Solutions Acids Bases Acids Bases Acids Bases Acids

Solutions, Acids, & Bases

Acids & Bases

Acids & Bases Acids • substances that donate hydrogen ions to form hydronium ions (H 3 O 1+) when dissolved in water

Acids & Bases Acids • substances that donate hydrogen ions to form hydronium ions (H 3 O 1+) when dissolved in water • strong acids fully ionize in water • weak acids do not fully ionize in water

Acids & Bases • substances that either contain hydroxide ions or react with water to form hydroxide ions

Acids & Bases • substances that either contain hydroxide ions or react with water to form hydroxide ions • strong bases fully ionize in water • weak bases do not fully ionize in water
Acids & Bases • degrees of acidity or basicity –measured by p. H • concentration of hydronium ions in solution
Acids & Bases • degrees of acidity or basicity –measured by p. H • concentration of hydronium ions in solution • p. H < 7. 0 → acidic • p. H = 7. 0 → neutral • p. H > 7. 0 → basic
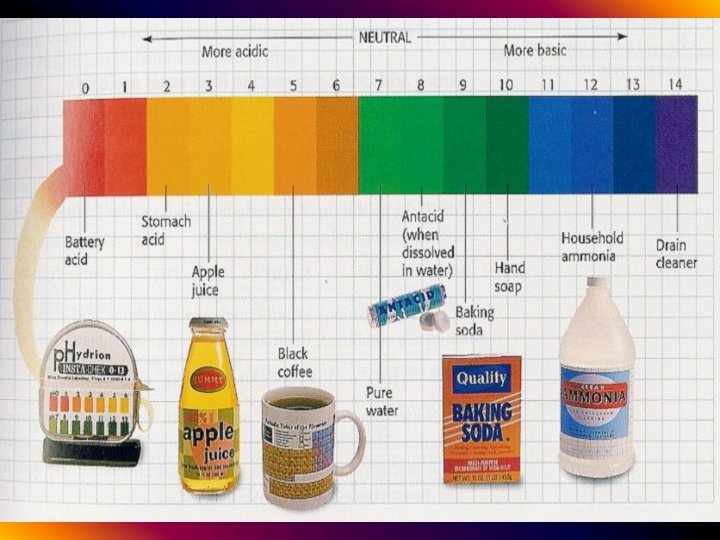

Acids & Bases • at home… –cleaning products are often basic –food products are often acidic

Acids & Bases • mixing acids & bases may cause neutralization, forming water and a salt –Ex: HCl(aq) + Na. OH(aq) Na. Cl(aq) + H 2 O

Homework Acids/Bases • Pg 491: #1 • Pg 523: #6(f, g) • Pg 527: #1, 9, 10(only tell if solution is acidic or basic for #10) Due 2/26
- Slides: 12