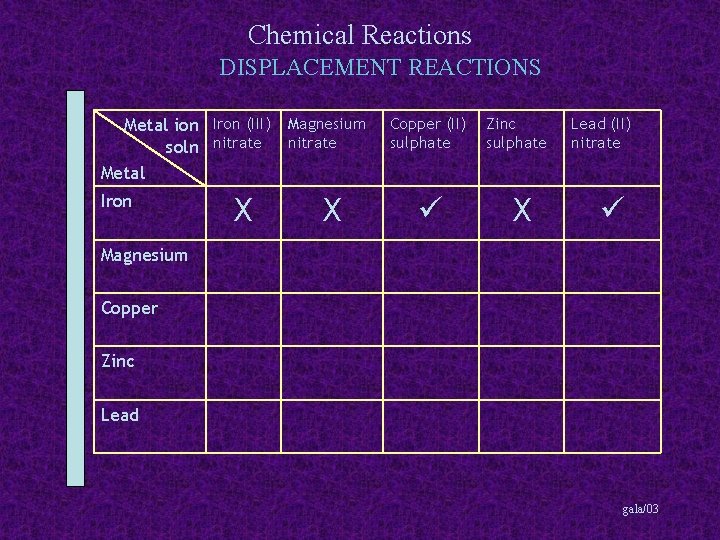
Chemical Reactions DISPLACEMENT REACTIONS Metal ion Iron (III) Magnesium soln nitrate Metal Iron X X Copper (II) sulphate Zinc sulphate X Lead (II) nitrate Magnesium Copper Zinc Lead gala/03

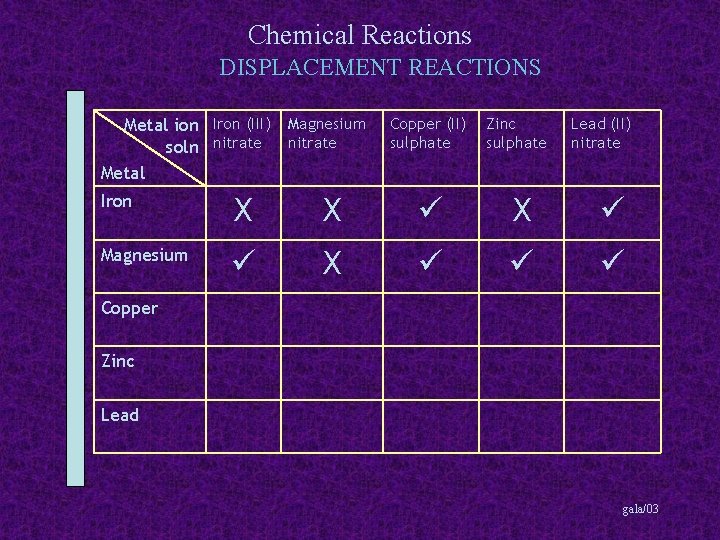
Chemical Reactions DISPLACEMENT REACTIONS Metal ion Iron (III) Magnesium soln nitrate Metal Copper (II) sulphate Zinc sulphate Lead (II) nitrate Iron X X X Magnesium X Copper Zinc Lead gala/03

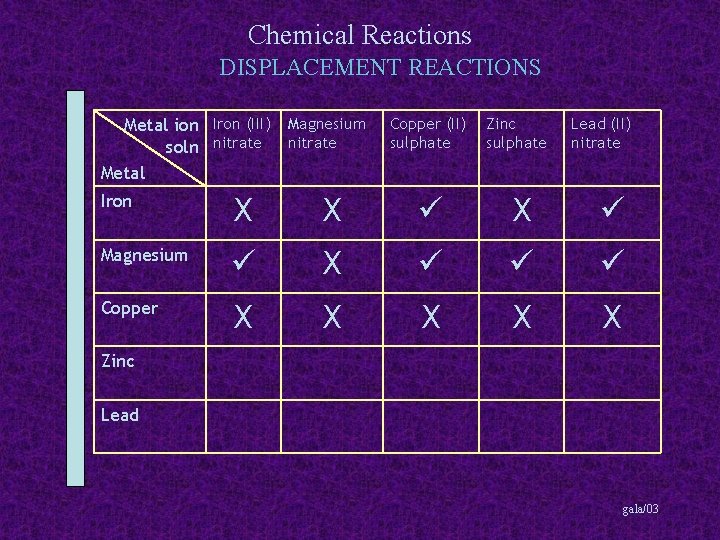
Chemical Reactions DISPLACEMENT REACTIONS Metal ion Iron (III) Magnesium soln nitrate Metal Copper (II) sulphate Zinc sulphate Lead (II) nitrate Iron X X X Magnesium X Copper X X X Zinc Lead gala/03

Chemical Reactions DISPLACEMENT REACTIONS Metal ion Iron (III) Magnesium soln nitrate Metal Copper (II) sulphate Zinc sulphate Lead (II) nitrate Iron X X X Magnesium X Copper X X X Zinc X X Lead gala/03

Chemical Reactions DISPLACEMENT REACTIONS Metal ion Iron (III) Magnesium soln nitrate Metal Copper (II) sulphate Zinc sulphate Lead (II) nitrate Iron X X X Magnesium X Copper X X X Zinc X X Lead X X gala/03

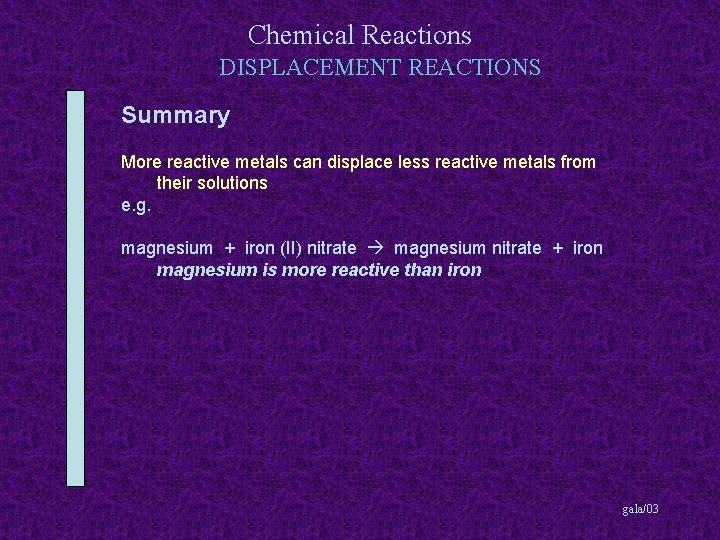
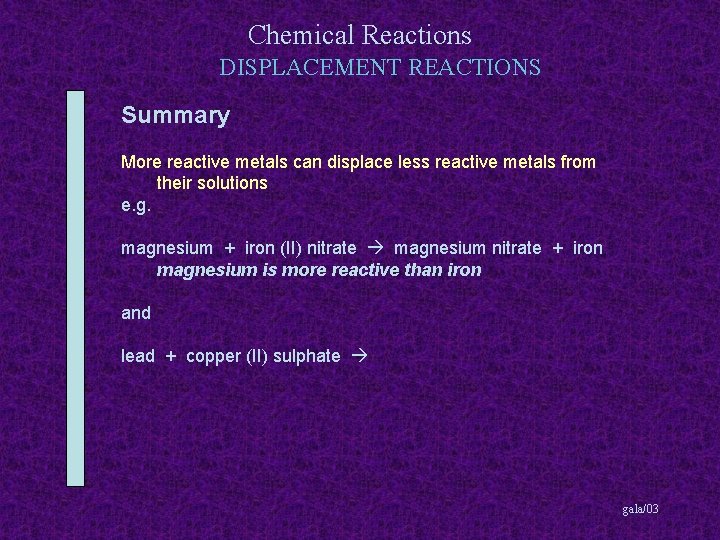
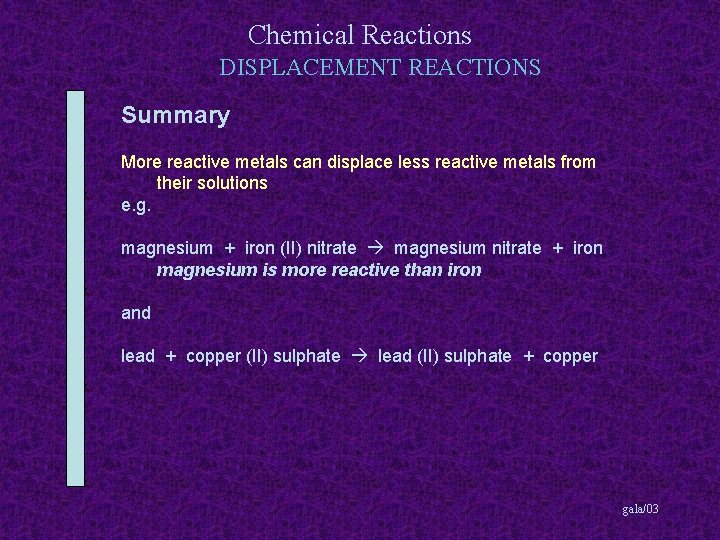
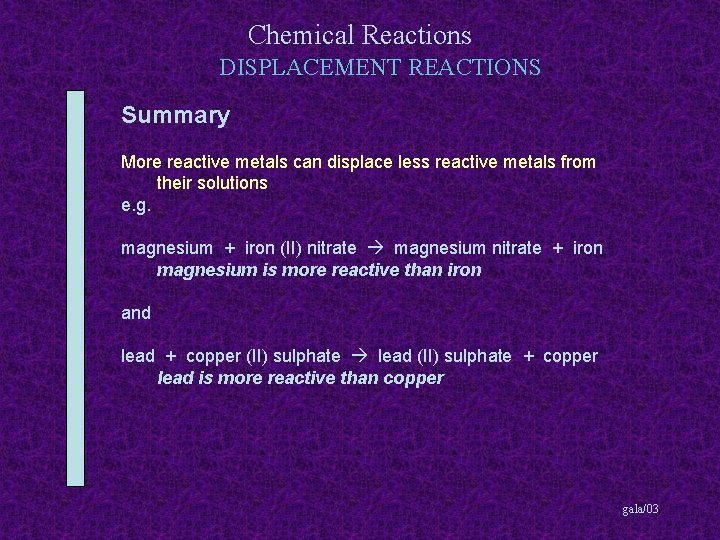
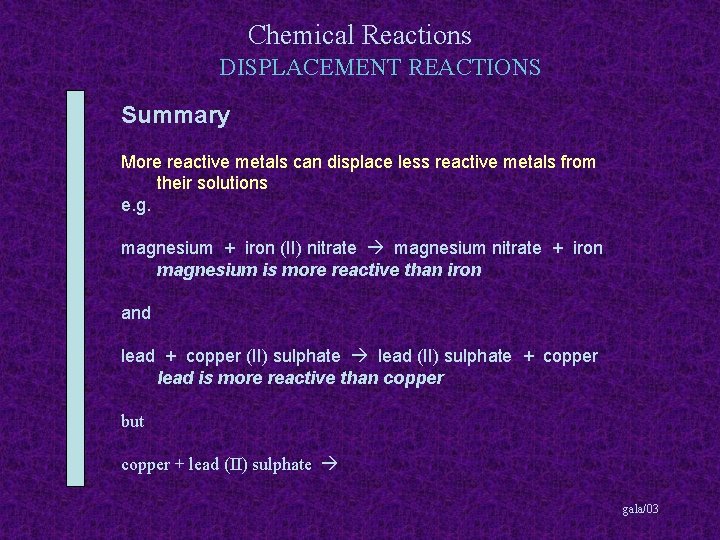
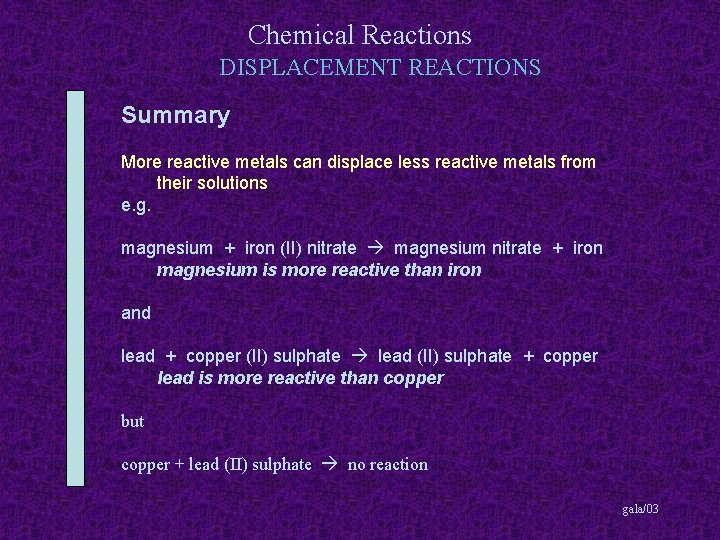
Chemical Reactions DISPLACEMENT REACTIONS Summary More reactive metals can displace less reactive metals from their solutions gala/03

Chemical Reactions DISPLACEMENT REACTIONS Summary More reactive metals can displace less reactive metals from their solutions e. g. magnesium + iron (II) nitrate gala/03

Chemical Reactions DISPLACEMENT REACTIONS Summary More reactive metals can displace less reactive metals from their solutions e. g. magnesium + iron (II) nitrate magnesium nitrate + iron gala/03

Chemical Reactions DISPLACEMENT REACTIONS Summary More reactive metals can displace less reactive metals from their solutions e. g. magnesium + iron (II) nitrate magnesium nitrate + iron magnesium is more reactive than iron gala/03

Chemical Reactions DISPLACEMENT REACTIONS Summary More reactive metals can displace less reactive metals from their solutions e. g. magnesium + iron (II) nitrate magnesium nitrate + iron magnesium is more reactive than iron and lead + copper (II) sulphate gala/03

Chemical Reactions DISPLACEMENT REACTIONS Summary More reactive metals can displace less reactive metals from their solutions e. g. magnesium + iron (II) nitrate magnesium nitrate + iron magnesium is more reactive than iron and lead + copper (II) sulphate lead (II) sulphate + copper gala/03

Chemical Reactions DISPLACEMENT REACTIONS Summary More reactive metals can displace less reactive metals from their solutions e. g. magnesium + iron (II) nitrate magnesium nitrate + iron magnesium is more reactive than iron and lead + copper (II) sulphate lead (II) sulphate + copper lead is more reactive than copper gala/03

Chemical Reactions DISPLACEMENT REACTIONS Summary More reactive metals can displace less reactive metals from their solutions e. g. magnesium + iron (II) nitrate magnesium nitrate + iron magnesium is more reactive than iron and lead + copper (II) sulphate lead (II) sulphate + copper lead is more reactive than copper but copper + lead (II) sulphate gala/03

Chemical Reactions DISPLACEMENT REACTIONS Summary More reactive metals can displace less reactive metals from their solutions e. g. magnesium + iron (II) nitrate magnesium nitrate + iron magnesium is more reactive than iron and lead + copper (II) sulphate lead (II) sulphate + copper lead is more reactive than copper but copper + lead (II) sulphate no reaction gala/03

Chemical Reactions DISPLACEMENT REACTIONS Summary More reactive metals can displace less reactive metals from their solutions e. g. magnesium + iron (II) nitrate magnesium nitrate + iron magnesium is more reactive than iron and lead + copper (II) sulphate lead (II) sulphate + copper lead is more reactive than copper but copper + lead (II) sulphate no reaction copper is not more reactive than lead gala/03

Chemical Reactions This powerpoint was kindly donated to www. worldofteaching. com http: //www. worldofteaching. com is home to over a thousand powerpoints submitted by teachers. This is a completely free site and requires no registration. Please visit and I hope it will help in your teaching. gala/03
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Section 2 reinforcement classifying chemical reactions
Section 2 reinforcement classifying chemical reactions Nonmetals on the periodic table
Nonmetals on the periodic table Characteristics of metals
Characteristics of metals Natural science grade 7 term 2
Natural science grade 7 term 2 Grade 7 term 2 natural science
Grade 7 term 2 natural science Example of metal
Example of metal Types of reactions
Types of reactions Examples of alloy metals
Examples of alloy metals Section 1 chemical changes
Section 1 chemical changes Are kc and kp equal
Are kc and kp equal Alkaline earth metals reactions
Alkaline earth metals reactions Non metals
Non metals Basic redox reactions
Basic redox reactions Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Alkali metals reactivity
Alkali metals reactivity All acids owe their chemical reactivity to
All acids owe their chemical reactivity to Chemical reactions reactants and products
Chemical reactions reactants and products Chapter 8 review describing chemical reactions
Chapter 8 review describing chemical reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Balancing equations chapter 8
Balancing equations chapter 8 Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes I intro
I intro Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Types of chemical reactions and solution stoichiometry
Types of chemical reactions and solution stoichiometry Percent yield of copper lab
Percent yield of copper lab What is released or absorbed whenever chemical
What is released or absorbed whenever chemical Section 2-4 chemical reactions and enzymes
Section 2-4 chemical reactions and enzymes Building vocabulary: chemical bonds and reactions
Building vocabulary: chemical bonds and reactions Stoichiometry mole island diagram
Stoichiometry mole island diagram Balancing redox reactions
Balancing redox reactions How to identify types of chemical reactions
How to identify types of chemical reactions 4 types of chemical reactions
4 types of chemical reactions
























































