Chapter 6 Modern Atomic Theory Review Dalton Thomson












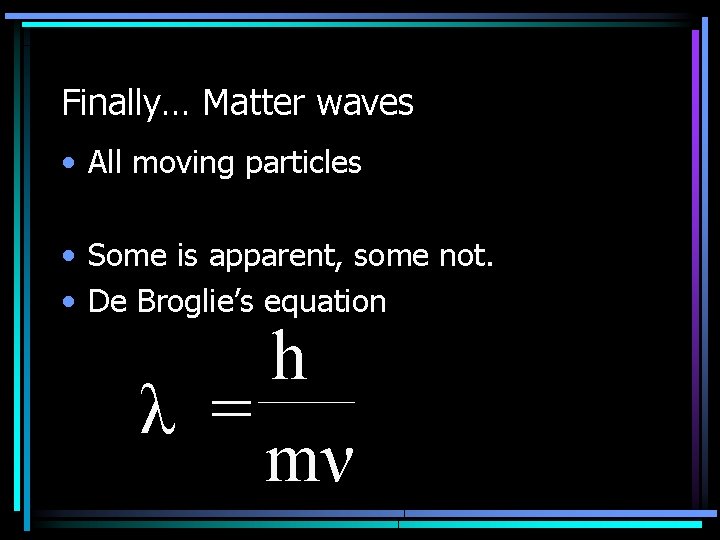








- Slides: 42

Chapter 6 Modern Atomic Theory

Review… • Dalton • Thomson • Rutherford – Model doesn’t explain how the negative electron can stay in orbit and not be attracted to the positive proton

Electromagnetic Radiation • Light travels in • Light is a form of – Form of energy that exhibits

Electromagnetic Radiation • All waves can be described in 3 ways: – Amplitude – – Wavelength (l): – Frequency (n):

Electromagnetic Radiation • Speed of light in air: Electromagnetic radiation moves through a vacuum at speed of • Since light moves at constant speed there is a relationship between wavelength and frequency: Wavelength and frequency are inversely proportional

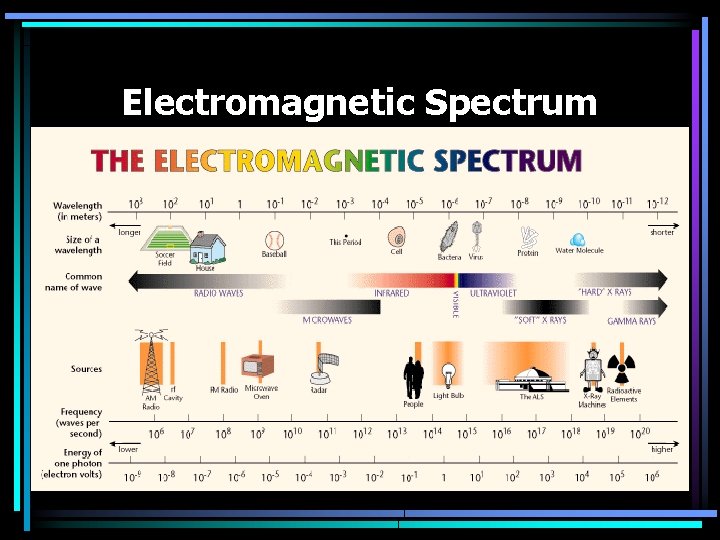
Electromagnetic Spectrum

Photoelectric Effect • The emission of – Albert Einstein (1905) used Planck’s equation to explain this phenomenon; • proposed that light consists of • Photon =

Photoelectric Effect • He (Einstein) explained that the photoelectric effect would not occur if the frequency and therefore • Analogy: – 70 cents placed in soda machine: no soda – 30 cents more and you will get your soda

Niels Henrik David Bohr • 1885 -1962 • Physicist • Worked with Rutherford – 1912 • Studying line spectra – of hydrogen

Niels Henrik David Bohr • 1913 – proposed new atomic structure – Electrons exist in – Electrons

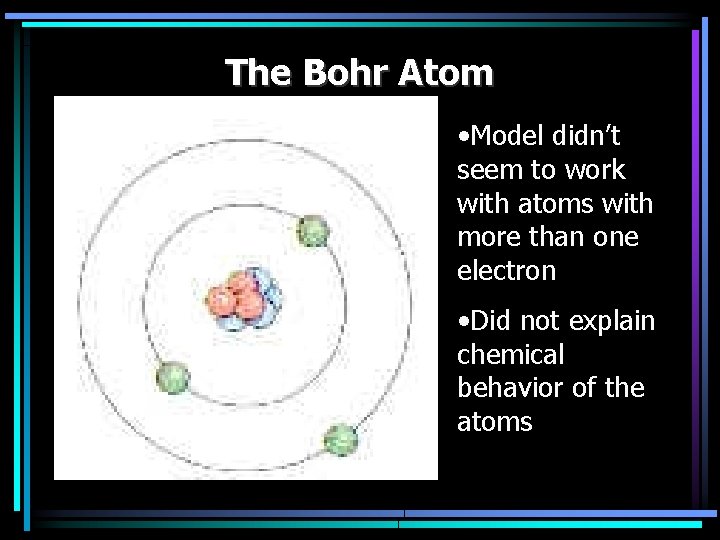
The Bohr Atom • Nucleus with • Electrons move in • When an electron moves from one state to another the energy lost or gained is in • Each line in a spectrum is produced when an electron moves from

The Bohr Atom • Model didn’t seem to work with atoms with more than one electron • Did not explain chemical behavior of the atoms

Now… • Light can be described as • • What does this mean for the atom? ? ?

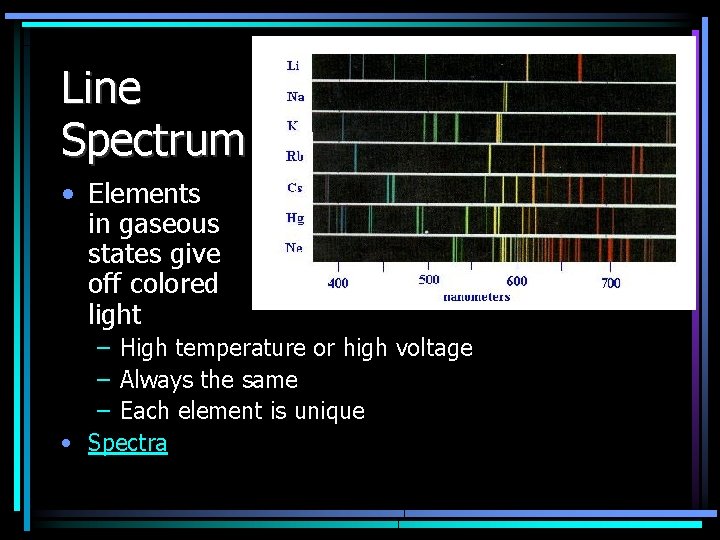
Line Spectrum • Elements in gaseous states give off colored light – High temperature or high voltage – Always the same – Each element is unique • Spectra

Line Spectrum • Ground state – • Excited state – – –

Line Spectrum • Electron • Color of light emitted depends on

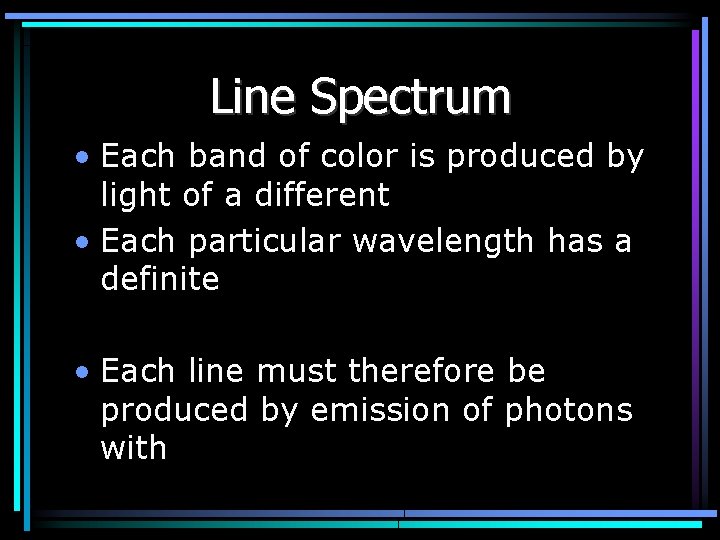
Line Spectrum • Each band of color is produced by light of a different • Each particular wavelength has a definite • Each line must therefore be produced by emission of photons with

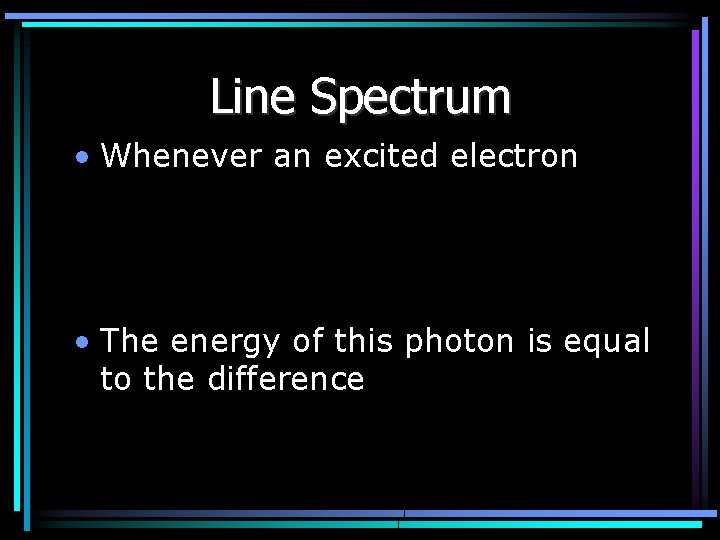
Line Spectrum • Whenever an excited electron • The energy of this photon is equal to the difference

Wave Matters… • Louis de Broglie (1924) • Proposed that electrons might have a • Used observations of normal wave activity

Problems… • Wave theory does not explain – Heated iron gives off heat • 1 st red glow yellow glow white glow – How elements such as barium and strontium give rise to green and red colors when heated

Beginnings… • Max Planck (1858 -1947) – Proposed that there is a fundamental restriction on the amounts of energy that an object emits or absorbs, and he called each of these pieces of energy • Energy is released in

Beginnings • A quantum is a finite quantity of energy that can be gained or lost by an atom • This constant, h, is the same for all electromagnetic radiation

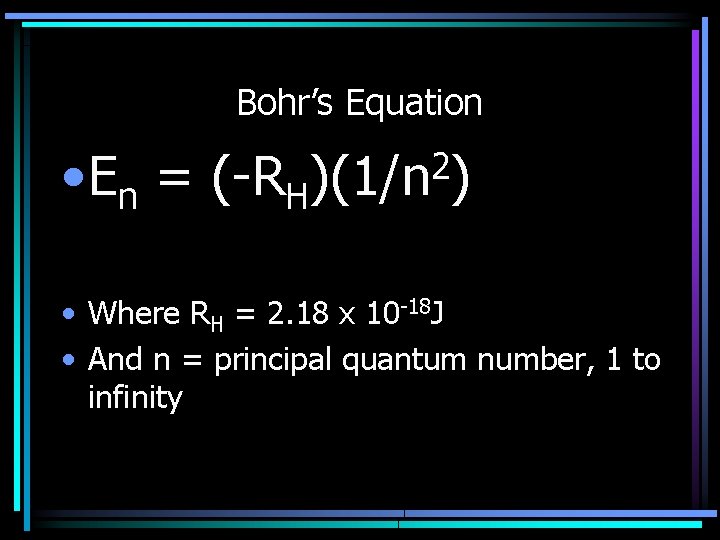
Bohr’s Equation • En = (-RH 2 )(1/n ) • Where RH = 2. 18 x 10 -18 J • And n = principal quantum number, 1 to infinity

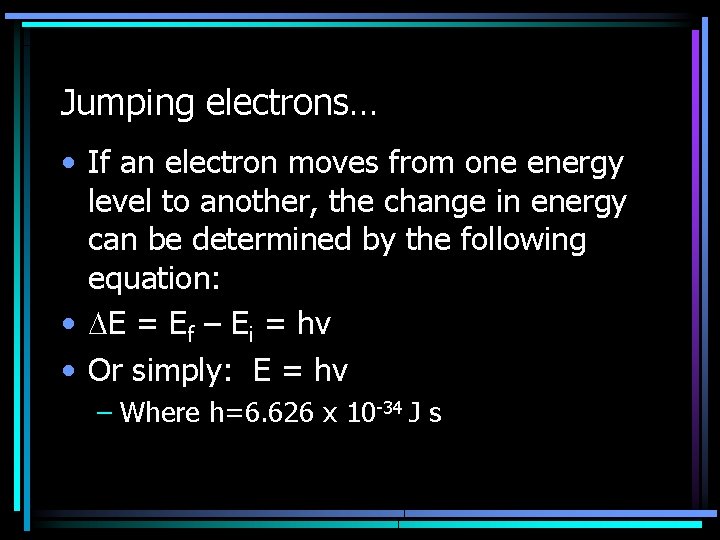
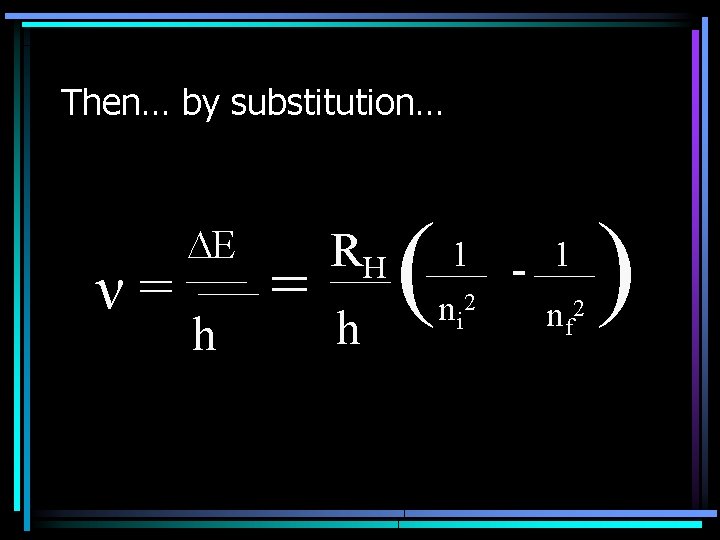
Jumping electrons… • If an electron moves from one energy level to another, the change in energy can be determined by the following equation: • E = Ef – Ei = hν • Or simply: E = hv – Where h=6. 626 x 10 -34 J s

Then… by substitution… h = RH h ( 1 ni 2 - 1 nf 2 ( ν= E
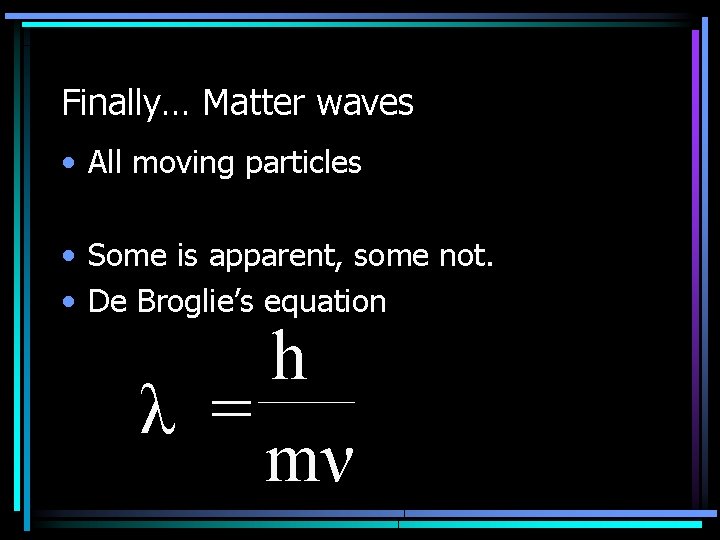
Finally… Matter waves • All moving particles • Some is apparent, some not. • De Broglie’s equation h λ = mν

Smart guy… • Erwin Schrodinger (1926) • Used mathematical understanding of wave behavior – devised an equation that treated electrons moving around nuclei as waves • Quantum Theory

Uncertainty principle • Heisenberg:

Quantum Theory • Describes mathematically the wave properties of electrons and other very small particles • Applies to all elements (not just H)

Quantum Numbers • Numbers that specify the • Principle Quantum Number: – Symbolized by n,

Energy Levels of Electrons • Principle energy levels – Designated by letter n – Corresponds to the – Each level divided into sublevels • 1 st energy level has • 2 nd energy level has • Etc.

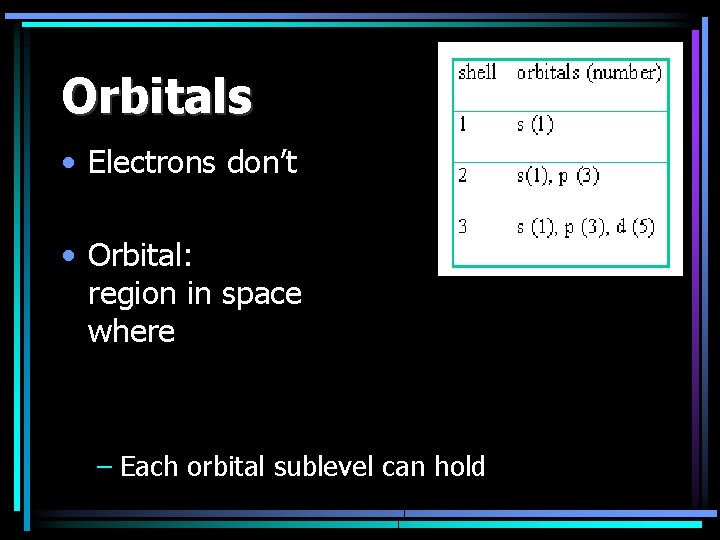
Orbitals • Electrons don’t • Orbital: region in space where – Each orbital sublevel can hold

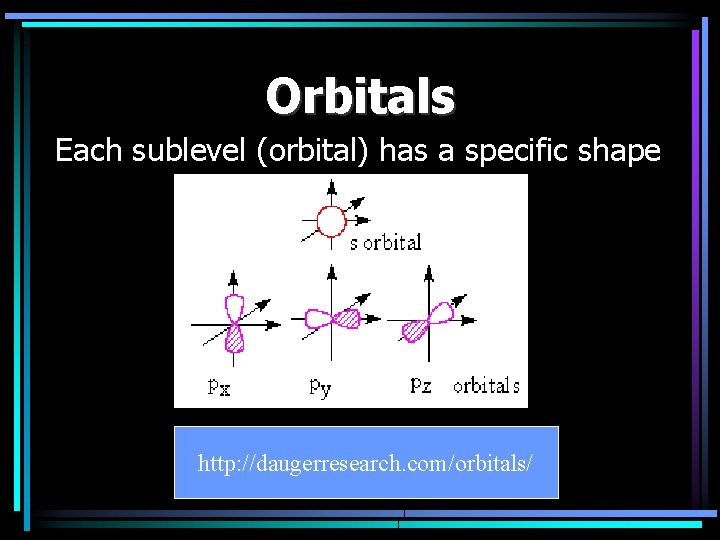
Orbitals Each sublevel (orbital) has a specific shape http: //daugerresearch. com/orbitals/

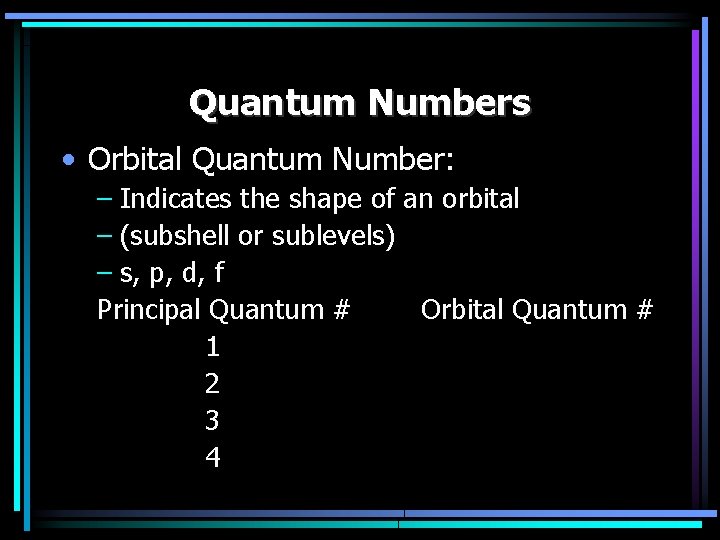
Quantum Numbers • Orbital Quantum Number: – Indicates the shape of an orbital – (subshell or sublevels) – s, p, d, f Principal Quantum # Orbital Quantum # 1 2 3 4

Quantum Numbers • Magnetic Quantum Number: – Indicates the – Orbital position with respect to

Orbitron • For a full view of the different orbital shapes, visit • http: //www. shef. ac. uk/chemistry/orbitr on/index. html

Orbitals • Pauli exclusion principle: • Electrons can only spin • Shown with

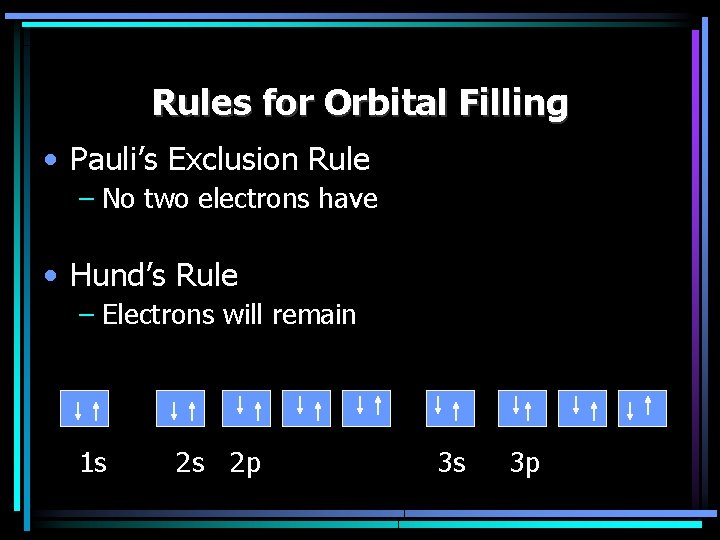
Rules for Orbital Filling • Pauli’s Exclusion Rule – No two electrons have • Hund’s Rule – Electrons will remain 1 s 2 s 2 p 3 s 3 p

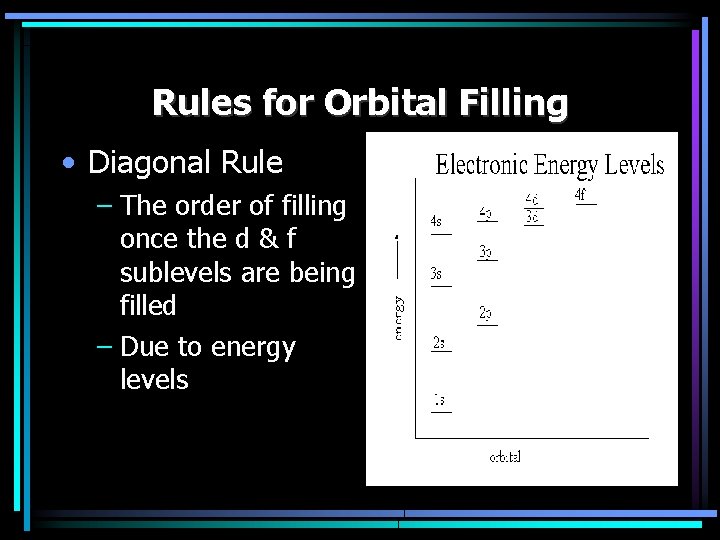
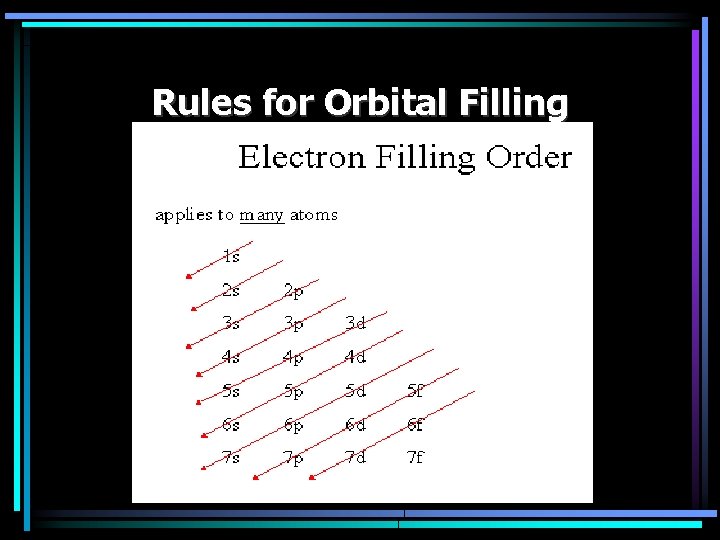
Rules for Orbital Filling • Diagonal Rule – The order of filling once the d & f sublevels are being filled – Due to energy levels

Rules for Orbital Filling

Application of Quantum Numbers • Several ways of writing the address or location of an electron • Lowest energy levels are filled first • Electron Configuration: 12 C: 32 S:

Application of Quantum Numbers • Orbital filling electron diagram: using Hund’s rule and the diagonal rule write out the location of all electrons • See examples on whiteboard