Chemical Equations Reactions Chemical Equations 4 Als 3






- Slides: 44

Chemical Equations & Reactions

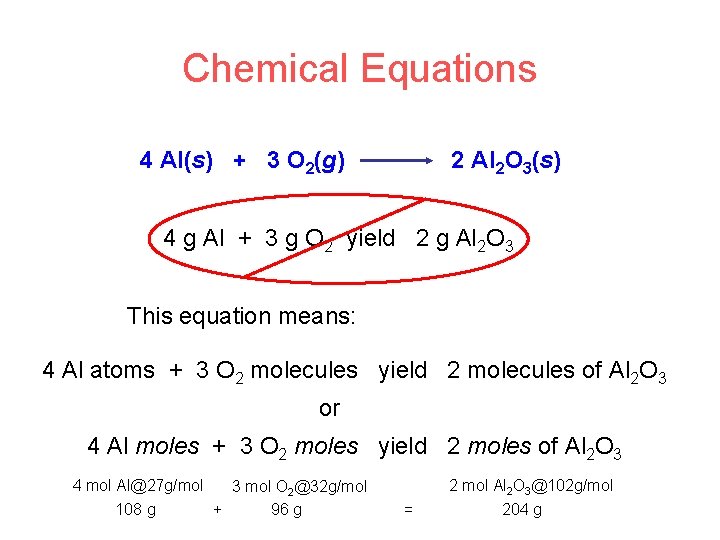
Chemical Equations 4 Al(s) + 3 O 2(g) 2 Al 2 O 3(s) 4 g Al + 3 g O 2 yield 2 g Al 2 O 3 This equation means: 4 Al atoms + 3 O 2 molecules yield 2 molecules of Al 2 O 3 or 4 Al moles + 3 O 2 moles yield 2 moles of Al 2 O 3 4 mol Al@27 g/mol 3 mol O 2@32 g/mol 108 g + 96 g 2 mol Al 2 O 3@102 g/mol = 204 g

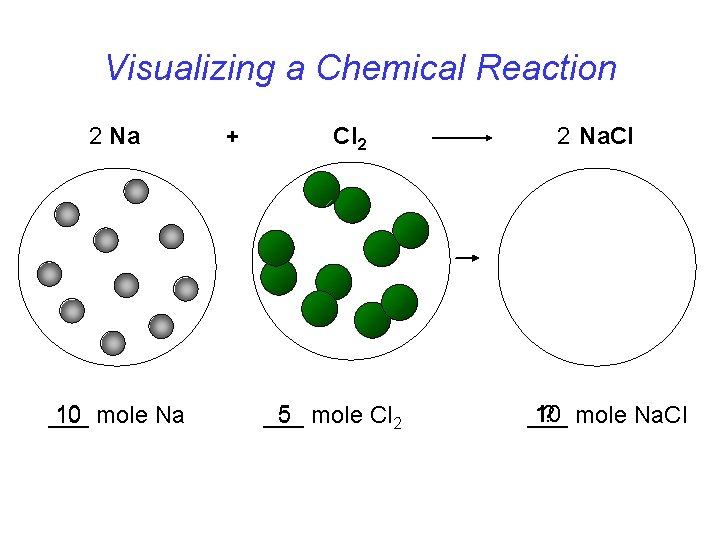
Visualizing a Chemical Reaction 2 Na 10 mole Na ___ + Cl 2 5 mole Cl 2 ___ 2 Na. Cl 10 ? mole Na. Cl ___

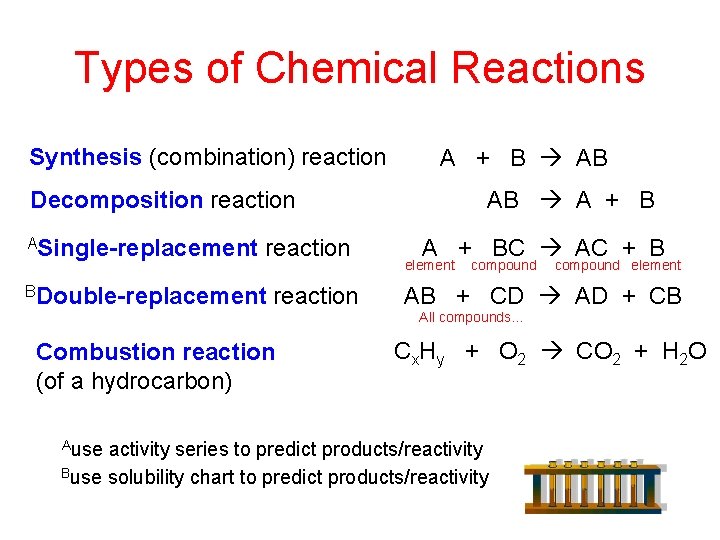
Types of Chemical Reactions Synthesis (combination) reaction A + B AB AB A + B Decomposition reaction ASingle-replacement reaction BDouble-replacement reaction Combustion reaction (of a hydrocarbon) Ause A + BC AC + B element compound element AB + CD AD + CB All compounds… Cx. Hy + O 2 CO 2 + H 2 O activity series to predict products/reactivity Buse solubility chart to predict products/reactivity

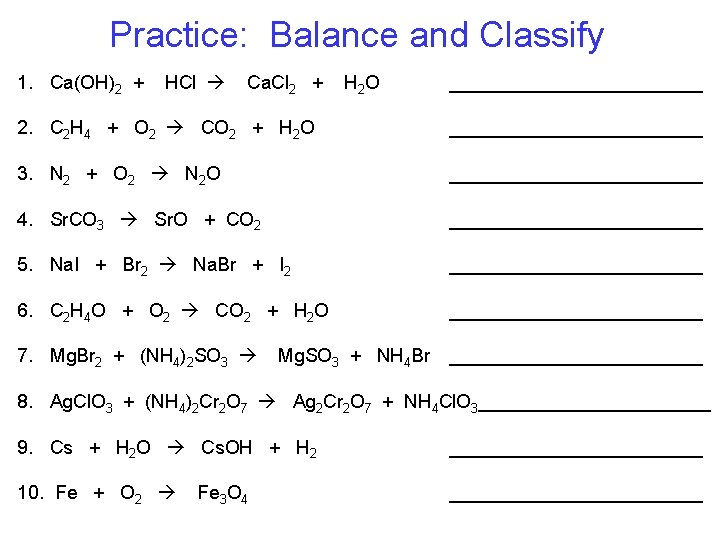
Practice: Balance and Classify 1. Ca(OH)2 + HCl Ca. Cl 2 + H 2 O ____________ 2. C 2 H 4 + O 2 CO 2 + H 2 O ____________ 3. N 2 + O 2 N 2 O ____________ 4. Sr. CO 3 Sr. O + CO 2 ____________ 5. Na. I + Br 2 Na. Br + I 2 ____________ 6. C 2 H 4 O + O 2 CO 2 + H 2 O ____________ 7. Mg. Br 2 + (NH 4)2 SO 3 ____________ Mg. SO 3 + NH 4 Br 8. Ag. Cl. O 3 + (NH 4)2 Cr 2 O 7 Ag 2 Cr 2 O 7 + NH 4 Cl. O 3___________ 9. Cs + H 2 O Cs. OH + H 2 ____________ 10. Fe + O 2 ____________ Fe 3 O 4

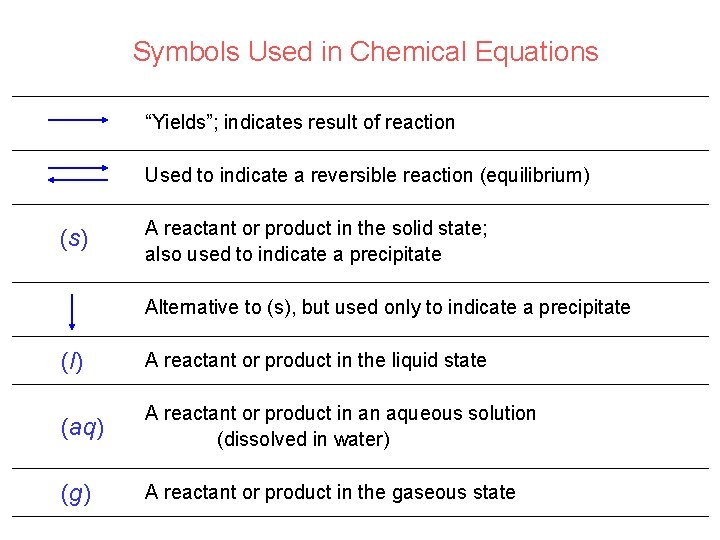
Symbols Used in Chemical Equations “Yields”; indicates result of reaction Used to indicate a reversible reaction (equilibrium) (s) A reactant or product in the solid state; also used to indicate a precipitate Alternative to (s), but used only to indicate a precipitate (l) A reactant or product in the liquid state (aq) A reactant or product in an aqueous solution (dissolved in water) (g) A reactant or product in the gaseous state

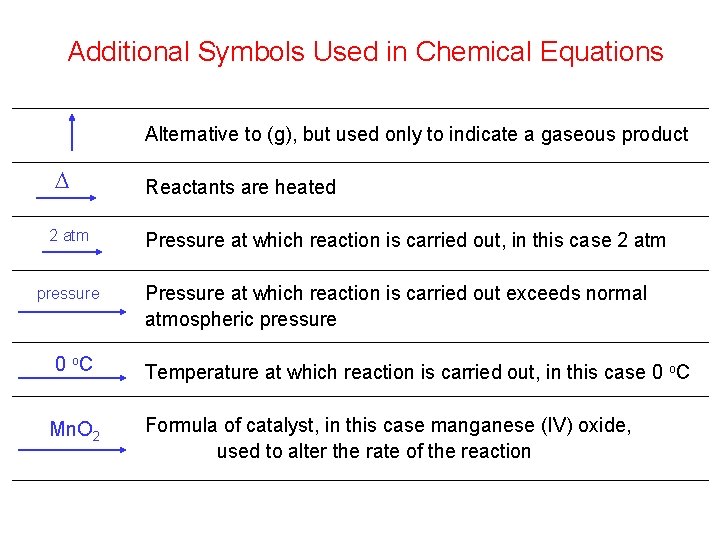
Additional Symbols Used in Chemical Equations Alternative to (g), but used only to indicate a gaseous product D 2 atm pressure Reactants are heated Pressure at which reaction is carried out, in this case 2 atm Pressure at which reaction is carried out exceeds normal atmospheric pressure 0 o. C Temperature at which reaction is carried out, in this case 0 o. C Mn. O 2 Formula of catalyst, in this case manganese (IV) oxide, used to alter the rate of the reaction

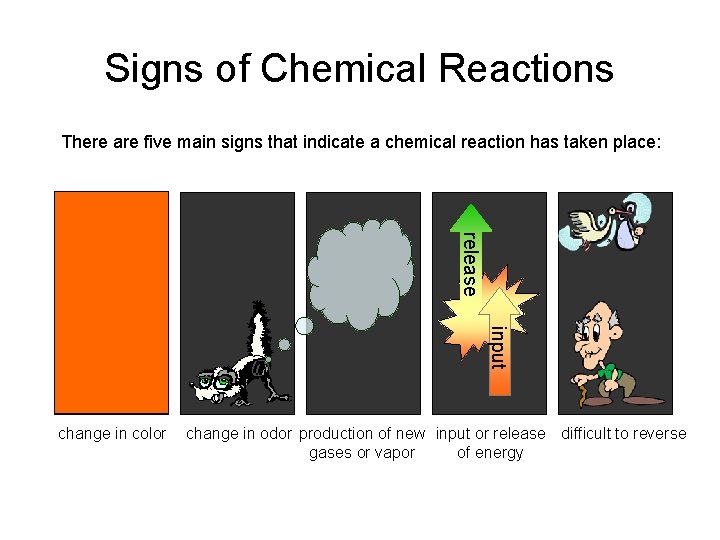
Signs of Chemical Reactions There are five main signs that indicate a chemical reaction has taken place: release input change in color change in odor production of new input or release difficult to reverse gases or vapor of energy


Combustion C 4 H 10 + 2 13/2 O 2 4 CO 2 + 13 8 General form: Cx. Hx carbon-hydrogen compound + O 2 oxygen 5 H 2 O 10 CO 2 carbon dioxide + H 2 O water

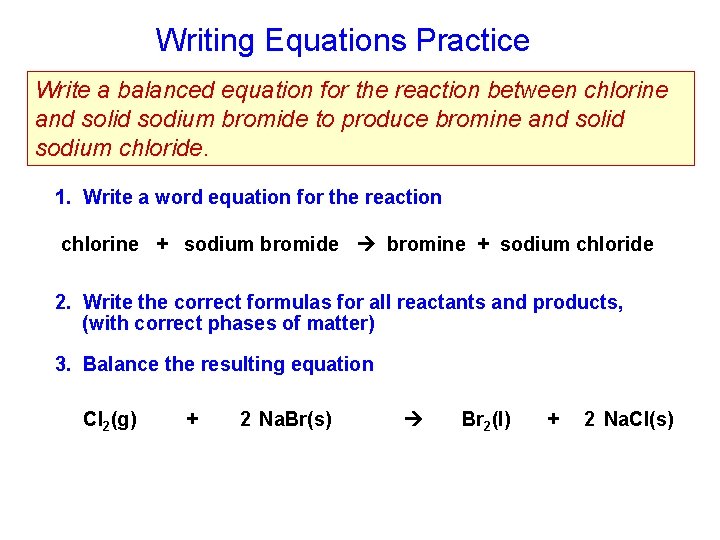
Writing Equations Practice Write a balanced equation for the reaction between chlorine and solid sodium bromide to produce bromine and solid sodium chloride. 1. Write a word equation for the reaction chlorine + sodium bromide bromine + sodium chloride 2. Write the correct formulas for all reactants and products, (with correct phases of matter) 3. Balance the resulting equation Cl 2(g) + 2 Na. Br(s) Br 2(l) + 2 Na. Cl(s)

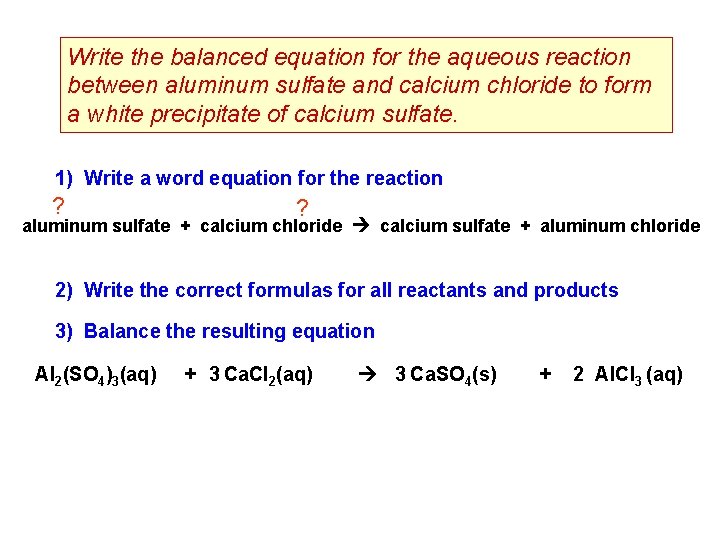
Write the balanced equation for the aqueous reaction between aluminum sulfate and calcium chloride to form a white precipitate of calcium sulfate. 1) Write a word equation for the reaction ? ? aluminum sulfate + calcium chloride calcium sulfate + aluminum chloride 2) Write the correct formulas for all reactants and products 3) Balance the resulting equation Al 2(SO 4)3(aq) + 3 Ca. Cl 2(aq) 3 Ca. SO 4(s) + 2 Al. Cl 3 (aq)

Oxidation-Reduction Reactions • “Redox” reactions involve the transfer of electrons (e-) • Reduction: gain e • Oxidation: lose e“LEO the lion says, ‘GER’” “OIL RIG” • Use oxidation states to keep track of the e-

My name is Leo. Grr-rrrr… Leo says Ger “Lose electron oxidation” Zn 2 e- + Zn 2+ “Gain electron reduction” 2 e- + Cu 2+ Cu

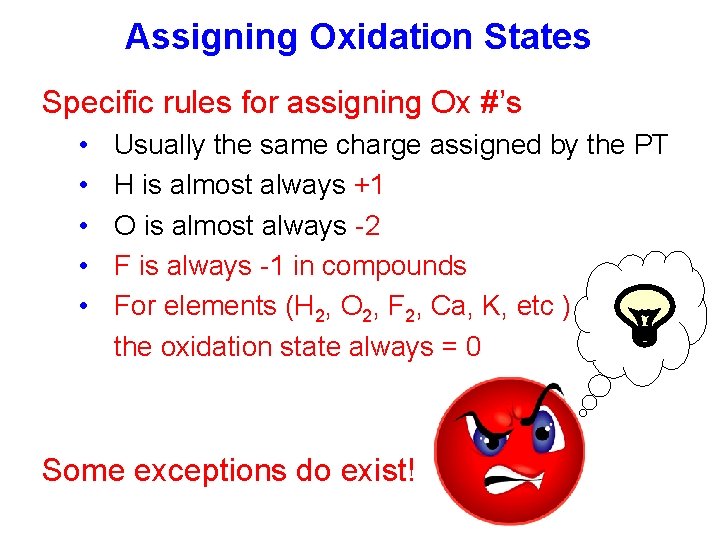
Assigning Oxidation States Specific rules for assigning Ox #’s • • • Usually the same charge assigned by the PT H is almost always +1 O is almost always -2 F is always -1 in compounds For elements (H 2, O 2, F 2, Ca, K, etc ) the oxidation state always = 0 Some exceptions do exist!

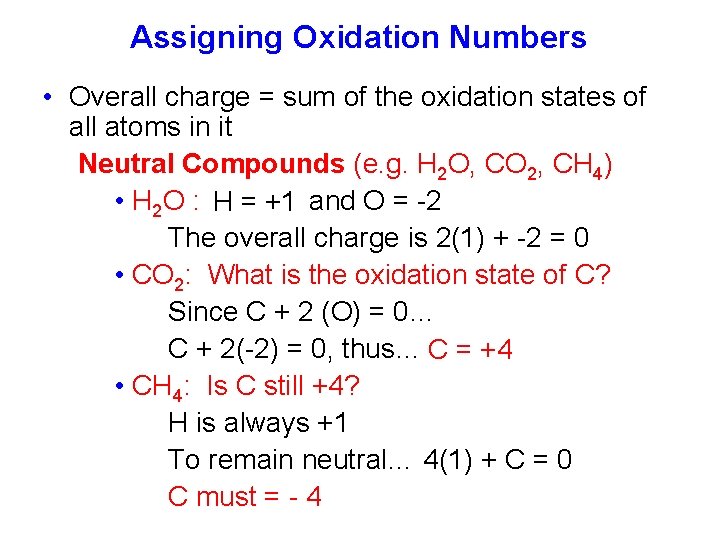
Assigning Oxidation Numbers • Overall charge = sum of the oxidation states of all atoms in it Neutral Compounds (e. g. H 2 O, CO 2, CH 4) • H 2 O : H = +1 and O = -2 The overall charge is 2(1) + -2 = 0 • CO 2: What is the oxidation state of C? Since C + 2 (O) = 0… C + 2(-2) = 0, thus… C = +4 • CH 4: Is C still +4? H is always +1 To remain neutral… 4(1) + C = 0 C must = - 4

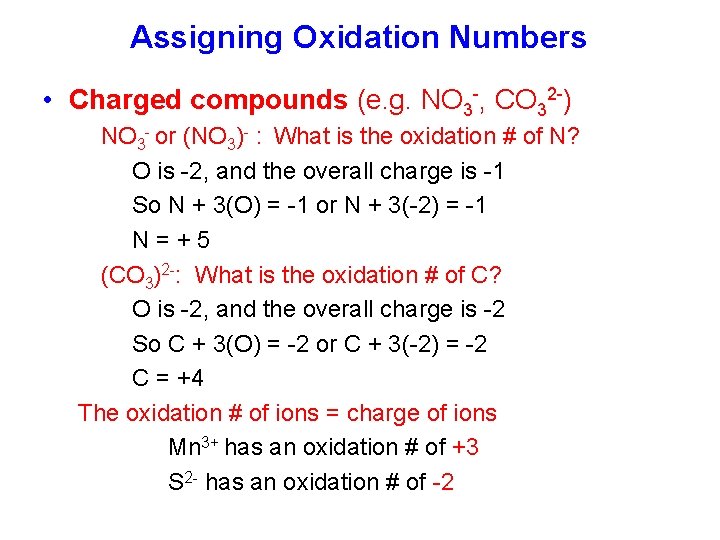
Assigning Oxidation Numbers • Charged compounds (e. g. NO 3 -, CO 32 -) NO 3 - or (NO 3)- : What is the oxidation # of N? O is -2, and the overall charge is -1 So N + 3(O) = -1 or N + 3(-2) = -1 N=+5 (CO 3)2 -: What is the oxidation # of C? O is -2, and the overall charge is -2 So C + 3(O) = -2 or C + 3(-2) = -2 C = +4 The oxidation # of ions = charge of ions Mn 3+ has an oxidation # of +3 S 2 - has an oxidation # of -2

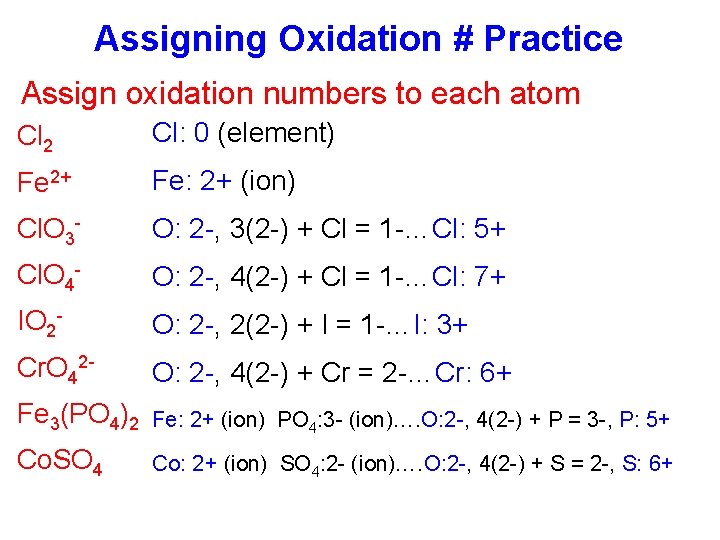
Assigning Oxidation # Practice Assign oxidation numbers to each atom Cl 2 Cl: 0 (element) Fe 2+ Fe: 2+ (ion) Cl. O 3 - O: 2 -, 3(2 -) + Cl = 1 -…Cl: 5+ Cl. O 4 - O: 2 -, 4(2 -) + Cl = 1 -…Cl: 7+ IO 2 - O: 2 -, 2(2 -) + I = 1 -…I: 3+ Cr. O 42 - O: 2 -, 4(2 -) + Cr = 2 -…Cr: 6+ Fe 3(PO 4)2 Fe: 2+ (ion) PO 4: 3 - (ion)…. O: 2 -, 4(2 -) + P = 3 -, P: 5+ Co. SO 4 Co: 2+ (ion) SO 4: 2 - (ion)…. O: 2 -, 4(2 -) + S = 2 -, S: 6+

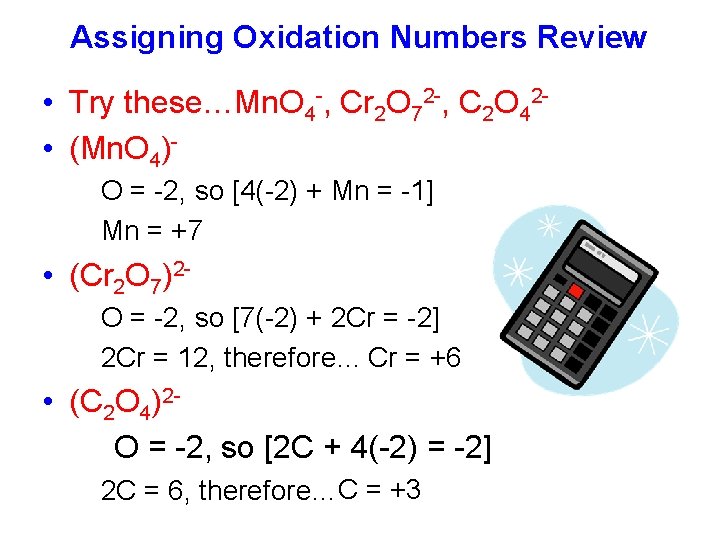
Assigning Oxidation Numbers Review • Try these…Mn. O 4 -, Cr 2 O 72 -, C 2 O 42 • (Mn. O 4)O = -2, so [4(-2) + Mn = -1] Mn = +7 • (Cr 2 O 7)2 O = -2, so [7(-2) + 2 Cr = -2] 2 Cr = 12, therefore… Cr = +6 • (C 2 O 4)2 O = -2, so [2 C + 4(-2) = -2] 2 C = 6, therefore…C = +3

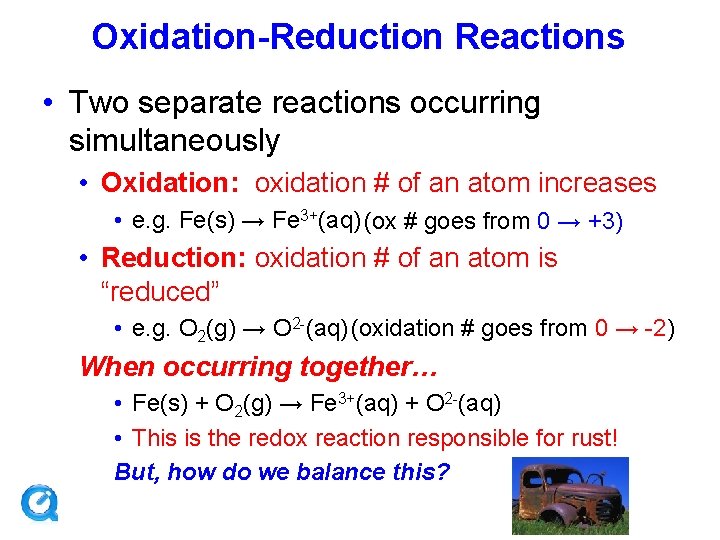
Oxidation-Reduction Reactions • Two separate reactions occurring simultaneously • Oxidation: oxidation # of an atom increases • e. g. Fe(s) → Fe 3+(aq) (ox # goes from 0 → +3) • Reduction: oxidation # of an atom is “reduced” • e. g. O 2(g) → O 2 -(aq) (oxidation # goes from 0 → -2) When occurring together… • Fe(s) + O 2(g) → Fe 3+(aq) + O 2 -(aq) • This is the redox reaction responsible for rust! But, how do we balance this?

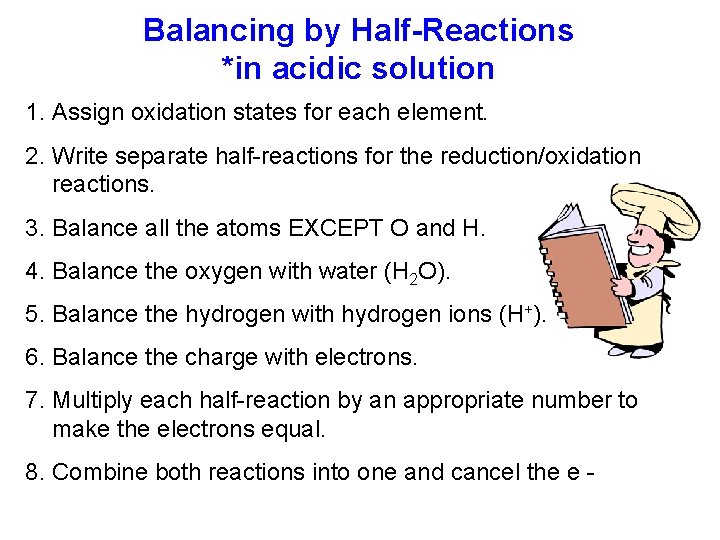
Balancing by Half-Reactions *in acidic solution 1. Assign oxidation states for each element. 2. Write separate half-reactions for the reduction/oxidation reactions. 3. Balance all the atoms EXCEPT O and H. 4. Balance the oxygen with water (H 2 O). 5. Balance the hydrogen with hydrogen ions (H+). 6. Balance the charge with electrons. 7. Multiply each half-reaction by an appropriate number to make the electrons equal. 8. Combine both reactions into one and cancel the e -

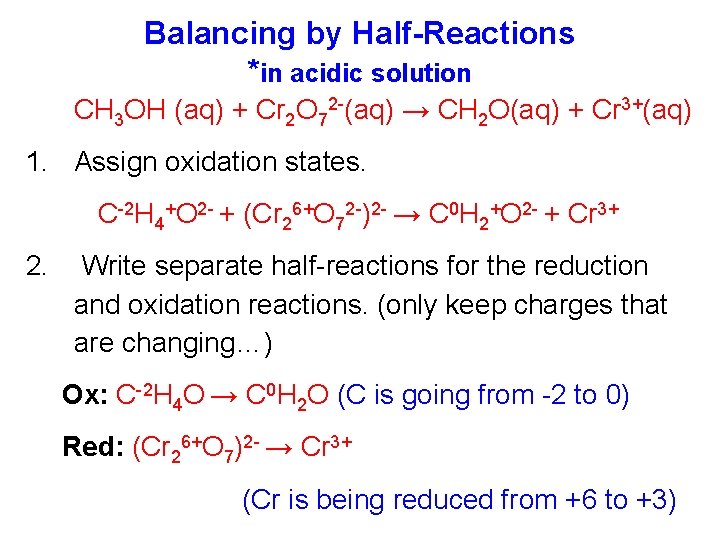
Balancing by Half-Reactions *in acidic solution CH 3 OH (aq) + Cr 2 O 72 -(aq) → CH 2 O(aq) + Cr 3+(aq) 1. Assign oxidation states. C-2 H 4+O 2 - + (Cr 26+O 72 -)2 - → C 0 H 2+O 2 - + Cr 3+ 2. Write separate half-reactions for the reduction and oxidation reactions. (only keep charges that are changing…) Ox: C-2 H 4 O → C 0 H 2 O (C is going from -2 to 0) Red: (Cr 26+O 7)2 - → Cr 3+ (Cr is being reduced from +6 to +3)

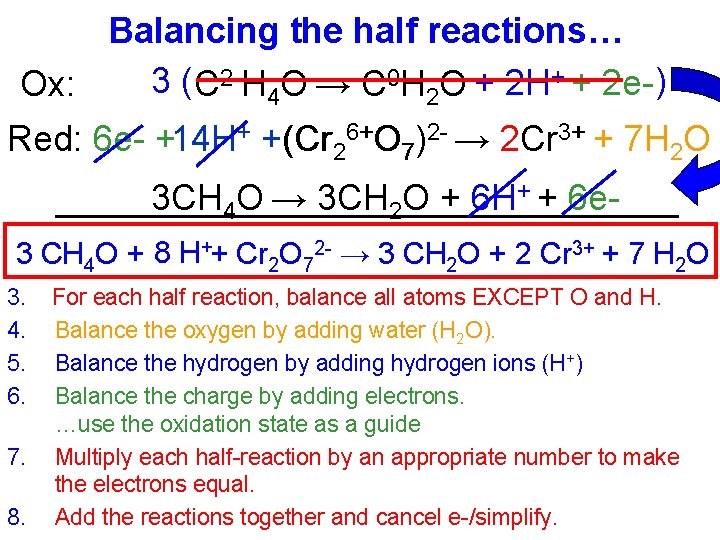
Balancing the half reactions… 3 ( C 2 -H 4 O → C 0 H 2 O + 2 H+ + 2 e- ) Ox: Red: 6 e- +14 H+ +(Cr 26+O 7)2 - → 2 Cr 3+ + 7 H 2 O 3 CH 4 O → 3 CH 2 O + 6 H+ + 6 e 3 CH 4 O + 8 H++ Cr 2 O 72 - → 3 CH 2 O + 2 Cr 3+ + 7 H 2 O 3. 4. 5. 6. 7. 8. For each half reaction, balance all atoms EXCEPT O and H. Balance the oxygen by adding water (H 2 O). Balance the hydrogen by adding hydrogen ions (H+) Balance the charge by adding electrons. …use the oxidation state as a guide Multiply each half-reaction by an appropriate number to make the electrons equal. Add the reactions together and cancel e-/simplify.

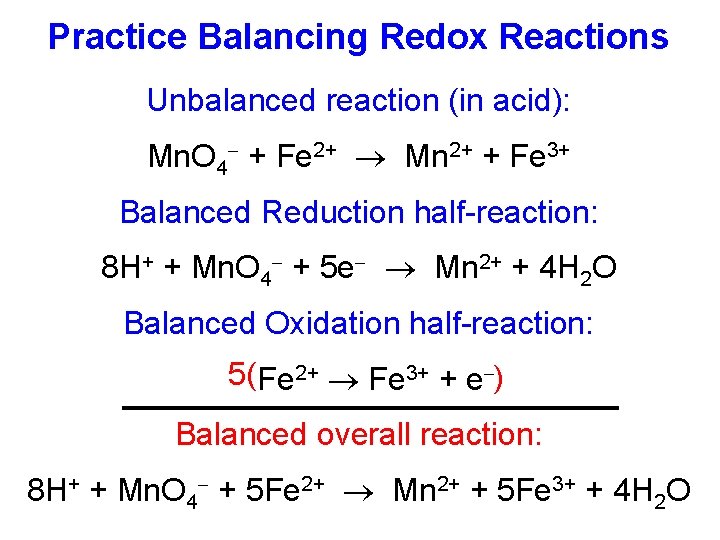
Practice Balancing Redox Reactions Unbalanced reaction (in acid): Mn. O 4 + Fe 2+ Mn 2+ + Fe 3+ Balanced Reduction half-reaction: 8 H+ + Mn. O 4 + 5 e Mn 2+ + 4 H 2 O Balanced Oxidation half-reaction: 5(Fe 2+ Fe 3+ + e ) Balanced overall reaction: 8 H+ + Mn. O 4 + 5 Fe 2+ Mn 2+ + 5 Fe 3+ + 4 H 2 O

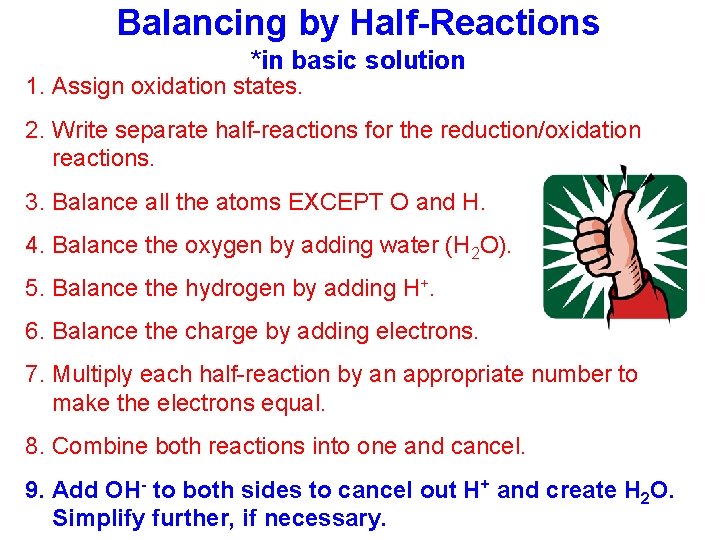
Balancing by Half-Reactions *in basic solution 1. Assign oxidation states. 2. Write separate half-reactions for the reduction/oxidation reactions. 3. Balance all the atoms EXCEPT O and H. 4. Balance the oxygen by adding water (H 2 O). 5. Balance the hydrogen by adding H+. 6. Balance the charge by adding electrons. 7. Multiply each half-reaction by an appropriate number to make the electrons equal. 8. Combine both reactions into one and cancel. 9. Add OH- to both sides to cancel out H+ and create H 2 O. Simplify further, if necessary.

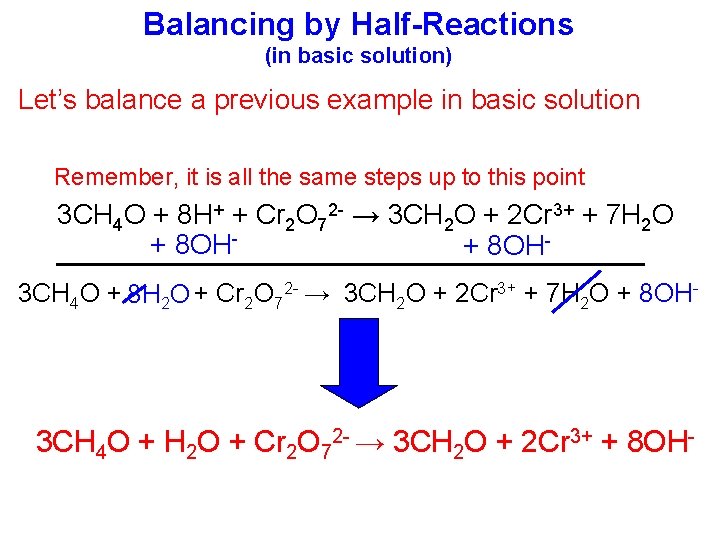
Balancing by Half-Reactions (in basic solution) Let’s balance a previous example in basic solution Remember, it is all the same steps up to this point 3 CH 4 O + 8 H+ + Cr 2 O 72 - → 3 CH 2 O + 2 Cr 3+ + 7 H 2 O + 8 OH 3 CH 4 O + 8 H 2 O + Cr 2 O 72 - → 3 CH 2 O + 2 Cr 3+ + 7 H 2 O + 8 OH- 3 CH 4 O + H 2 O + Cr 2 O 72 - → 3 CH 2 O + 2 Cr 3+ + 8 OH-

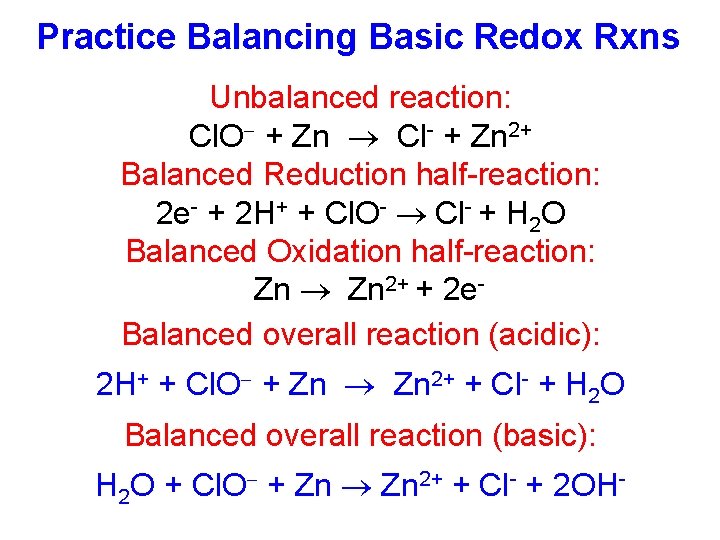
Practice Balancing Basic Redox Rxns Unbalanced reaction: Cl. O + Zn Cl- + Zn 2+ Balanced Reduction half-reaction: 2 e- + 2 H+ + Cl. O- Cl- + H 2 O Balanced Oxidation half-reaction: Zn 2+ + 2 e. Balanced overall reaction (acidic): 2 H+ + Cl. O + Zn 2+ + Cl- + H 2 O Balanced overall reaction (basic): H 2 O + Cl. O + Zn 2+ + Cl- + 2 OH-

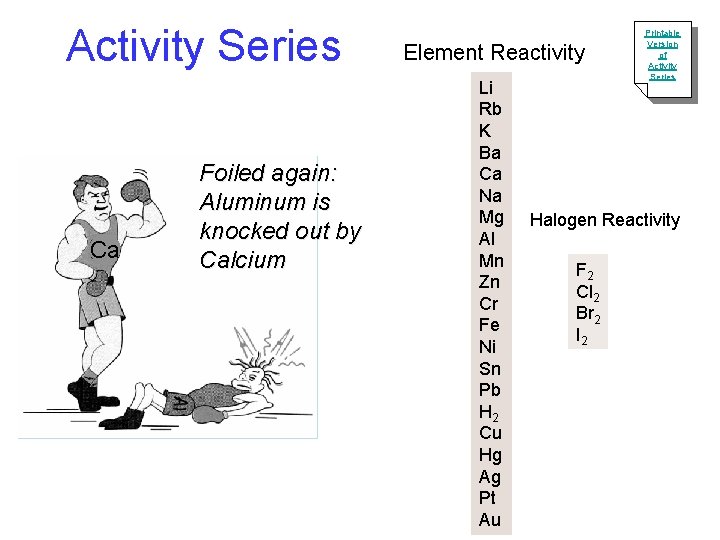
Activity Series Ca Foiled again: Aluminum is knocked out by Calcium Element Reactivity Li Rb K Ba Ca Na Mg Al Mn Zn Cr Fe Ni Sn Pb H 2 Cu Hg Ag Pt Au Printable Version of Activity Series Halogen Reactivity F 2 Cl 2 Br 2 I 2

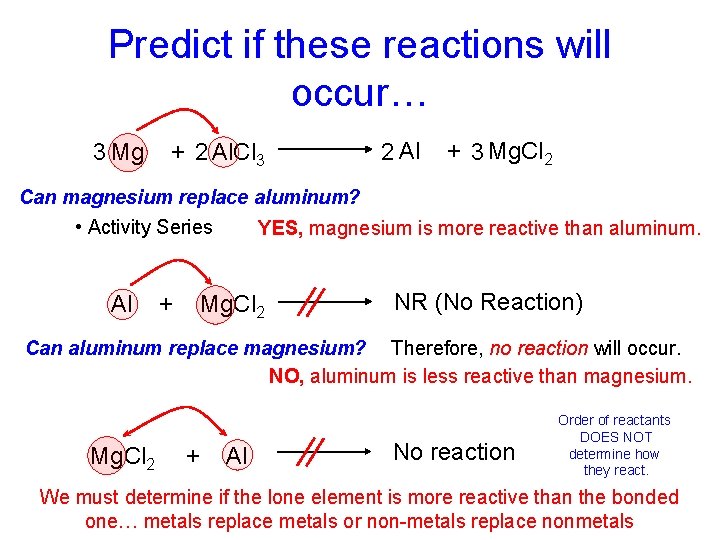
Predict if these reactions will occur… 3 Mg + 2 Al. Cl 3 2 Al + 3 Mg. Cl 2 Can magnesium replace aluminum? • Activity Series Al + YES, magnesium is more reactive than aluminum. Mg. Cl 2 NR (No Reaction) Can aluminum replace magnesium? Therefore, no reaction will occur. NO, aluminum is less reactive than magnesium. Mg. Cl 2 + Al No reaction Order of reactants DOES NOT determine how they react. We must determine if the lone element is more reactive than the bonded one… metals replace metals or non-metals replace nonmetals

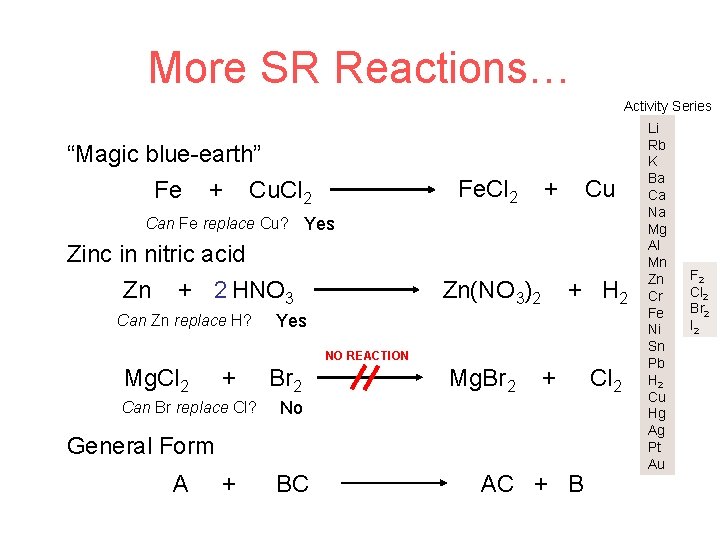
More SR Reactions… Activity Series “Magic blue-earth” Fe + Cu. Cl 2 Fe. Cl 2 + Cu Can Fe replace Cu? Yes Zinc in nitric acid Zn + 2 HNO 3 Can Zn replace H? Zn(NO 3)2 + H 2 Yes NO REACTION Mg. Cl 2 + Can Br replace Cl? Br 2 Mg. Br 2 + No General Form A + BC AC + B Cl 2 Li Rb K Ba Ca Na Mg Al Mn Zn Cr Fe Ni Sn Pb H 2 Cu Hg Ag Pt Au F 2 Cl 2 Br 2 I 2

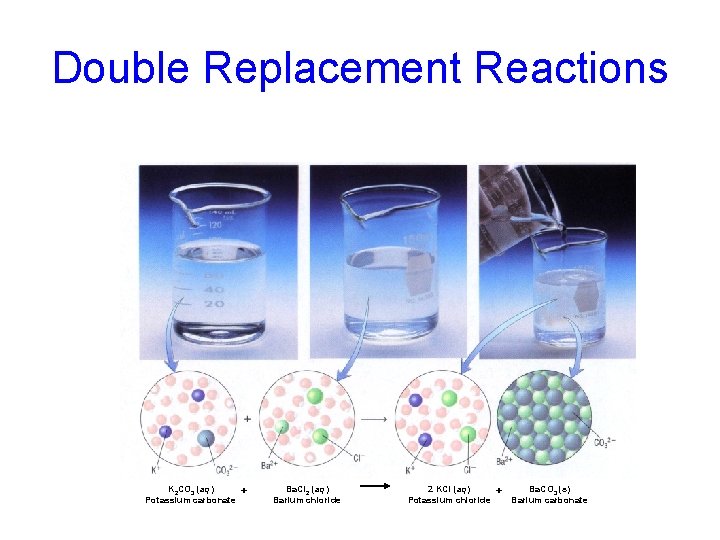
Double Replacement Reactions K 2 CO 3 (aq) Potassium carbonate + Ba. Cl 2 (aq) Barium chloride 2 KCl (aq) Potassium chloride + Ba. CO 3 (s) Barium carbonate

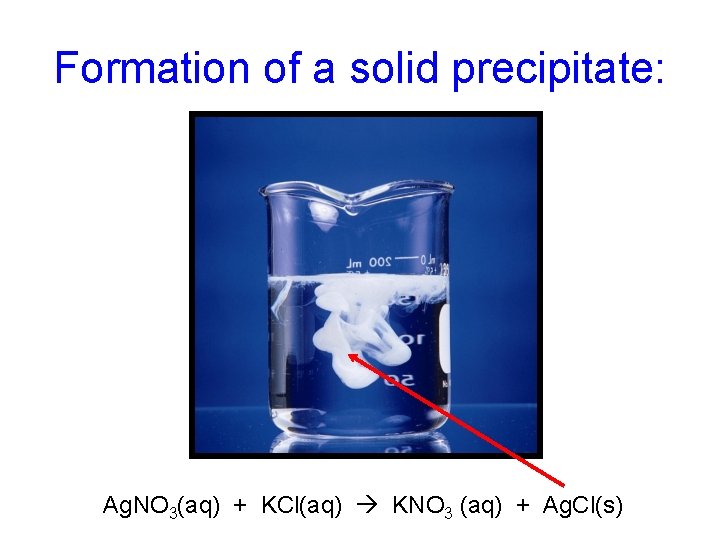
Formation of a solid precipitate: Ag. NO 3(aq) + KCl(aq) KNO 3 (aq) + Ag. Cl(s)

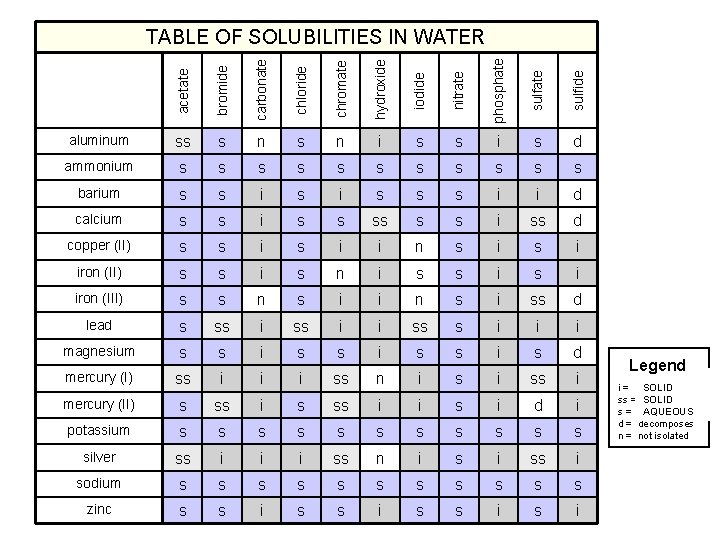
acetate bromide carbonate chloride chromate hydroxide iodide nitrate phosphate sulfide TABLE OF SOLUBILITIES IN WATER aluminum ss s n i s s i s d ammonium s s s barium s s i s s s i i d calcium s s i ss d copper (II) s s i i n s i iron (II) s s i s n i s s i iron (III) s s n s i i n s i ss d lead s ss i i ss s i i i magnesium s s i s d mercury (I) ss i i i ss n i ss i mercury (II) s ss i i s i d i potassium s s silver ss i i i ss n i ss i sodium s s s zinc s s i Legend SOLID i = insoluble SOLIDsoluble ss = slightly AQUEOUS s = soluble d = decomposes n = not isolated

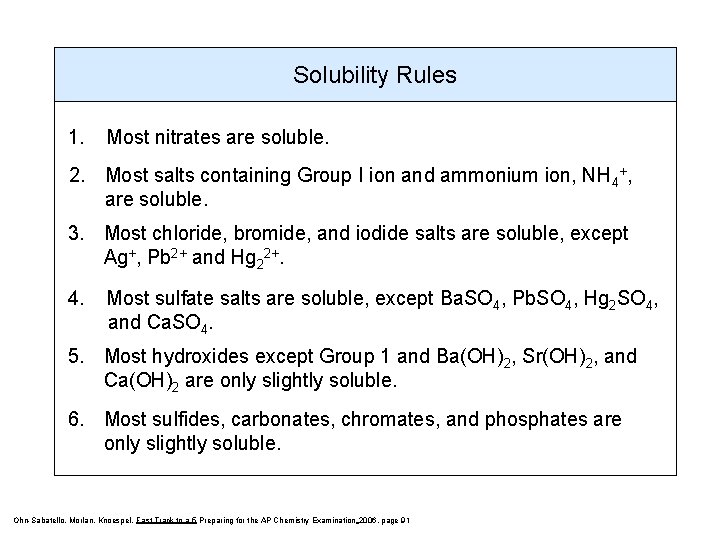
Solubility Rules 1. Most nitrates are soluble. 2. Most salts containing Group I ion and ammonium ion, NH 4+, are soluble. 3. Most chloride, bromide, and iodide salts are soluble, except Ag+, Pb 2+ and Hg 22+. 4. Most sulfate salts are soluble, except Ba. SO 4, Pb. SO 4, Hg 2 SO 4, and Ca. SO 4. 5. Most hydroxides except Group 1 and Ba(OH)2, Sr(OH)2, and Ca(OH)2 are only slightly soluble. 6. Most sulfides, carbonates, chromates, and phosphates are only slightly soluble. Ohn-Sabatello, Morlan, Knoespel, Fast Track to a 5 Preparing for the AP Chemistry Examination 2006, page 91

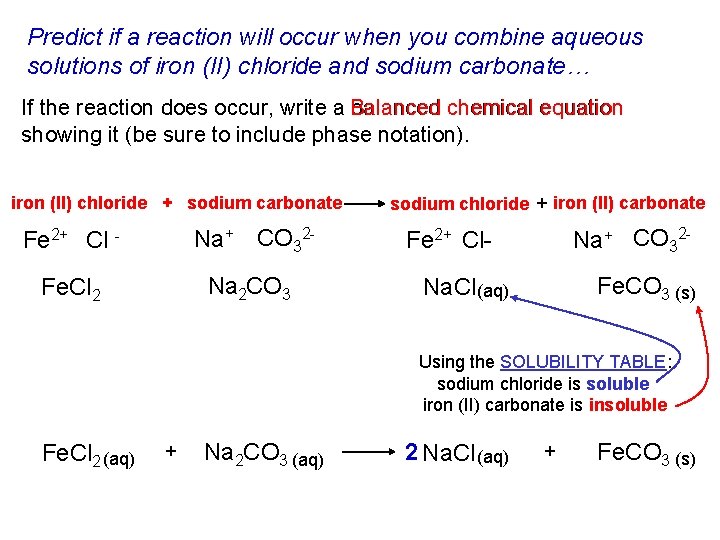
Predict if a reaction will occur when you combine aqueous solutions of iron (II) chloride and sodium carbonate… If the reaction does occur, write a Balanced balanced chemical equation showing it (be sure to include phase notation). iron (II) chloride + sodium carbonate Fe 2+ Cl - Na+ CO 32 - Fe. Cl 2 Na 2 CO 3 sodium chloride + iron (II) carbonate Na+ CO 32 - Fe 2+ Cl- Fe. CO 3 (s) Na. Cl (aq) Using the SOLUBILITY TABLE: sodium chloride is soluble iron (II) carbonate is insoluble Fe. Cl 2 (aq) + Na 2 CO 3 (aq) 2 Na. Cl (aq) + Fe. CO 3 (s)

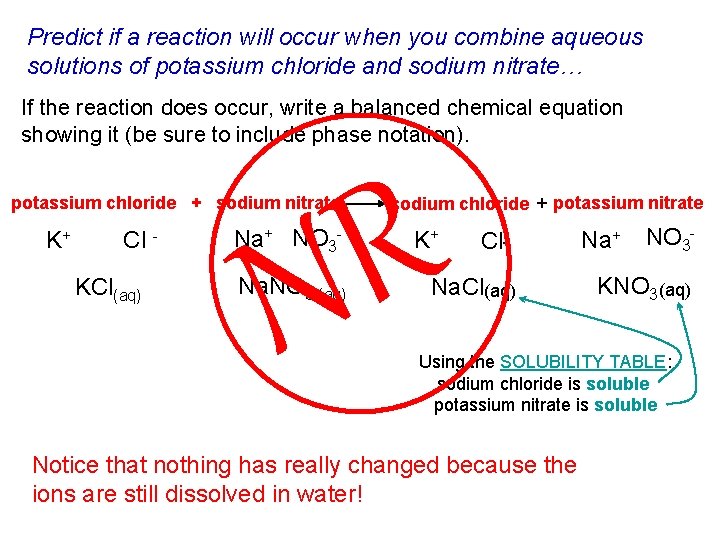
Predict if a reaction will occur when you combine aqueous solutions of potassium chloride and sodium nitrate… If the reaction does occur, write a balanced chemical equation showing it (be sure to include phase notation). R N potassium chloride + sodium nitrate K+ Cl KCl(aq) Na+ NO 3 - Na. NO 3 (aq) sodium chloride + potassium nitrate K+ Cl- Na. Cl(aq) Na+ NO 3 - KNO 3(aq) Using the SOLUBILITY TABLE: sodium chloride is soluble potassium nitrate is soluble Notice that nothing has really changed because the ions are still dissolved in water!

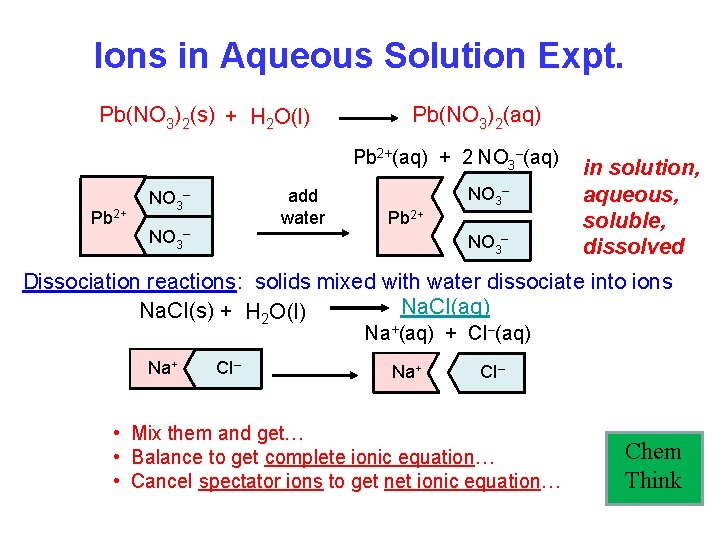
Ions in Aqueous Solution Expt. Pb(NO 3)2(s) + H 2 O(l) Pb(NO 3)2(aq) Pb 2+(aq) + 2 NO 3–(aq) Pb 2+ add water NO 3– Pb 2+ NO 3– in solution, aqueous, soluble, dissolved Dissociation reactions: solids mixed with water dissociate into ions Na. CI(aq) Na. CI(s) + H 2 O(l) Na+(aq) + CI–(aq) Na+ CI– • Mix them and get… • Balance to get complete ionic equation… • Cancel spectator ions to get net ionic equation… Chem Think

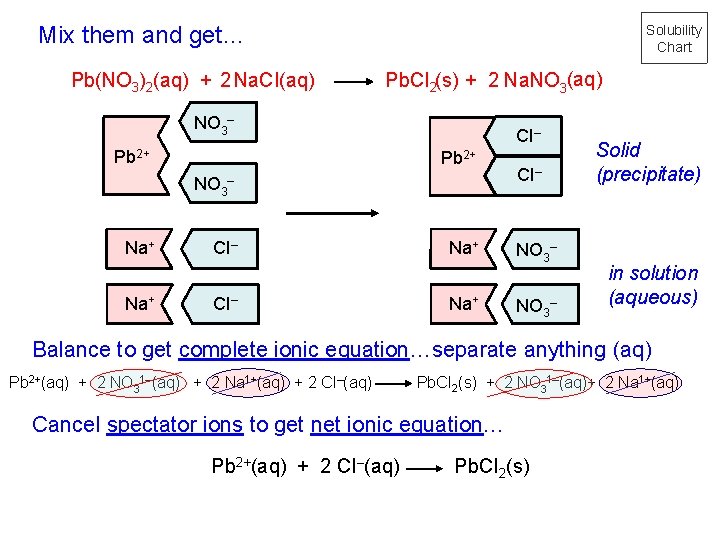
Mix them and get… Pb(NO 3)2(aq) + 2 Na. CI(aq) Solubility Chart Pb. CI 2(s) + 2 Na. NO 3(aq) NO 3– Pb 2+ NO 3 Na+ CI– – CI– Na+ CI– NO 3– Solid (precipitate) in solution (aqueous) Balance to get complete ionic equation…separate anything (aq) Pb 2+(aq) + 2 NO 31–(aq) + 2 Na 1+(aq) + 2 CI–(aq) Pb. CI 2(s) + 2 NO 31–(aq)+ 2 Na 1+(aq) Cancel spectator ions to get net ionic equation… Pb 2+(aq) + 2 CI–(aq) Pb. CI 2(s)

Pre-lab: 1. What ions are present in the following solutions? Na+(aq) Cl-(aq) Ag+(aq) NO 3 -(aq) Na. Cl(aq) __________ Ag. NO 3(aq) __________ 2. When these solutions are mixed together, a precipitate is seen. What are the new combinations of ions that could have formed the precipitate? Ag (aq) Na (aq) NO 3 (aq) and __________ Cl (aq) __________ + + - - 3. Using the solubility table, which new combination will form a precipitate? Ag+(aq) Cl-(aq) __________ Ag. Cl(s) 4. Which new combination will remain in solution? Na (aq) NO 3 (aq) __________ + - 5. Write the complete ionic equation for this reaction. Be sure to indicate the correct phase (reaction condition) for each reactant and each product. Na+(aq) + Cl-(aq) + Ag+(aq) + NO 3 -(aq) Ag. Cl(s) + Na+(aq) + NO 3 -(aq) 6. Write the net ionic equation for this reaction by canceling out spectators. Again, include the phases (reaction conditions). Ag+(aq) + Cl-(aq) Ag. Cl(s) 7. Explain why you would expect no reaction between solutions of KOH(aq) and Na. OH(aq). When the cations switch places they end with a hydroxide (no new combination is formed)

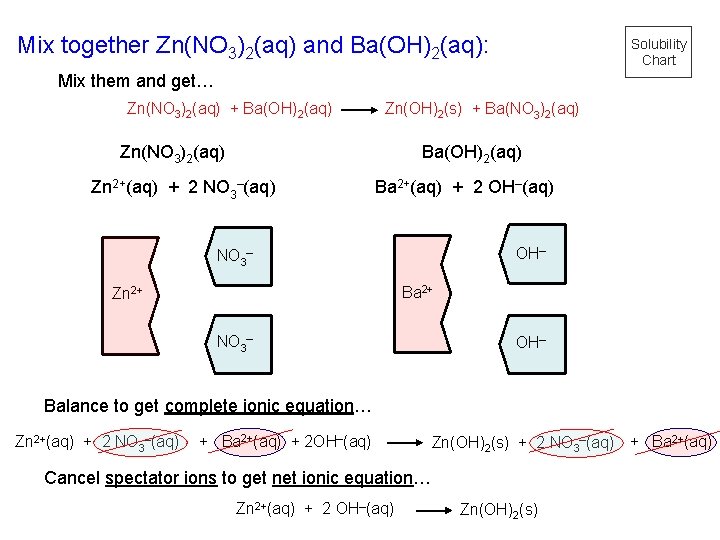
Mix together Zn(NO 3)2(aq) and Ba(OH)2(aq): Solubility Chart Mix them and get… Zn(NO 3)2(aq) + Ba(OH)2(aq) Zn(OH)2(s) + Ba(NO 3)2(aq) Zn(NO 3)2(aq) Ba(OH)2(aq) Zn 2+(aq) + 2 NO 3–(aq) Ba 2+(aq) + 2 OH–(aq) OH– NO 3– Ba 2+ Zn 2+ NO 3– OH– Balance to get complete ionic equation… Zn 2+(aq) + 2 NO 3–(aq) + Ba 2+(aq) + 2 OH–(aq) Zn(OH)2(s) + 2 NO 3–(aq) + Ba 2+(aq) Cancel spectator ions to get net ionic equation… Zn 2+(aq) + 2 OH–(aq) Zn(OH)2(s)

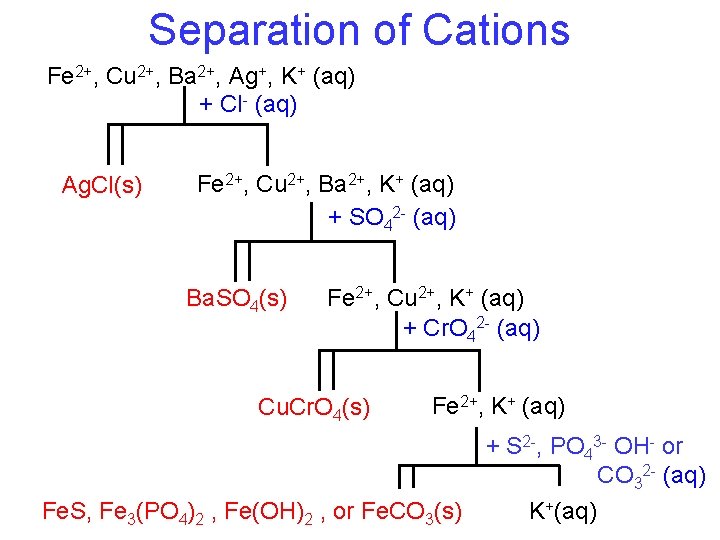
Separation of Cations You have a solution containing Fe 2+, Cu 2+, Ba 2+, Ag+ and K+ ions. By what means could you separate these ions from each other? • In Chem I, we discussed various ways to separate things… • • Distillation Filtration Centrifugation Reactivity Will any of these work to separate aqueous ions?

Separation of Cations Fe 2+, Cu 2+, Ba 2+, Ag+, K+ (aq) + Cl- (aq) Ag. Cl(s) Fe 2+, Cu 2+, Ba 2+, K+ (aq) + SO 42 - (aq) Ba. SO 4(s) Fe 2+, Cu 2+, K+ (aq) + Cr. O 42 - (aq) Cu. Cr. O 4(s) Fe 2+, K+ (aq) + S 2 -, PO 43 - OH- or CO 32 - (aq) Fe. S, Fe 3(PO 4)2 , Fe(OH)2 , or Fe. CO 3(s) K+(aq)

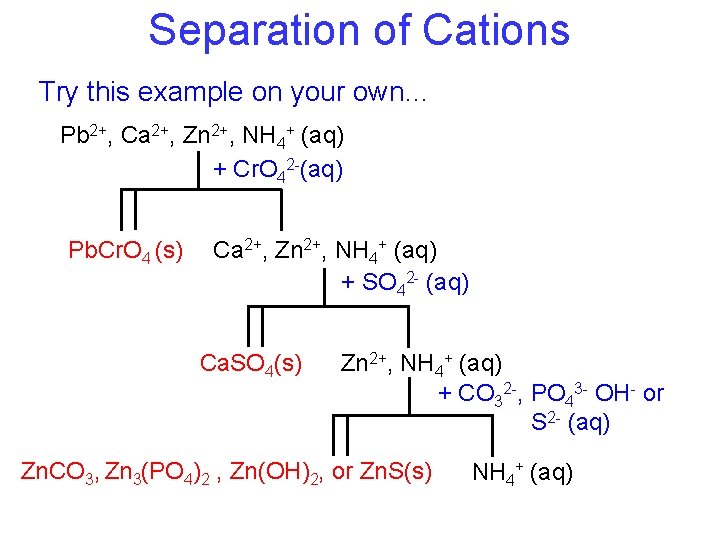
Separation of Cations Try this example on your own… Pb 2+, Ca 2+, Zn 2+, NH 4+ (aq) + Cr. O 42 -(aq) Pb. Cr. O 4 (s) Ca 2+, Zn 2+, NH 4+ (aq) + SO 42 - (aq) Ca. SO 4(s) Zn 2+, NH 4+ (aq) + CO 32 -, PO 43 - OH- or S 2 - (aq) Zn. CO 3, Zn 3(PO 4)2 , Zn(OH)2, or Zn. S(s) NH 4+ (aq)

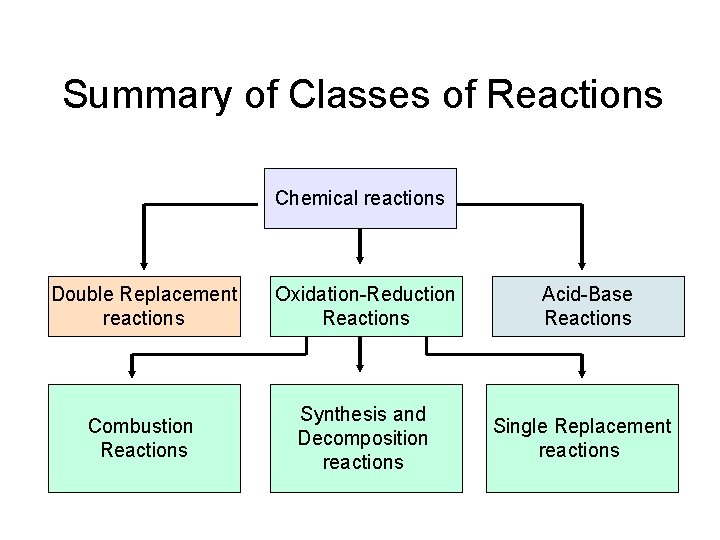
Summary of Classes of Reactions Chemical reactions Double Replacement reactions Oxidation-Reduction Reactions Combustion Reactions Synthesis and Decomposition reactions Acid-Base Reactions Single Replacement reactions
 Section 2 classifying chemical reactions
Section 2 classifying chemical reactions Chemical reactions section 2 classifying chemical reactions
Chemical reactions section 2 classifying chemical reactions Are kc and kp equal
Are kc and kp equal Types of reactions
Types of reactions Section 1 chemical changes
Section 1 chemical changes Lesson 68 toxic reactions chemical equations answer key
Lesson 68 toxic reactions chemical equations answer key Chapter 8 review chemical equations and reactions
Chapter 8 review chemical equations and reactions Chapter 8 section 1 chemical equations and reactions
Chapter 8 section 1 chemical equations and reactions Balancing equations chapter 8
Balancing equations chapter 8 Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Unit 5 chemical equations and reactions
Unit 5 chemical equations and reactions Chapter 19 chemical reactions answer key
Chapter 19 chemical reactions answer key Toxic reactions chemical equations
Toxic reactions chemical equations What are redox reactions examples
What are redox reactions examples Unit 5 chemical reactions answers
Unit 5 chemical reactions answers Translating chemical equations
Translating chemical equations Proportional relationships in chemical reactions
Proportional relationships in chemical reactions Types of chemical reactions redox
Types of chemical reactions redox Identify types of reactions
Identify types of reactions Types of reactions chemistry
Types of reactions chemistry Types of reactions
Types of reactions Predicting products of chemical reactions
Predicting products of chemical reactions 4 types of chemical reactions
4 types of chemical reactions Non examples of chemical reactions
Non examples of chemical reactions Chapter 10 chapter assessment chemical reactions answers
Chapter 10 chapter assessment chemical reactions answers The calculations of quantities in chemical reactions
The calculations of quantities in chemical reactions Ouchterlony
Ouchterlony Predicting products of chemical reactions
Predicting products of chemical reactions Chemistry predicting products
Chemistry predicting products Activity series of metals
Activity series of metals Unit 11 chemical reactions
Unit 11 chemical reactions Four types of chemical reactions
Four types of chemical reactions Www.biology-roots.com
Www.biology-roots.com Describing chemical reactions
Describing chemical reactions Chemical reactions classification
Chemical reactions classification Examples of chemical reactions in everyday life
Examples of chemical reactions in everyday life Five chemical changes
Five chemical changes Chemical reactions reactants and products
Chemical reactions reactants and products 5 general types of chemical reactions
5 general types of chemical reactions Solubility rules
Solubility rules Chapter 9 chemical reactions study guide
Chapter 9 chemical reactions study guide Rate constant and equilibrium constant
Rate constant and equilibrium constant Chapter 9 chemical reactions
Chapter 9 chemical reactions What are the 4 types of chemical reactions
What are the 4 types of chemical reactions Four types of chemical reactions
Four types of chemical reactions