Acids and Metals Acids react with certain metals




- Slides: 29

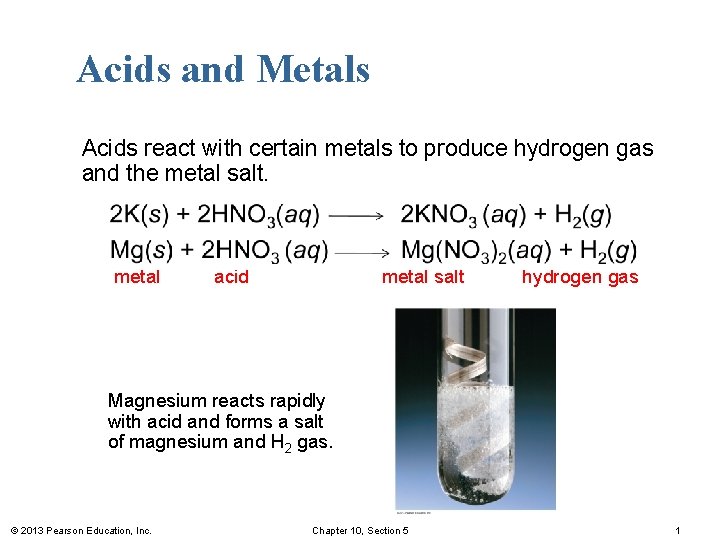
Acids and Metals Acids react with certain metals to produce hydrogen gas and the metal salt. metal acid metal salt hydrogen gas Magnesium reacts rapidly with acid and forms a salt of magnesium and H 2 gas. © 2013 Pearson Education, Inc. Chapter 10, Section 5 1

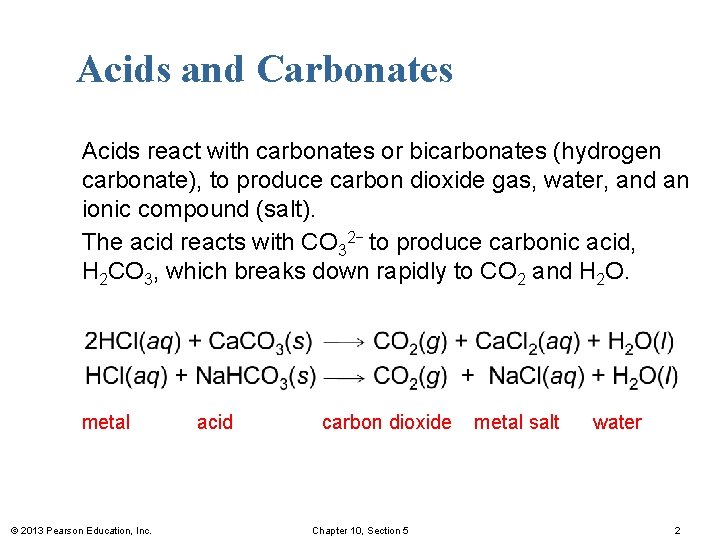
Acids and Carbonates Acids react with carbonates or bicarbonates (hydrogen carbonate), to produce carbon dioxide gas, water, and an ionic compound (salt). The acid reacts with CO 32− to produce carbonic acid, H 2 CO 3, which breaks down rapidly to CO 2 and H 2 O. metal © 2013 Pearson Education, Inc. acid carbon dioxide Chapter 10, Section 5 metal salt water 2

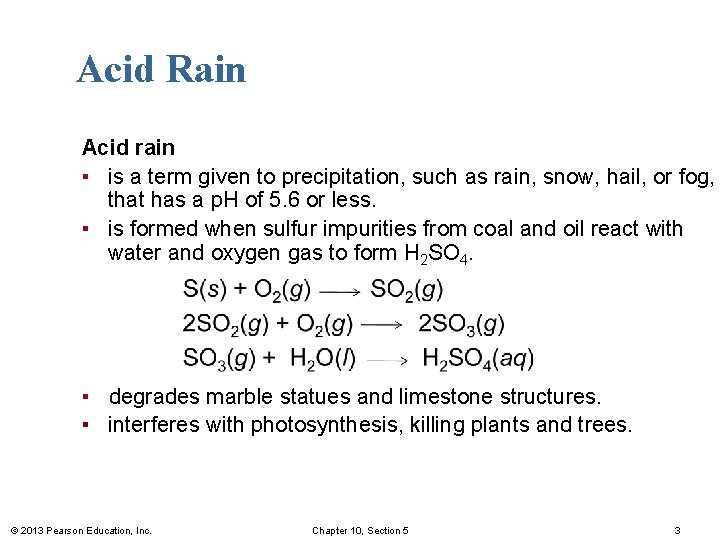
Acid Rain Acid rain ▪ is a term given to precipitation, such as rain, snow, hail, or fog, that has a p. H of 5. 6 or less. ▪ is formed when sulfur impurities from coal and oil react with water and oxygen gas to form H 2 SO 4. ▪ degrades marble statues and limestone structures. ▪ interferes with photosynthesis, killing plants and trees. © 2013 Pearson Education, Inc. Chapter 10, Section 5 3

Acid Rain A marble statue in Washington Square Park has been eroded by acid rain. © 2013 Pearson Education, Inc. Acid rain has severely damaged forests in Eastern Europe. Chapter 10, Section 5 4

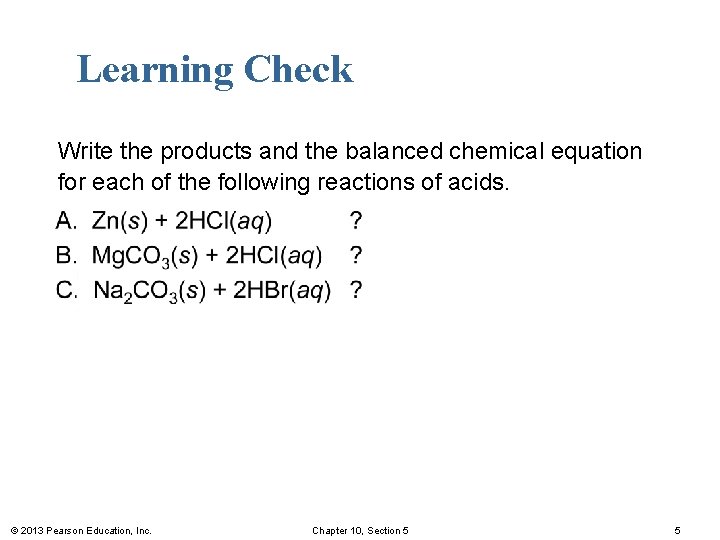
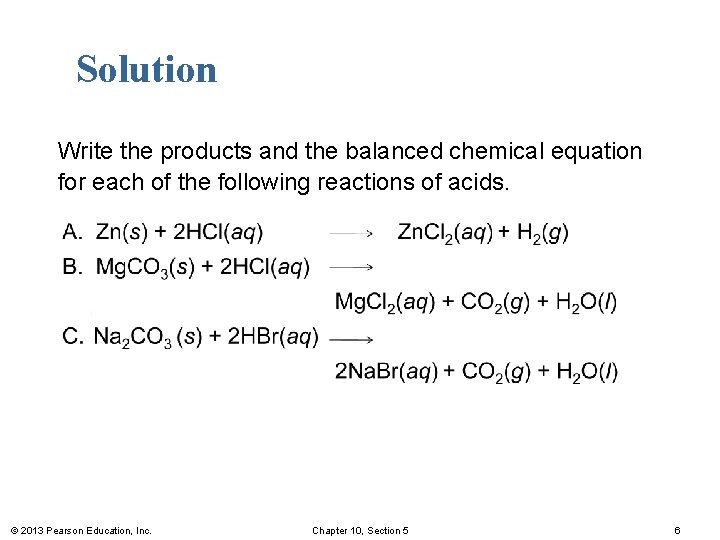
Learning Check Write the products and the balanced chemical equation for each of the following reactions of acids. © 2013 Pearson Education, Inc. Chapter 10, Section 5 5

Solution Write the products and the balanced chemical equation for each of the following reactions of acids. © 2013 Pearson Education, Inc. Chapter 10, Section 5 6

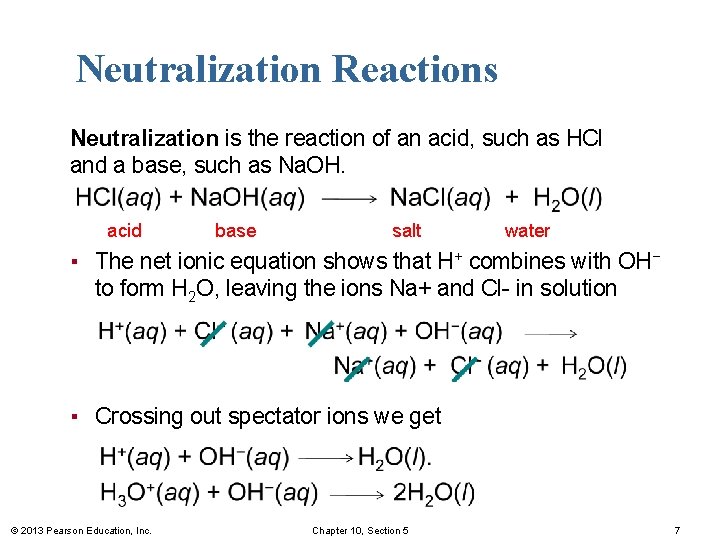
Neutralization Reactions Neutralization is the reaction of an acid, such as HCl and a base, such as Na. OH. acid base salt water ▪ The net ionic equation shows that H+ combines with OH− to form H 2 O, leaving the ions Na+ and Cl- in solution ▪ Crossing out spectator ions we get © 2013 Pearson Education, Inc. Chapter 10, Section 5 7

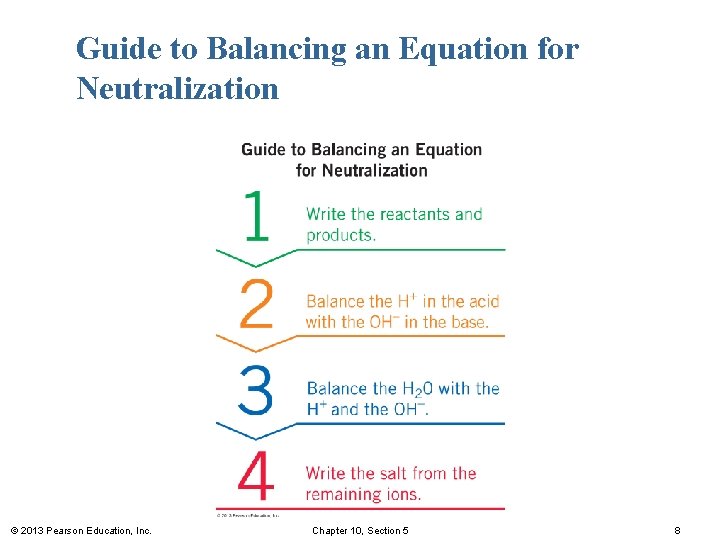
Guide to Balancing an Equation for Neutralization © 2013 Pearson Education, Inc. Chapter 10, Section 5 8

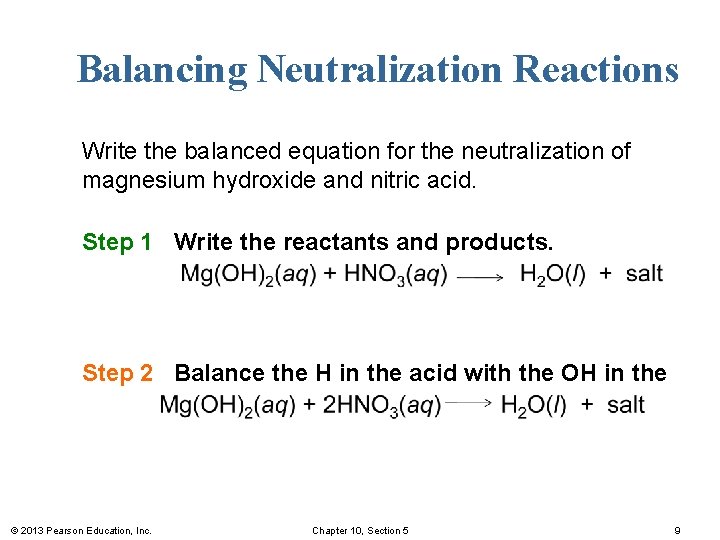
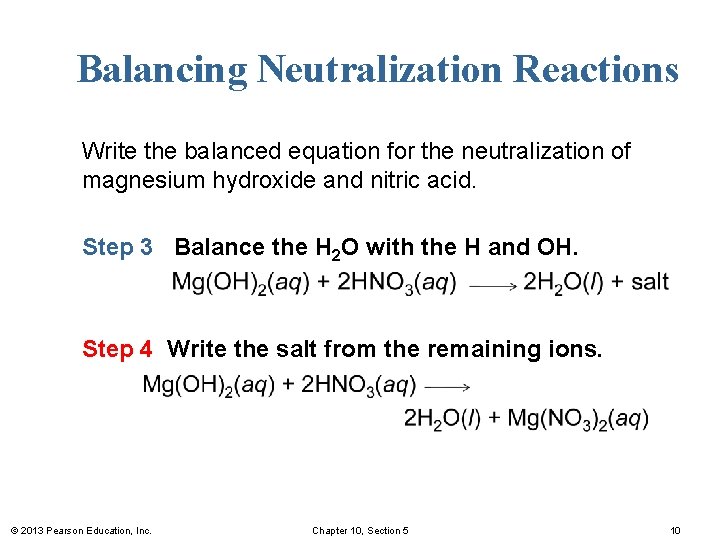
Balancing Neutralization Reactions Write the balanced equation for the neutralization of magnesium hydroxide and nitric acid. Step 1 Write the reactants and products. Step 2 Balance the H in the acid with the OH in the base. © 2013 Pearson Education, Inc. Chapter 10, Section 5 9

Balancing Neutralization Reactions Write the balanced equation for the neutralization of magnesium hydroxide and nitric acid. Step 3 Balance the H 2 O with the H and OH. Step 4 Write the salt from the remaining ions. © 2013 Pearson Education, Inc. Chapter 10, Section 5 10

Learning Check Write the balanced equation for the reaction of the base KOH with the strong acid, H 2 SO 4. © 2013 Pearson Education, Inc. Chapter 10, Section 5 11

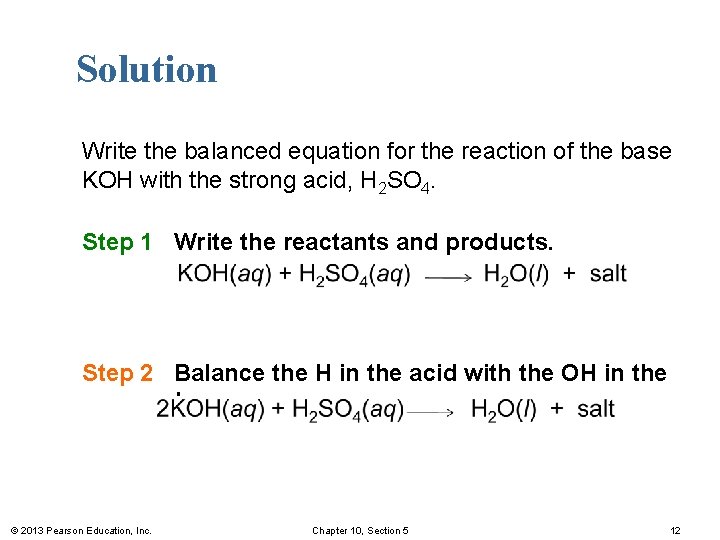
Solution Write the balanced equation for the reaction of the base KOH with the strong acid, H 2 SO 4. Step 1 Write the reactants and products. Step 2 Balance the H in the acid with the OH in the base. © 2013 Pearson Education, Inc. Chapter 10, Section 5 12

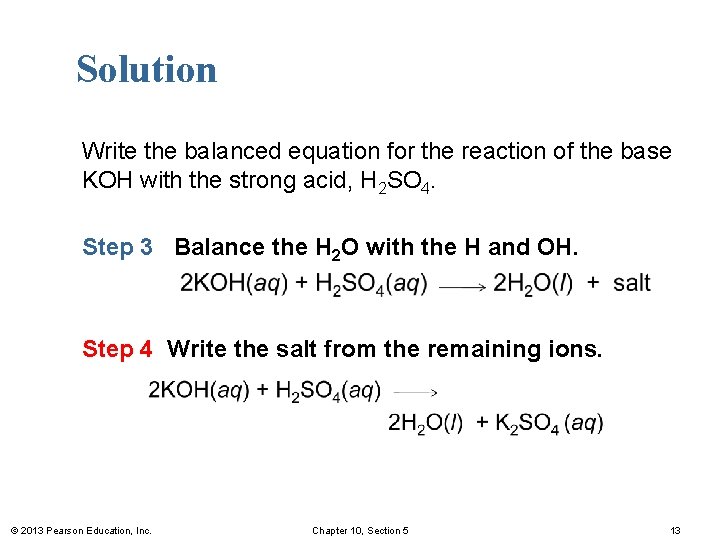
Solution Write the balanced equation for the reaction of the base KOH with the strong acid, H 2 SO 4. Step 3 Balance the H 2 O with the H and OH. Step 4 Write the salt from the remaining ions. © 2013 Pearson Education, Inc. Chapter 10, Section 5 13

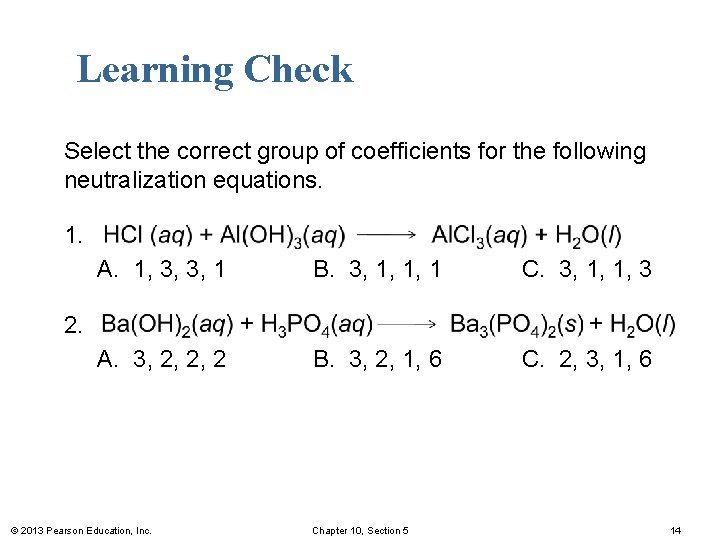
Learning Check Select the correct group of coefficients for the following neutralization equations. 1. A. 1, 3, 3, 1 B. 3, 1, 1, 1 C. 3, 1, 1, 3 A. 3, 2, 2, 2 B. 3, 2, 1, 6 C. 2, 3, 1, 6 2. © 2013 Pearson Education, Inc. Chapter 10, Section 5 14

Solution Select the correct group of coefficients for the following neutralization equations. 1. Answer is C. 3, 1, 1, 3. 2. Answer is B. 3, 2, 1, 6. © 2013 Pearson Education, Inc. Chapter 10, Section 5 15

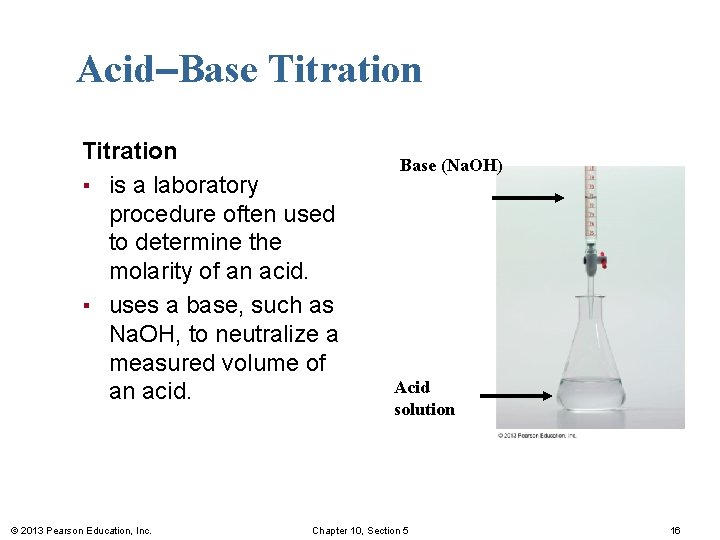
Acid–Base Titration ▪ is a laboratory procedure often used to determine the molarity of an acid. ▪ uses a base, such as Na. OH, to neutralize a measured volume of an acid. © 2013 Pearson Education, Inc. Base (Na. OH) Acid solution Chapter 10, Section 5 16

Indicator An indicator ▪ is added to the acid in the flask. ▪ causes the solution to change color when the acid is neutralized. © 2013 Pearson Education, Inc. Chapter 10, Section 5 17

End Point of Titration At the end point, ▪ moles of OH− equal moles of H 3 O+ in the acid, and ▪ the indicator has a faint, permanent pink color. © 2013 Pearson Education, Inc. Chapter 10, Section 5 18

Concentration of the Acid From the measured volume of the Na. OH solution at the end point and its molarity, we calculate ▪ the number of moles of Na. OH used, ▪ the moles of acid in the flask, and ▪ the concentration of the acid. © 2013 Pearson Education, Inc. Chapter 10, Section 5 19

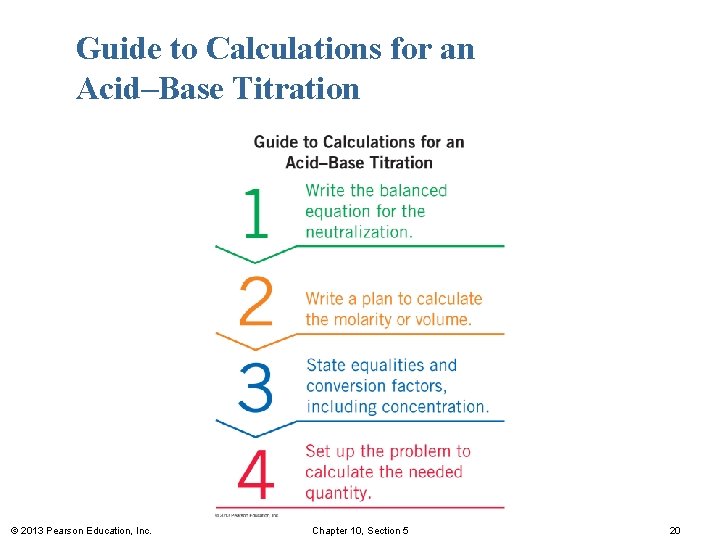
Guide to Calculations for an Acid–Base Titration © 2013 Pearson Education, Inc. Chapter 10, Section 5 20

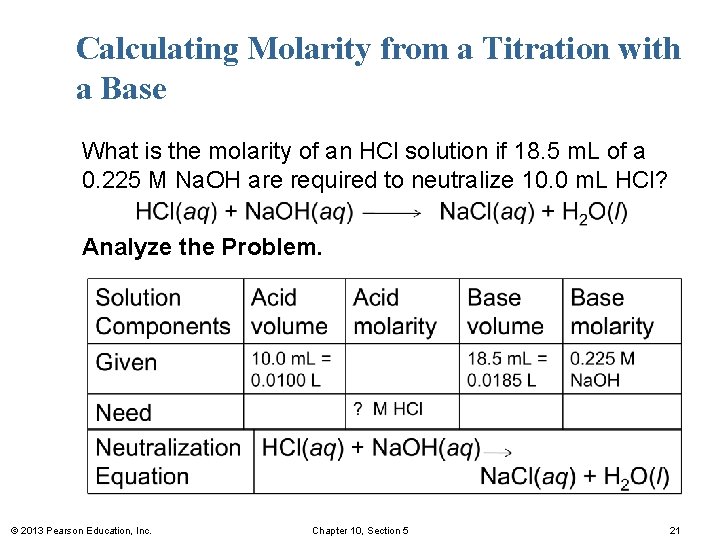
Calculating Molarity from a Titration with a Base What is the molarity of an HCl solution if 18. 5 m. L of a 0. 225 M Na. OH are required to neutralize 10. 0 m. L HCl? Analyze the Problem. © 2013 Pearson Education, Inc. Chapter 10, Section 5 21

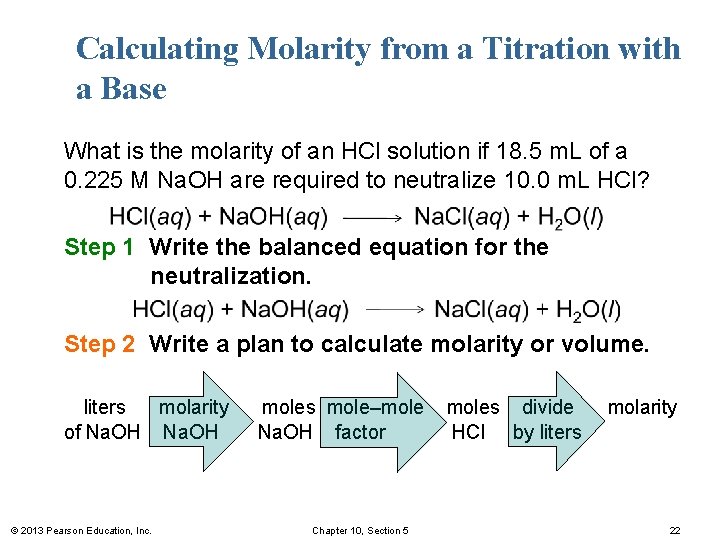
Calculating Molarity from a Titration with a Base What is the molarity of an HCl solution if 18. 5 m. L of a 0. 225 M Na. OH are required to neutralize 10. 0 m. L HCl? Step 1 Write the balanced equation for the neutralization. Step 2 Write a plan to calculate molarity or volume. liters molarity of Na. OH © 2013 Pearson Education, Inc. moles mole–mole Na. OH factor Chapter 10, Section 5 moles divide HCl by liters molarity 22

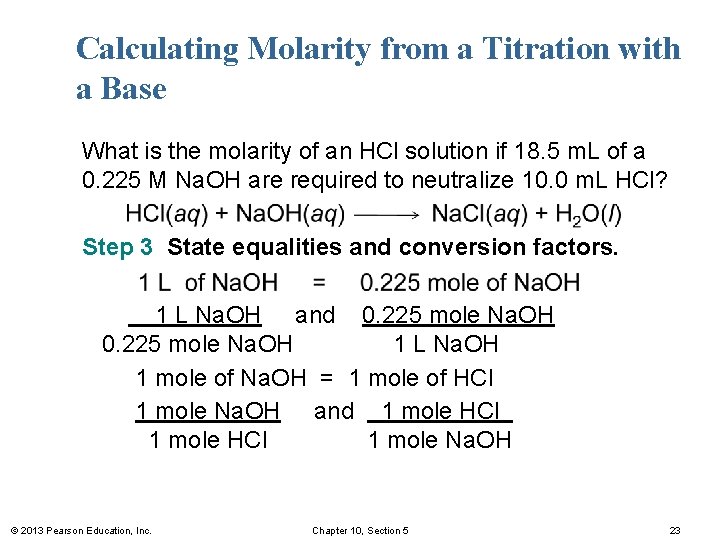
Calculating Molarity from a Titration with a Base What is the molarity of an HCl solution if 18. 5 m. L of a 0. 225 M Na. OH are required to neutralize 10. 0 m. L HCl? Step 3 State equalities and conversion factors. 1 L Na. OH and 0. 225 mole Na. OH 1 L Na. OH 1 mole of Na. OH = 1 mole of HCl 1 mole Na. OH and 1 mole HCl 1 mole Na. OH © 2013 Pearson Education, Inc. Chapter 10, Section 5 23

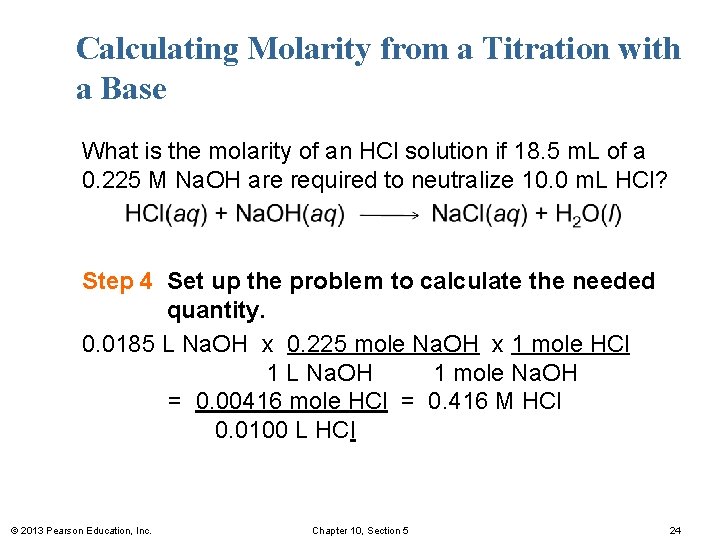
Calculating Molarity from a Titration with a Base What is the molarity of an HCl solution if 18. 5 m. L of a 0. 225 M Na. OH are required to neutralize 10. 0 m. L HCl? Step 4 Set up the problem to calculate the needed quantity. 0. 0185 L Na. OH x 0. 225 mole Na. OH x 1 mole HCl 1 L Na. OH 1 mole Na. OH = 0. 00416 mole HCl = 0. 416 M HCl 0. 0100 L HCl © 2013 Pearson Education, Inc. Chapter 10, Section 5 24

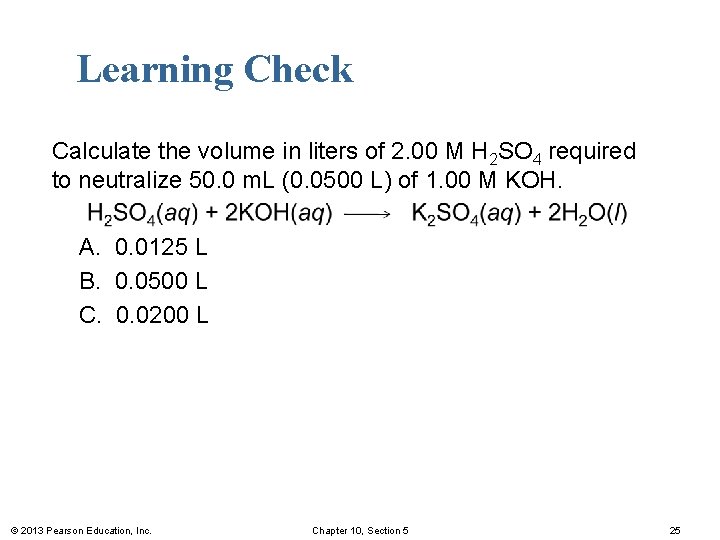
Learning Check Calculate the volume in liters of 2. 00 M H 2 SO 4 required to neutralize 50. 0 m. L (0. 0500 L) of 1. 00 M KOH. A. 0. 0125 L B. 0. 0500 L C. 0. 0200 L © 2013 Pearson Education, Inc. Chapter 10, Section 5 25

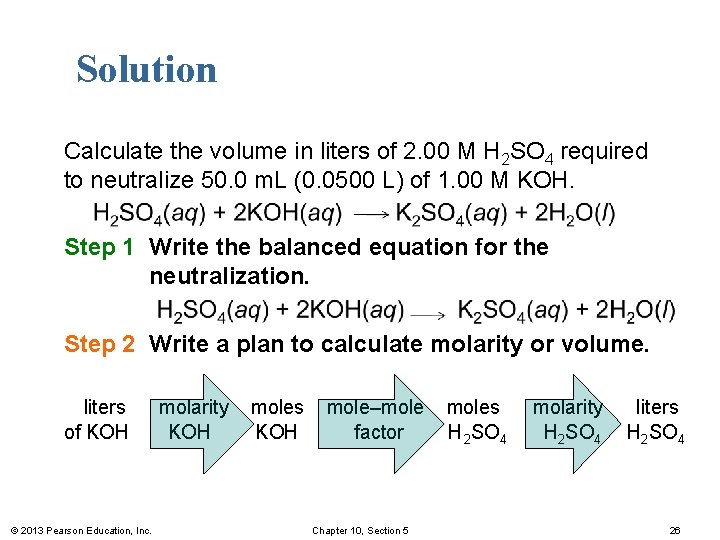
Solution Calculate the volume in liters of 2. 00 M H 2 SO 4 required to neutralize 50. 0 m. L (0. 0500 L) of 1. 00 M KOH. H 2 SO 4(aq) + 2 KOH(aq) K 2 SO 4(aq) + 2 H 2 O(l) Step 1 Write the balanced equation for the neutralization. H 2 SO 4(aq) + 2 KOH(aq) K 2 SO 4(aq) + 2 H 2 O(l) Step 2 Write a plan to calculate molarity or volume. liters of KOH © 2013 Pearson Education, Inc. molarity KOH moles KOH mole–mole factor Chapter 10, Section 5 moles H 2 SO 4 molarity H 2 SO 4 liters H 2 SO 4 26

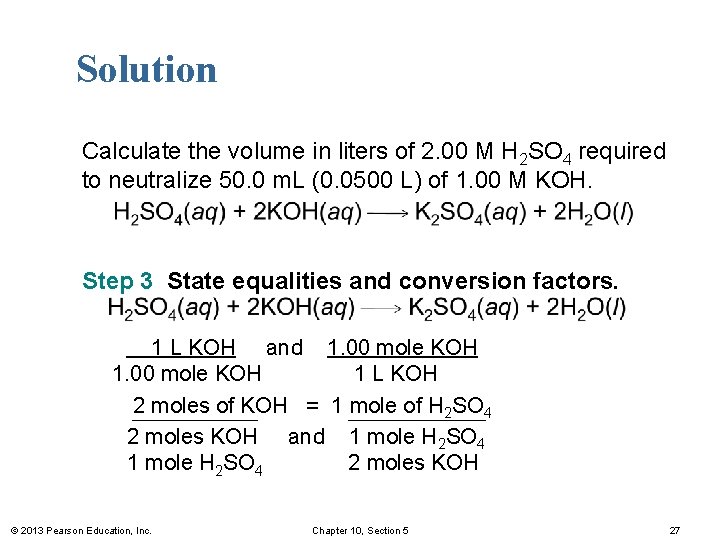
Solution Calculate the volume in liters of 2. 00 M H 2 SO 4 required to neutralize 50. 0 m. L (0. 0500 L) of 1. 00 M KOH. Step 3 State equalities and conversion factors. 1 L KOH and 1. 00 mole KOH 1 L KOH 2 moles of KOH = 1 mole of H 2 SO 4 2 moles KOH and 1 mole H 2 SO 4 2 moles KOH © 2013 Pearson Education, Inc. Chapter 10, Section 5 27

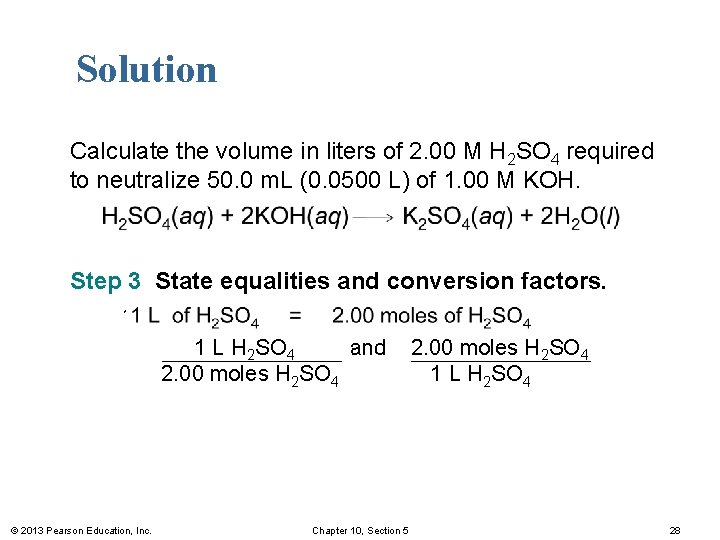
Solution Calculate the volume in liters of 2. 00 M H 2 SO 4 required to neutralize 50. 0 m. L (0. 0500 L) of 1. 00 M KOH. Step 3 State equalities and conversion factors. 1 L of H 2 SO 4 = 2. 00 moles of H 2 SO 4 1 L H 2 SO 4 and 2. 00 moles H 2 SO 4 1 L H 2 SO 4 © 2013 Pearson Education, Inc. Chapter 10, Section 5 28

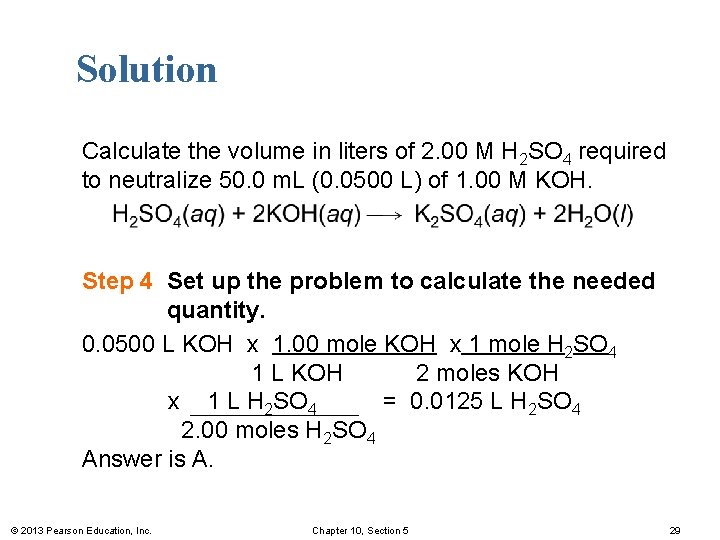
Solution Calculate the volume in liters of 2. 00 M H 2 SO 4 required to neutralize 50. 0 m. L (0. 0500 L) of 1. 00 M KOH. Step 4 Set up the problem to calculate the needed quantity. 0. 0500 L KOH x 1. 00 mole KOH x 1 mole H 2 SO 4 1 L KOH 2 moles KOH x 1 L H 2 SO 4 = 0. 0125 L H 2 SO 4 2. 00 moles H 2 SO 4 Answer is A. © 2013 Pearson Education, Inc. Chapter 10, Section 5 29