Review Modern Physics Ph 311 It was found









- Slides: 91

Review Modern Physics, Ph 311 It was found that there was no displacement of the interference fringes, so that the result of the experiment was negative and would, therefore, show that there is still a difficulty in theory itself… - Albert Michelson, 1907 1/ 2 to / of our 3 3 modern economy !!! 1

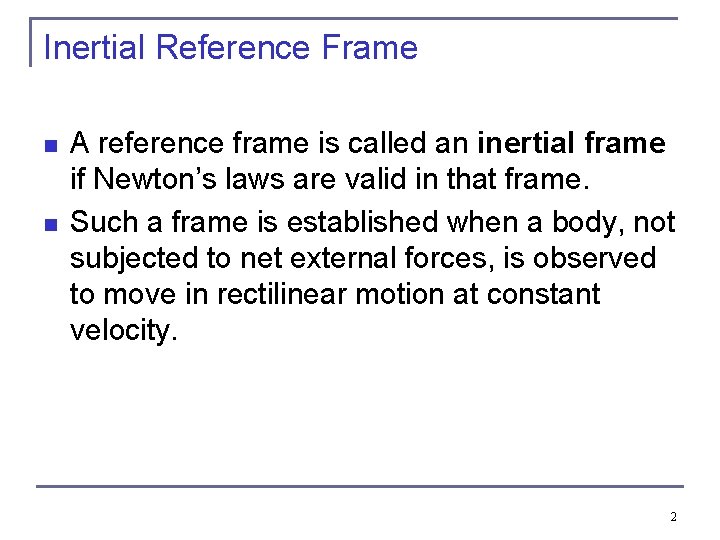
Inertial Reference Frame n n A reference frame is called an inertial frame if Newton’s laws are valid in that frame. Such a frame is established when a body, not subjected to net external forces, is observed to move in rectilinear motion at constant velocity. 2

Newtonian Principle of Relativity n If Newton’s laws are valid in one inertial reference frame, then they are also valid in another inertial reference frame moving at a uniform velocity relative to the first system. n This is referred to as the Newtonian principle of relativity or Galilean invariance/relativity. So the laws of mechanics are independent on the state of movement in a straight line at constant velocity 3

4

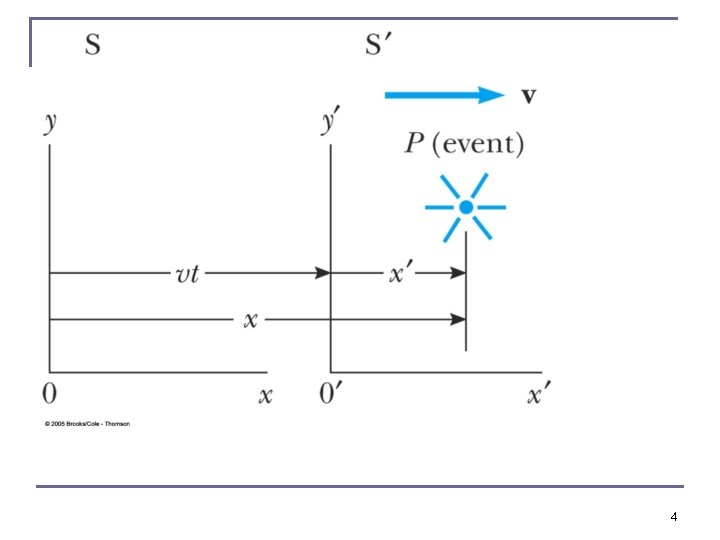
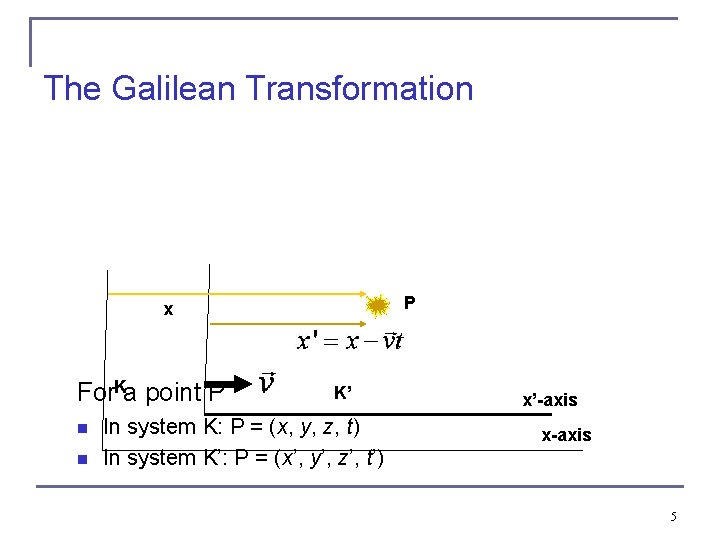
The Galilean Transformation P x K For a point P n n K’ In system K: P = (x, y, z, t) In system K’: P = (x’, y’, z’, t’) x’-axis x-axis 5

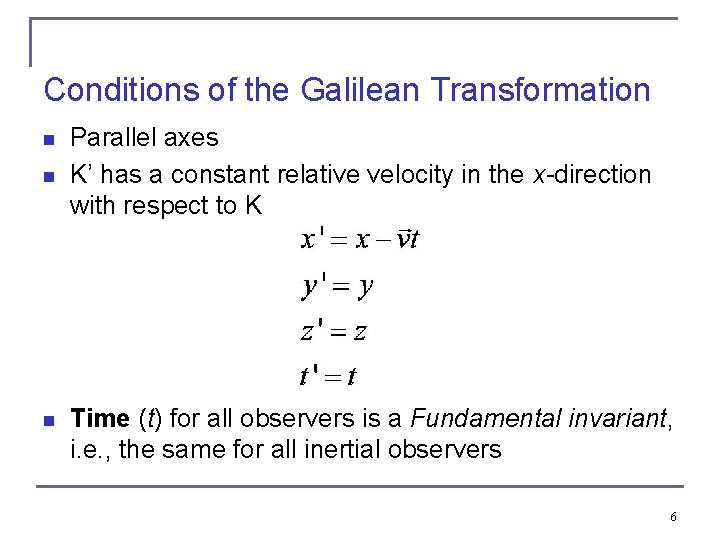
Conditions of the Galilean Transformation n Parallel axes K’ has a constant relative velocity in the x-direction with respect to K Time (t) for all observers is a Fundamental invariant, i. e. , the same for all inertial observers 6

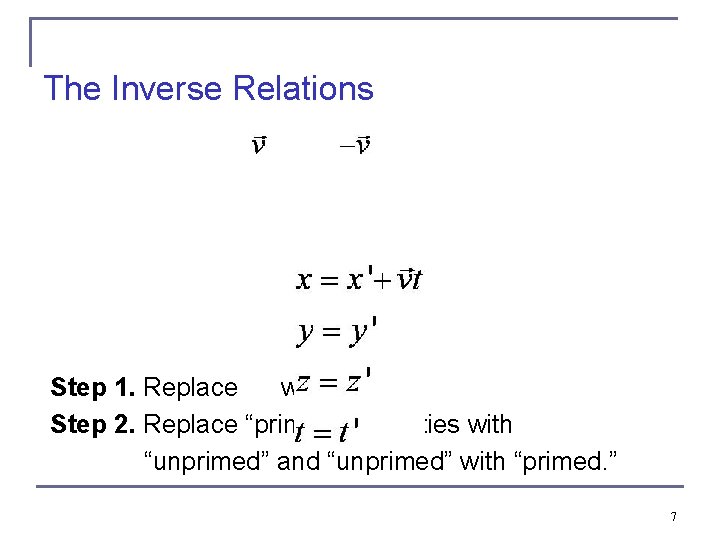
The Inverse Relations Step 1. Replace with . Step 2. Replace “primed” quantities with “unprimed” and “unprimed” with “primed. ” 7

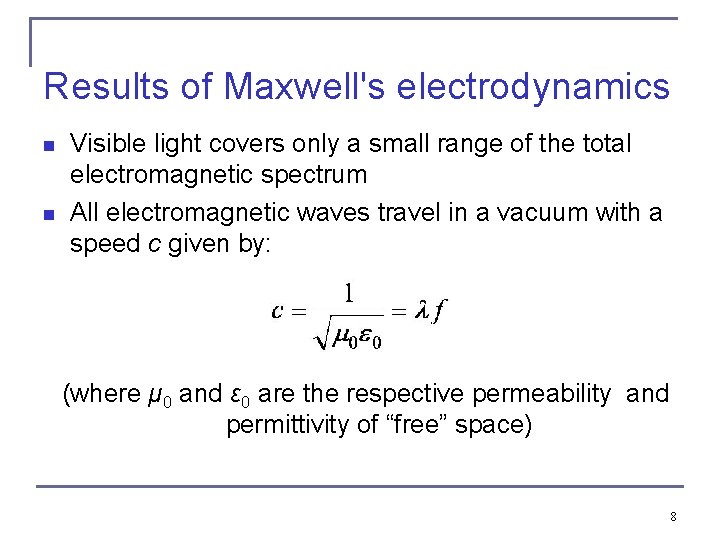
Results of Maxwell's electrodynamics n n Visible light covers only a small range of the total electromagnetic spectrum All electromagnetic waves travel in a vacuum with a speed c given by: (where μ 0 and ε 0 are the respective permeability and permittivity of “free” space) 8

Need for Ether n The wave nature of light suggested that there existed a propagation medium called the luminiferous ether or just ether. q q q Ether had to have such a low density that the planets could move through it without loss of energy It also had to have an enormous elasticity/toughness to support the high velocity of light waves According to classical physics ideas, the ether frame would be a preferred frame, the only one in which Maxwell’s equation would be valid as derived 9

An Absolute Reference System n n Ether was proposed as an absolute reference system in which the speed of light was this constant and in all frames moving with respect to that frame, there needed to be modifications of Maxwell’s laws. The Michelson-Morley experiment was an attempt to figure out Earth’s relatives movement through (with respect to) the ether so that Maxwell’s equations could be corrected for this effect. 10

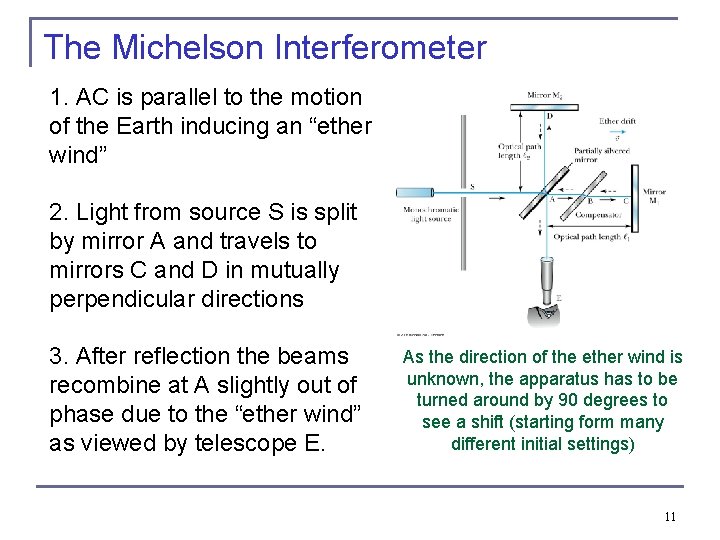
The Michelson Interferometer 1. AC is parallel to the motion of the Earth inducing an “ether wind” 2. Light from source S is split by mirror A and travels to mirrors C and D in mutually perpendicular directions 3. After reflection the beams recombine at A slightly out of phase due to the “ether wind” as viewed by telescope E. As the direction of the ether wind is unknown, the apparatus has to be turned around by 90 degrees to see a shift (starting form many different initial settings) 11

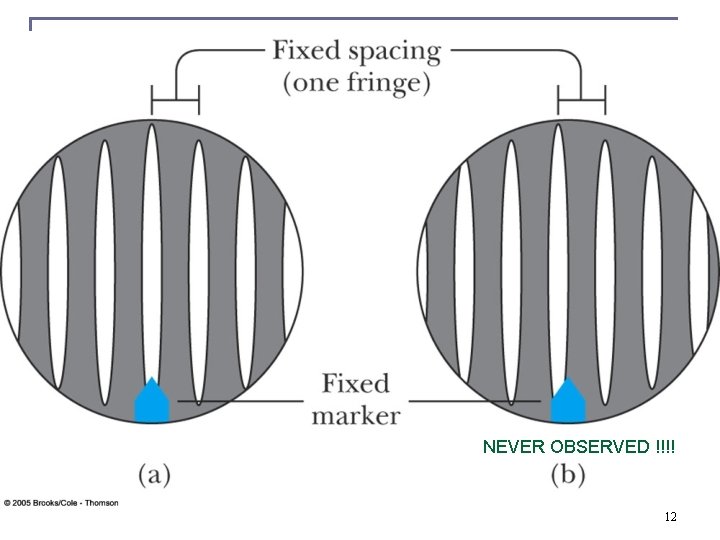
NEVER OBSERVED !!!! 12

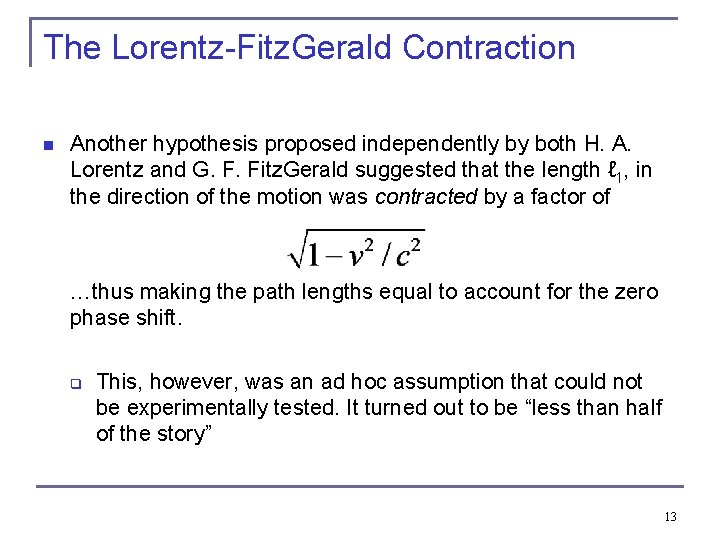
The Lorentz-Fitz. Gerald Contraction n Another hypothesis proposed independently by both H. A. Lorentz and G. F. Fitz. Gerald suggested that the length ℓ 1, in the direction of the motion was contracted by a factor of …thus making the path lengths equal to account for the zero phase shift. q This, however, was an ad hoc assumption that could not be experimentally tested. It turned out to be “less than half of the story” 13

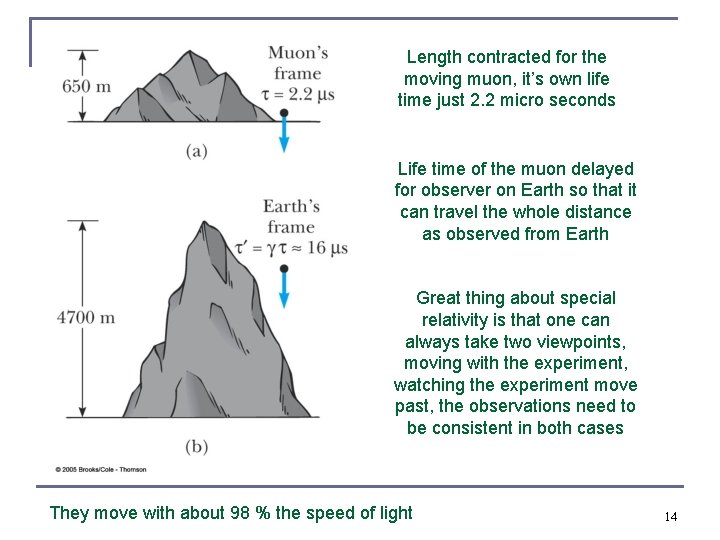
Length contracted for the moving muon, it’s own life time just 2. 2 micro seconds Life time of the muon delayed for observer on Earth so that it can travel the whole distance as observed from Earth Great thing about special relativity is that one can always take two viewpoints, moving with the experiment, watching the experiment move past, the observations need to be consistent in both cases They move with about 98 % the speed of light 14

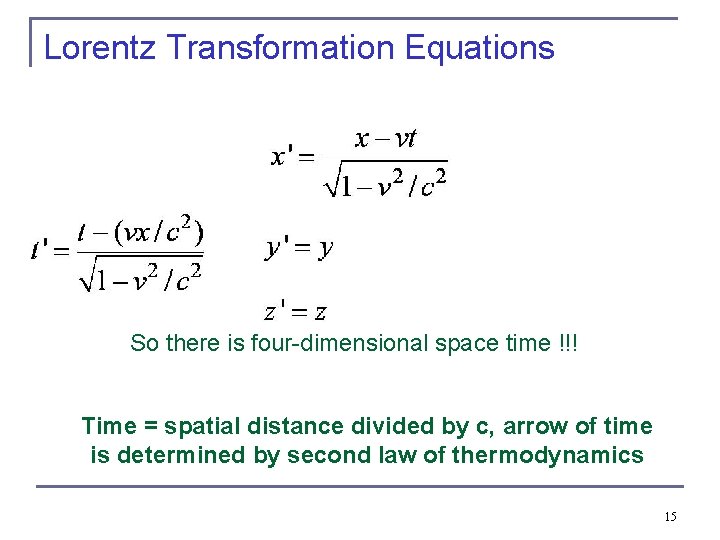
Lorentz Transformation Equations So there is four-dimensional space time !!! Time = spatial distance divided by c, arrow of time is determined by second law of thermodynamics 15

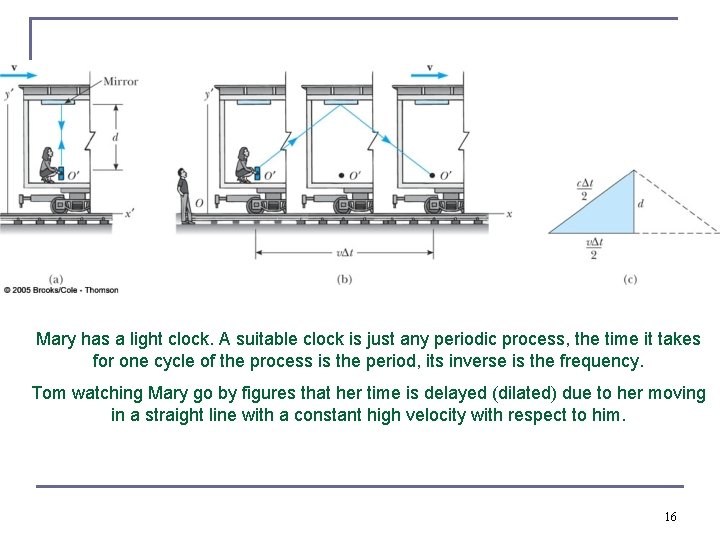
Mary has a light clock. A suitable clock is just any periodic process, the time it takes for one cycle of the process is the period, its inverse is the frequency. Tom watching Mary go by figures that her time is delayed (dilated) due to her moving in a straight line with a constant high velocity with respect to him. 16

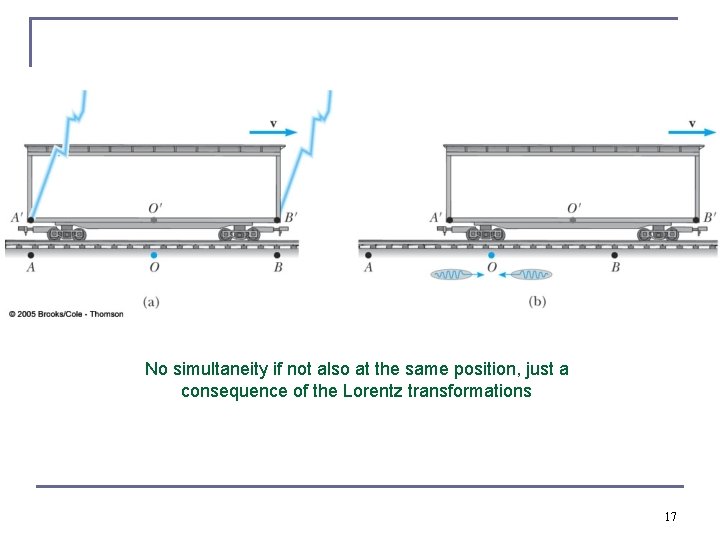
No simultaneity if not also at the same position, just a consequence of the Lorentz transformations 17

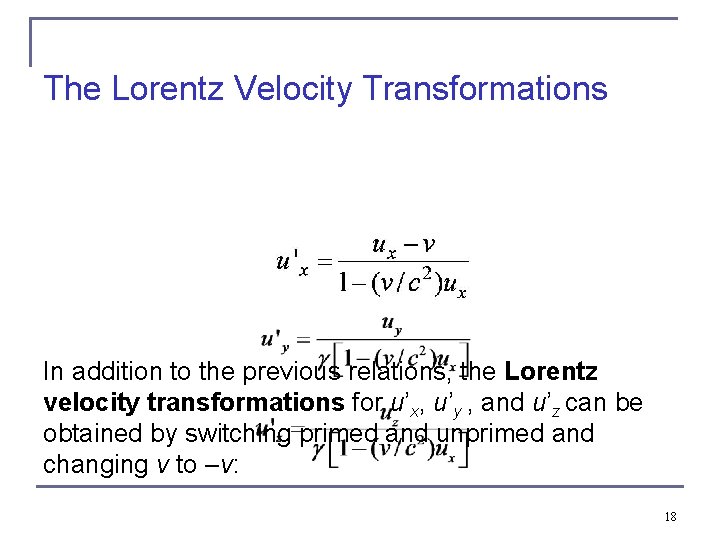
The Lorentz Velocity Transformations In addition to the previous relations, the Lorentz velocity transformations for u’x, u’y , and u’z can be obtained by switching primed and unprimed and changing v to –v: 18

Einstein’s Two Postulates With the belief that Maxwell’s equations (and with it all of the known physics of the time) must be valid in all inertial frames, Einstein proposes the following postulates: 1) The principle of (special) relativity: The laws of physics are the same in all inertial systems. There is no way to detect absolute motion, and no preferred inertial system exists. 2) The constancy of the speed of light: Observers in all inertial systems measure the same value for the speed of light in a vacuum. 19

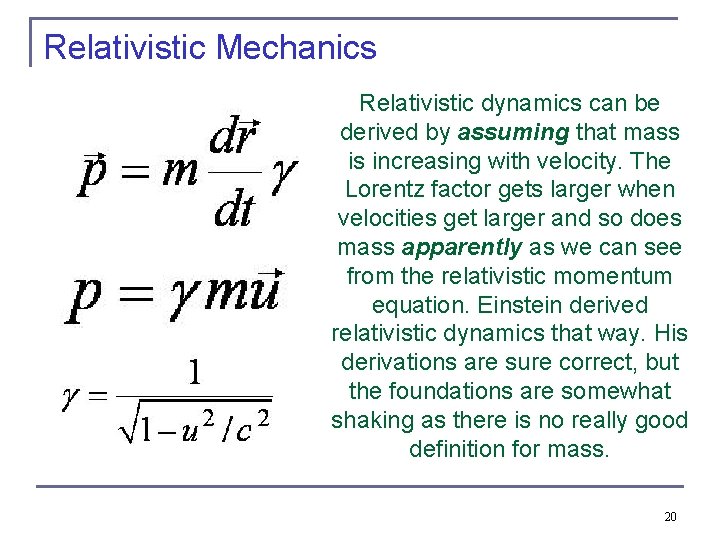
Relativistic Mechanics Relativistic dynamics can be derived by assuming that mass is increasing with velocity. The Lorentz factor gets larger when velocities get larger and so does mass apparently as we can see from the relativistic momentum equation. Einstein derived relativistic dynamics that way. His derivations are sure correct, but the foundations are somewhat shaking as there is no really good definition for mass. 20

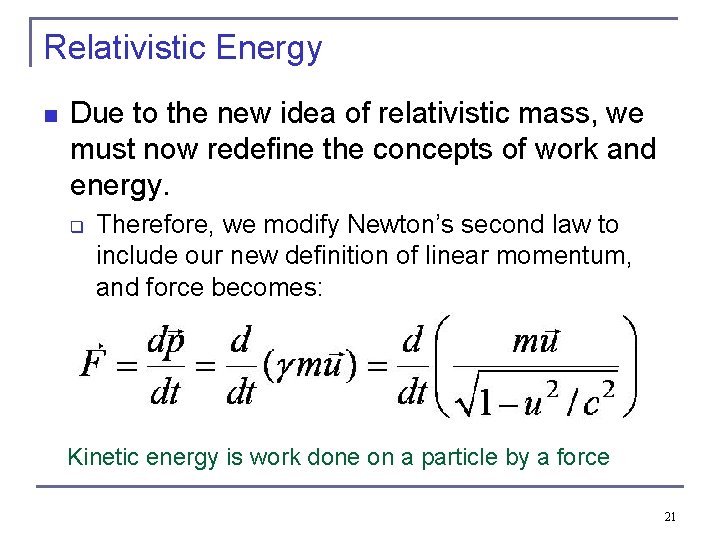
Relativistic Energy n Due to the new idea of relativistic mass, we must now redefine the concepts of work and energy. q Therefore, we modify Newton’s second law to include our new definition of linear momentum, and force becomes: Kinetic energy is work done on a particle by a force 21

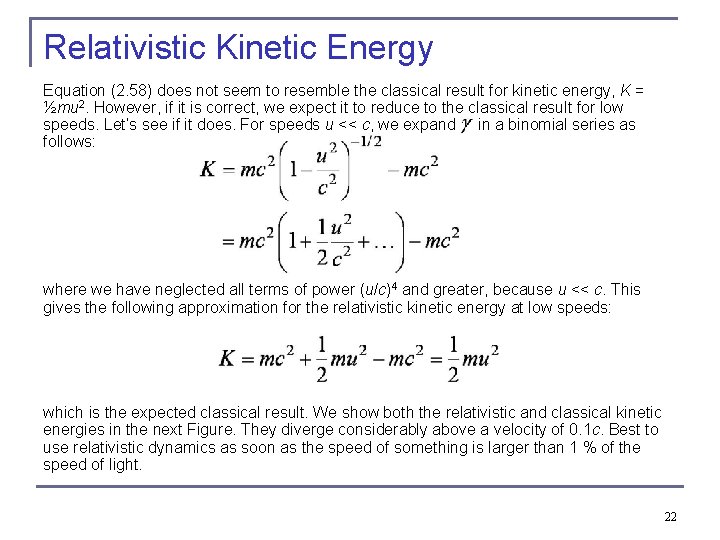
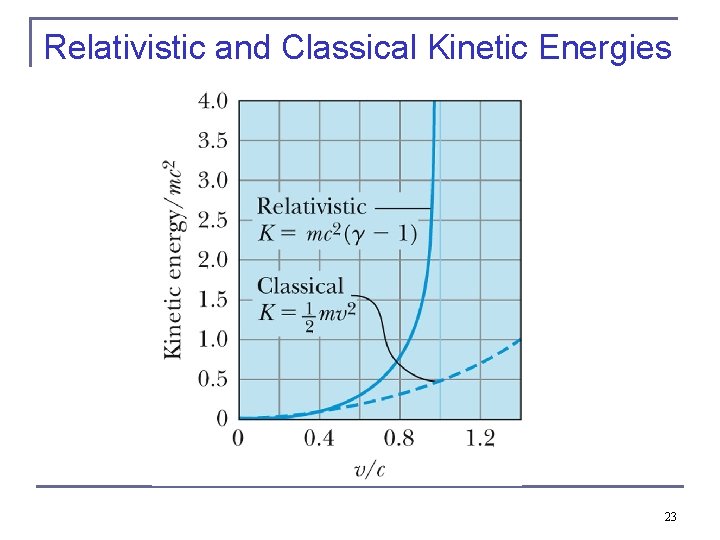
Relativistic Kinetic Energy Equation (2. 58) does not seem to resemble the classical result for kinetic energy, K = ½mu 2. However, if it is correct, we expect it to reduce to the classical result for low speeds. Let’s see if it does. For speeds u << c, we expand in a binomial series as follows: where we have neglected all terms of power (u/c)4 and greater, because u << c. This gives the following approximation for the relativistic kinetic energy at low speeds: which is the expected classical result. We show both the relativistic and classical kinetic energies in the next Figure. They diverge considerably above a velocity of 0. 1 c. Best to use relativistic dynamics as soon as the speed of something is larger than 1 % of the speed of light. 22

Relativistic and Classical Kinetic Energies 23

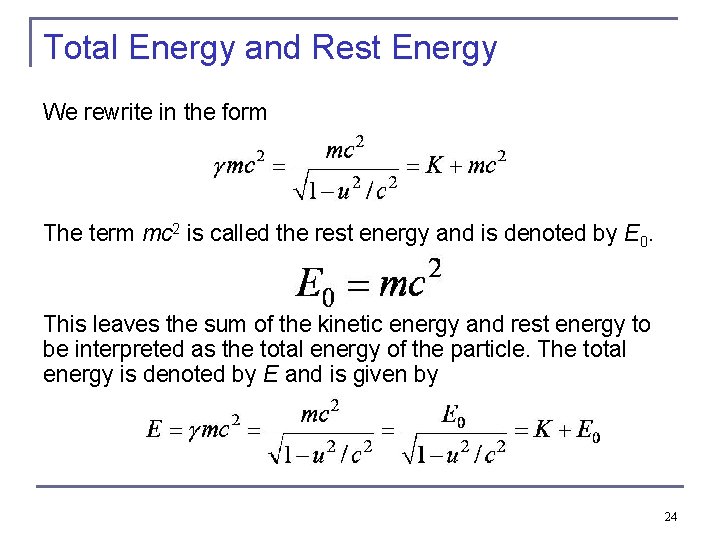
Total Energy and Rest Energy We rewrite in the form The term mc 2 is called the rest energy and is denoted by E 0. This leaves the sum of the kinetic energy and rest energy to be interpreted as the total energy of the particle. The total energy is denoted by E and is given by 24

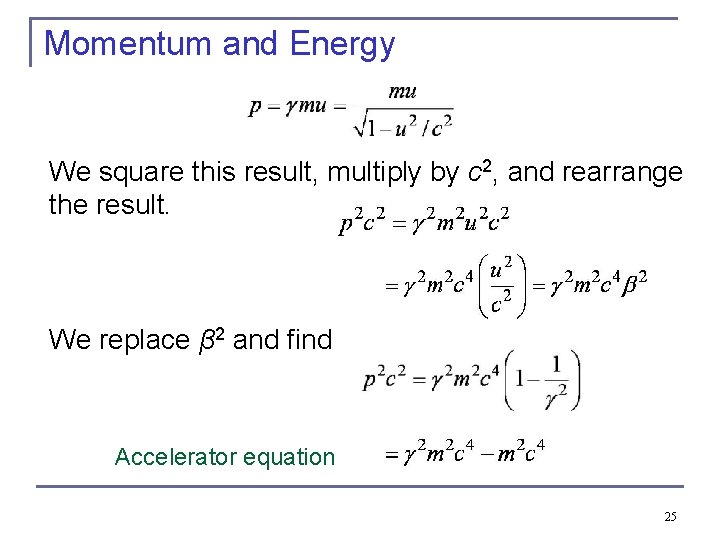
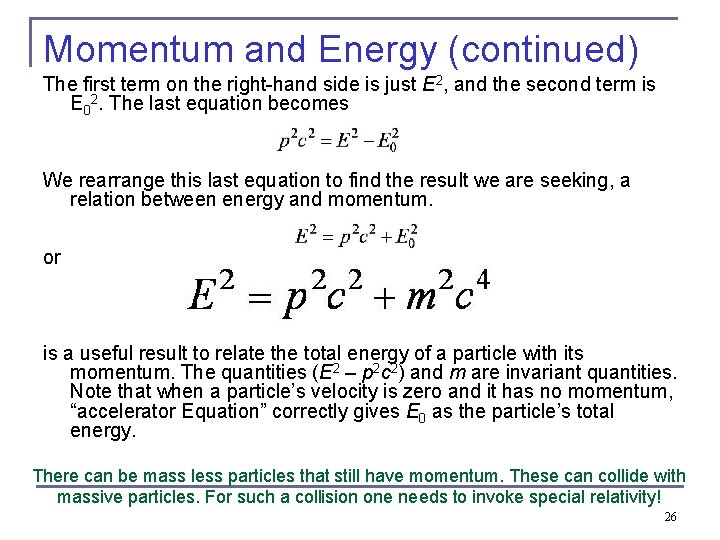
Momentum and Energy We square this result, multiply by c 2, and rearrange the result. We replace β 2 and find Accelerator equation 25

Momentum and Energy (continued) The first term on the right-hand side is just E 2, and the second term is E 02. The last equation becomes We rearrange this last equation to find the result we are seeking, a relation between energy and momentum. or is a useful result to relate the total energy of a particle with its momentum. The quantities (E 2 – p 2 c 2) and m are invariant quantities. Note that when a particle’s velocity is zero and it has no momentum, “accelerator Equation” correctly gives E 0 as the particle’s total energy. There can be mass less particles that still have momentum. These can collide with massive particles. For such a collision one needs to invoke special relativity! 26

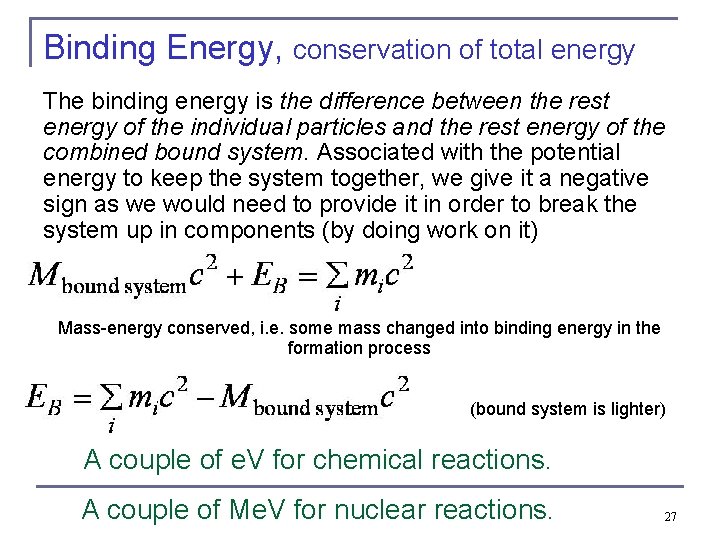
Binding Energy, conservation of total energy The binding energy is the difference between the rest energy of the individual particles and the rest energy of the combined bound system. Associated with the potential energy to keep the system together, we give it a negative sign as we would need to provide it in order to break the system up in components (by doing work on it) Mass-energy conserved, i. e. some mass changed into binding energy in the formation process (bound system is lighter) A couple of e. V for chemical reactions. A couple of Me. V for nuclear reactions. 27

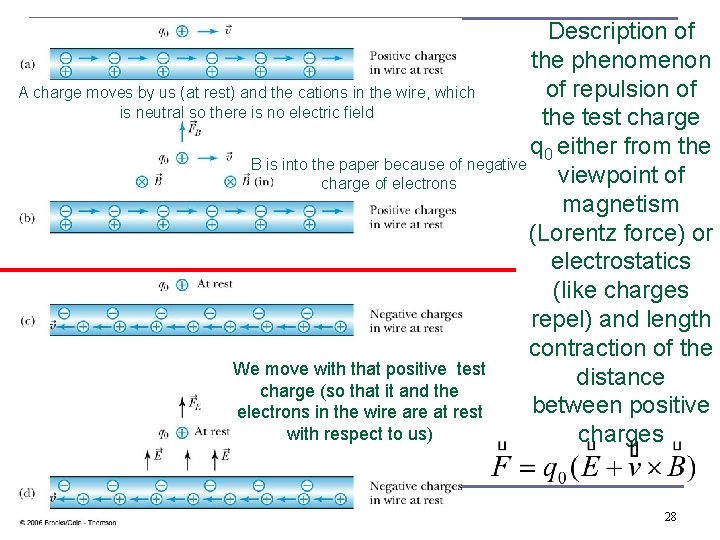
Description of the phenomenon of repulsion of A charge moves by us (at rest) and the cations in the wire, which is neutral so there is no electric field the test charge q 0 either from the B is into the paper because of negative viewpoint of charge of electrons magnetism (Lorentz force) or electrostatics (like charges repel) and length contraction of the We move with that positive test distance charge (so that it and the between positive electrons in the wire at rest with respect to us) charges 28

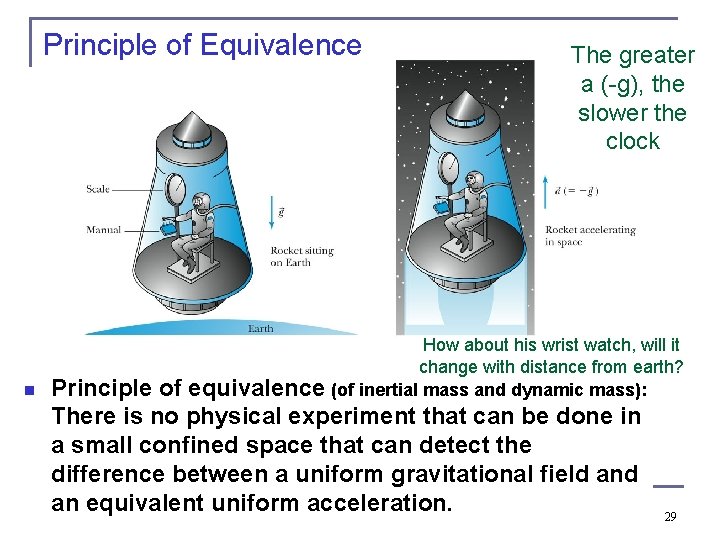
Principle of Equivalence n The greater a (-g), the slower the clock How about his wrist watch, will it change with distance from earth? equivalence (of inertial mass and dynamic mass): Principle of There is no physical experiment that can be done in a small confined space that can detect the difference between a uniform gravitational field an equivalent uniform acceleration. 29

Since the frequency of the clock decreases near the Gravitational Time Dilation Earth, a clock in a gravitational field runs more n slowly (it takes longer for a hand to move on a clock – so in aggregate the clock gets slower) according to gravitational time dilation. This is because 4 D space-time is “bend” – non-Euclidian, so there are no Euclidian straight lines to follow but Geodesics in a space with Riemann’s coordinates n n A very accurate experiment was done by comparing the frequency of an atomic clock flown on a Scout D rocket to an altitude of 10, 000 km with the frequency of a similar clock on the ground. The measurement agreed with Einstein’s general relativity theory to within 0. 02%. Now time differences due to height differences above the earth of 30 cm can be measured, (Nobel price 2012) 30

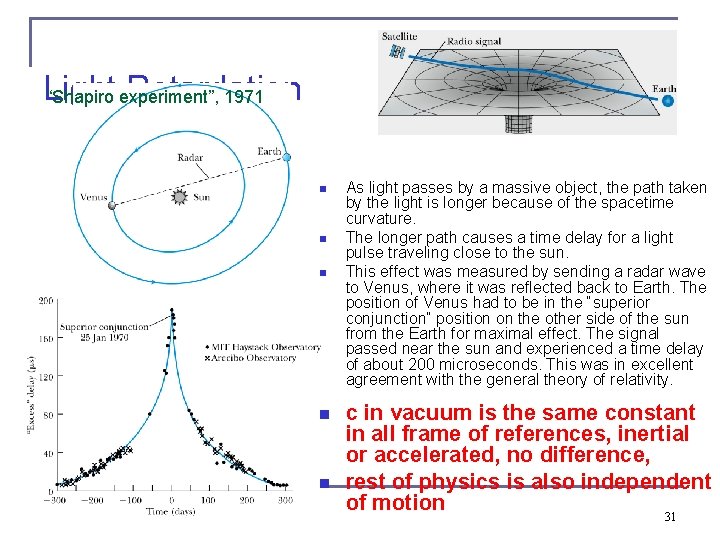
Light Retardation “Shapiro experiment”, 1971 n n n As light passes by a massive object, the path taken by the light is longer because of the spacetime curvature. The longer path causes a time delay for a light pulse traveling close to the sun. This effect was measured by sending a radar wave to Venus, where it was reflected back to Earth. The position of Venus had to be in the “superior conjunction” position on the other side of the sun from the Earth for maximal effect. The signal passed near the sun and experienced a time delay of about 200 microseconds. This was in excellent agreement with the general theory of relativity. c in vacuum is the same constant in all frame of references, inertial or accelerated, no difference, rest of physics is also independent of motion 31

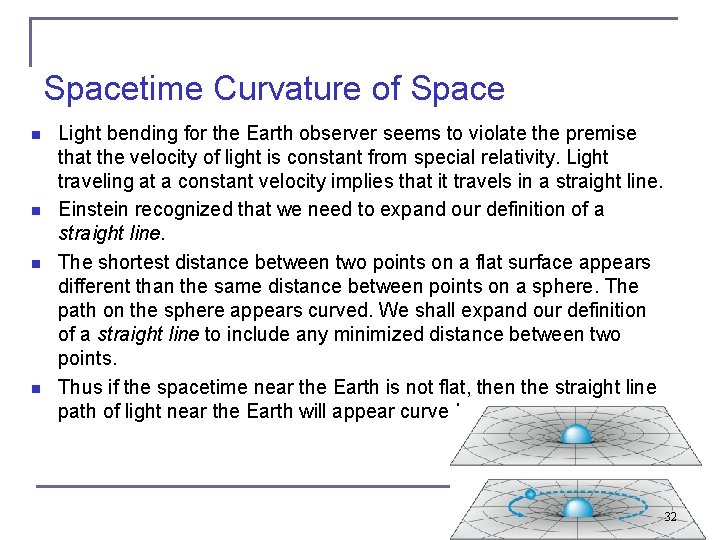
Spacetime Curvature of Space n n Light bending for the Earth observer seems to violate the premise that the velocity of light is constant from special relativity. Light traveling at a constant velocity implies that it travels in a straight line. Einstein recognized that we need to expand our definition of a straight line. The shortest distance between two points on a flat surface appears different than the same distance between points on a sphere. The path on the sphere appears curved. We shall expand our definition of a straight line to include any minimized distance between two points. Thus if the spacetime near the Earth is not flat, then the straight line path of light near the Earth will appear curved. 32

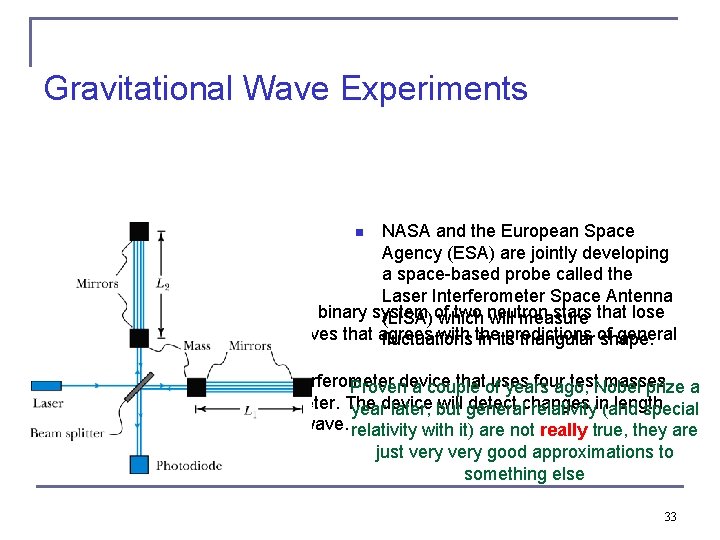
Gravitational Wave Experiments NASA and the European Space Agency (ESA) are jointly developing a space-based probe called the Laser Interferometer Space Antenna Taylor and Hulse discovered a binary system of two neutron stars that lose (LISA) which will measure energy due to gravitational waves that agrees with the predictions of general fluctuations in its triangular shape. relativity. LIGO is a large Michelson interferometer device that uses four test masses Proven a couple of years ago, Nobel prize a on two arms of the interferometer. The device will detect changes in length year later, but general relativity (and special of the arms due to a passing wave. relativity with it) are not really true, they are n n n just very good approximations to something else 33

Thank you very much indeed Albert !!! everybody loves this !!!! 34

Dual nature of light (electromagnetic radiation) both/neither wave and/nor particle http: //usatoday 30. usatoday. com/tech/science/genetics/2008 -05 -08 -platypus-geneticmap_N. htm “Australia's unique duck-billed platypus is part bird, part reptile and part mammal according to its gene map. The platypus is classed as a mammal because it has fur and feeds its young with milk. It flaps a beaver-like tail. But it also has bird and reptile features — a duck-like bill and webbed feet, and lives mostly underwater. Males have venom-filled spurs on their heels. ” 35

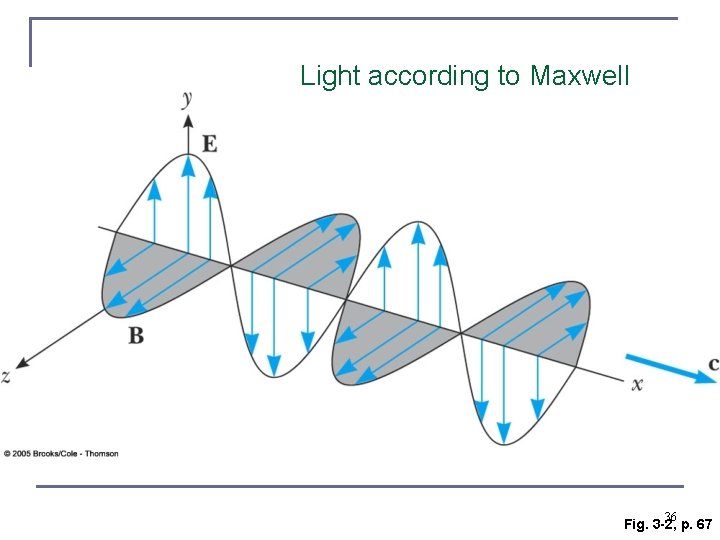
Light according to Maxwell 36 Fig. 3 -2, p. 67

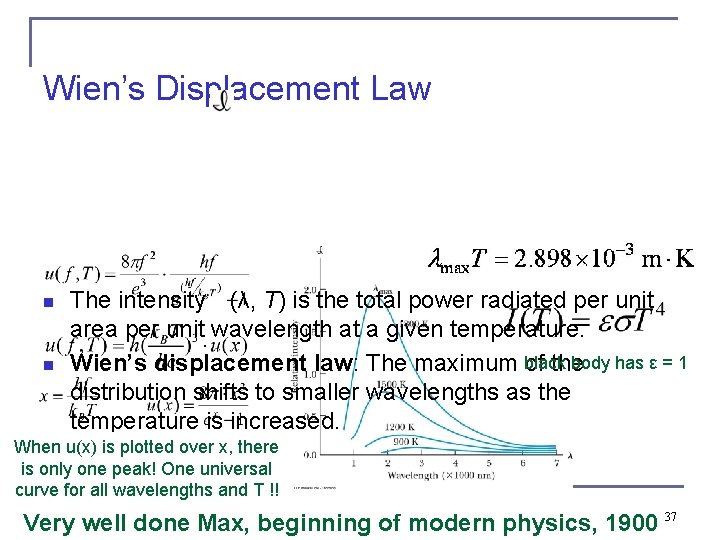
Wien’s Displacement Law n n The intensity (λ, T) is the total power radiated per unit area per unit wavelength at a given temperature. black body has ε = 1 Wien’s displacement law: The maximum of the distribution shifts to smaller wavelengths as the temperature is increased. When u(x) is plotted over x, there is only one peak! One universal curve for all wavelengths and T !! Very well done Max, beginning of modern physics, 1900 37

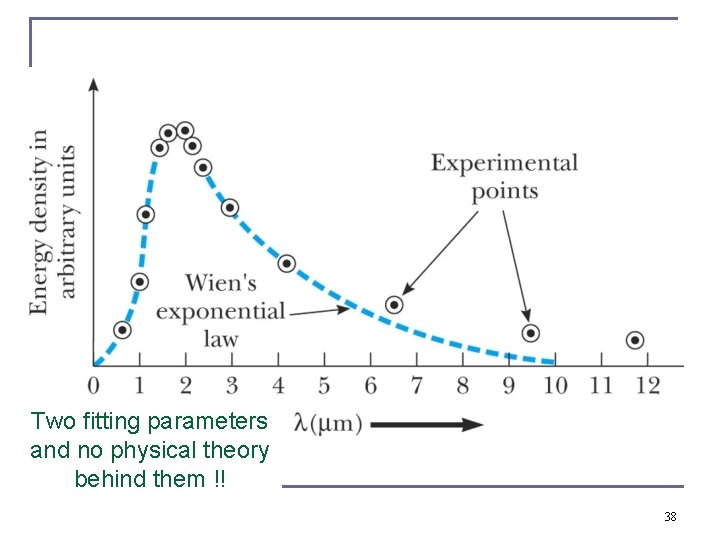
Two fitting parameters and no physical theory behind them !! 38

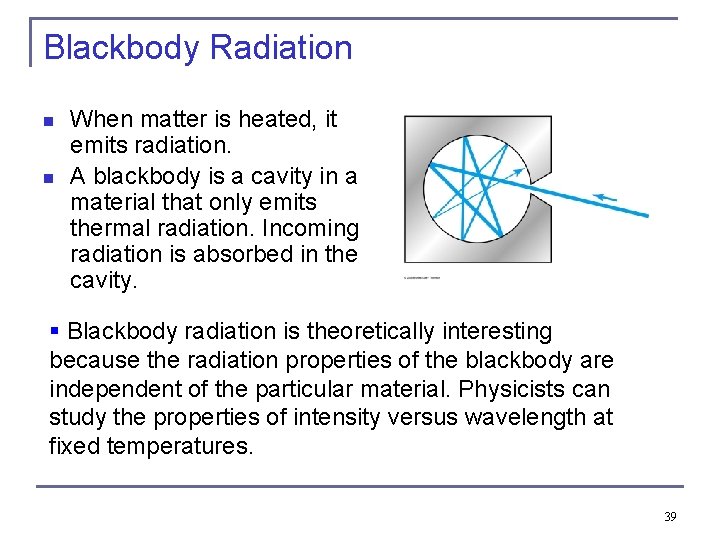
Blackbody Radiation n n When matter is heated, it emits radiation. A blackbody is a cavity in a material that only emits thermal radiation. Incoming radiation is absorbed in the cavity. § Blackbody radiation is theoretically interesting because the radiation properties of the blackbody are independent of the particular material. Physicists can study the properties of intensity versus wavelength at fixed temperatures. 39

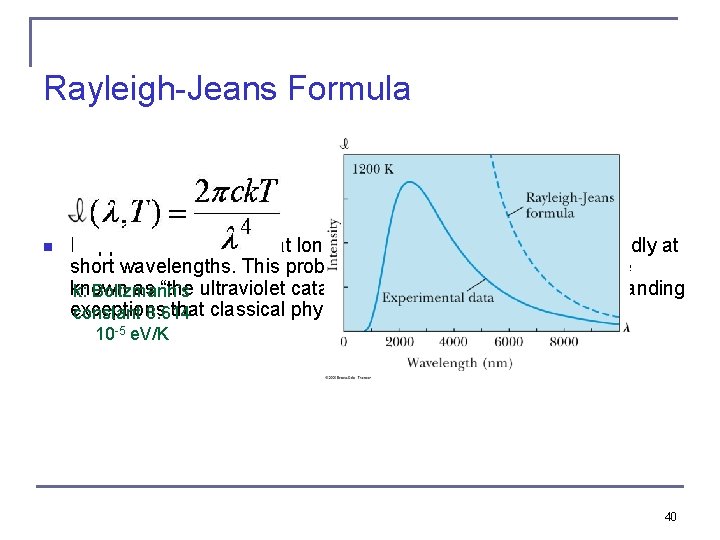
Rayleigh-Jeans Formula n It approaches the data at longer wavelengths, but it deviates badly at short wavelengths. This problem for small wavelengths became known as “the ultraviolet catastrophe” and was one of the outstanding k: Boltzmann’s exceptions that classical physics could not explain. constant 8. 614 10 -5 e. V/K 40

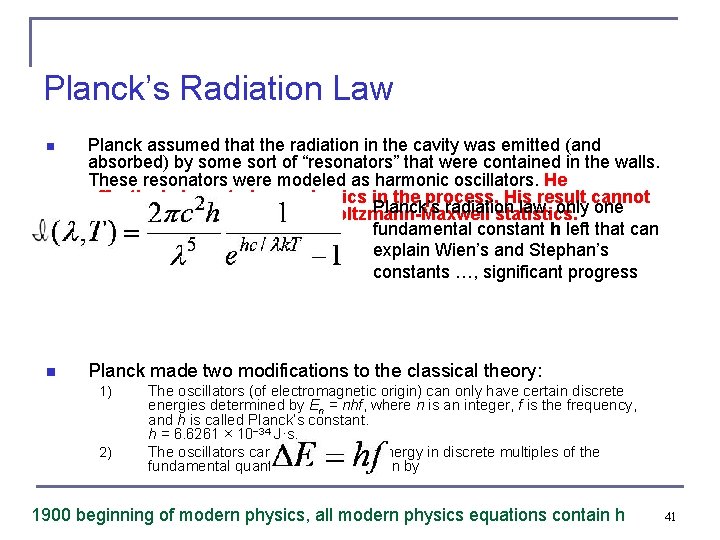
Planck’s Radiation Law n Planck assumed that the radiation in the cavity was emitted (and absorbed) by some sort of “resonators” that were contained in the walls. These resonators were modeled as harmonic oscillators. He effectively invented new physics in the process. His result cannot Planck’s radiation law, only one be explained with classical Boltzmann-Maxwell statistics. fundamental constant h left that can explain Wien’s and Stephan’s constants …, significant progress n Planck made two modifications to the classical theory: 1) 2) The oscillators (of electromagnetic origin) can only have certain discrete energies determined by En = nhf, where n is an integer, f is the frequency, and h is called Planck’s constant. h = 6. 6261 × 10− 34 J·s. The oscillators can absorb or emit energy in discrete multiples of the fundamental quantum of energy given by 1900 beginning of modern physics, all modern physics equations contain h 41

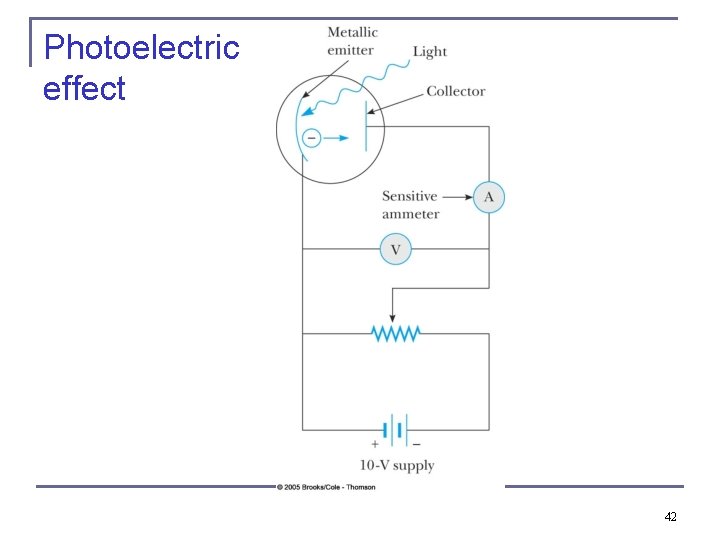
Photoelectric effect 42

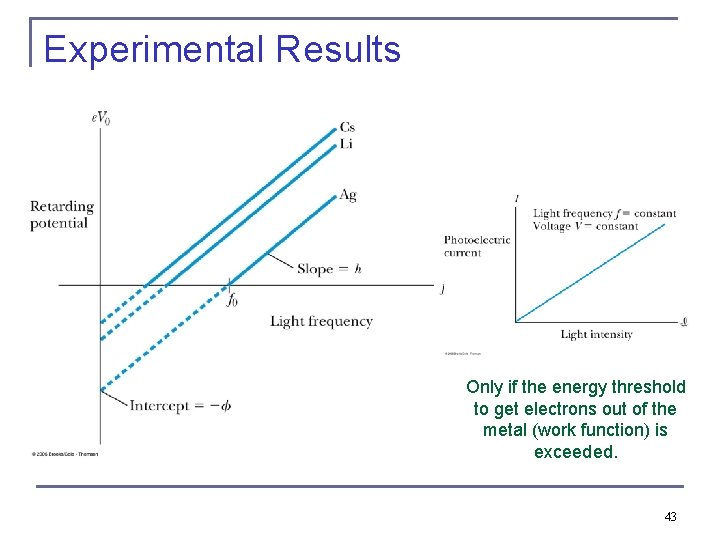
Experimental Results Only if the energy threshold to get electrons out of the metal (work function) is exceeded. 43

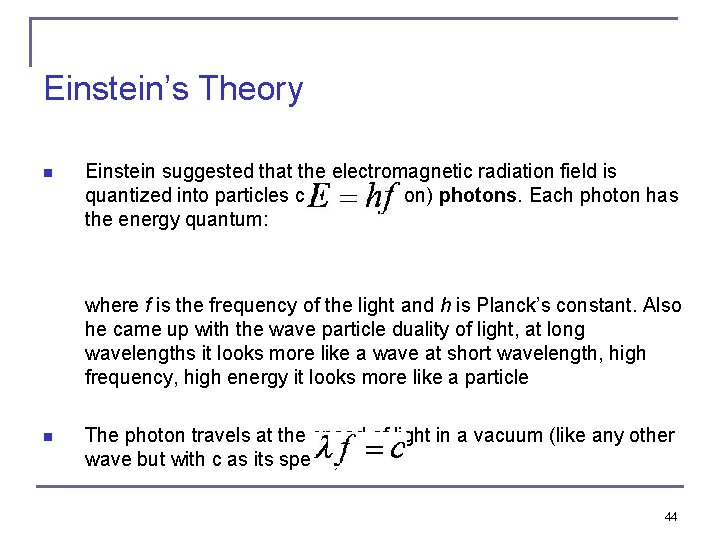
Einstein’s Theory n Einstein suggested that the electromagnetic radiation field is quantized into particles called (later on) photons. Each photon has the energy quantum: where f is the frequency of the light and h is Planck’s constant. Also he came up with the wave particle duality of light, at long wavelengths it looks more like a wave at short wavelength, high frequency, high energy it looks more like a particle n The photon travels at the speed of light in a vacuum (like any other wave but with c as its speed) 44

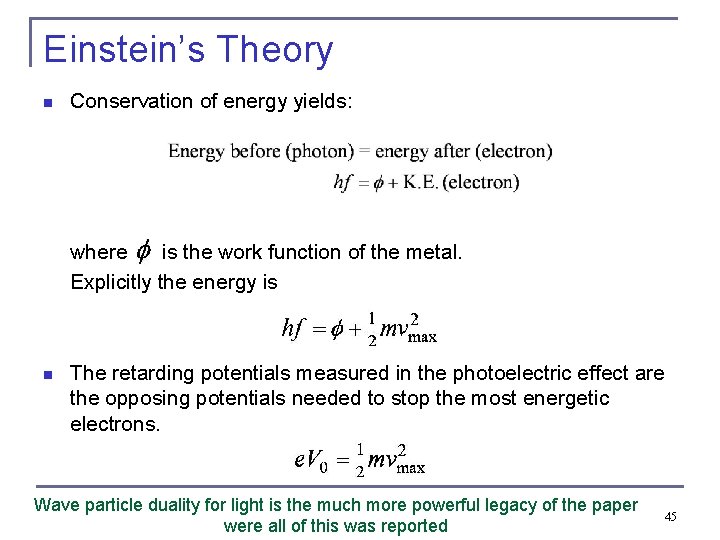
Einstein’s Theory n Conservation of energy yields: where is the work function of the metal. Explicitly the energy is n The retarding potentials measured in the photoelectric effect are the opposing potentials needed to stop the most energetic electrons. Wave particle duality for light is the much more powerful legacy of the paper were all of this was reported 45

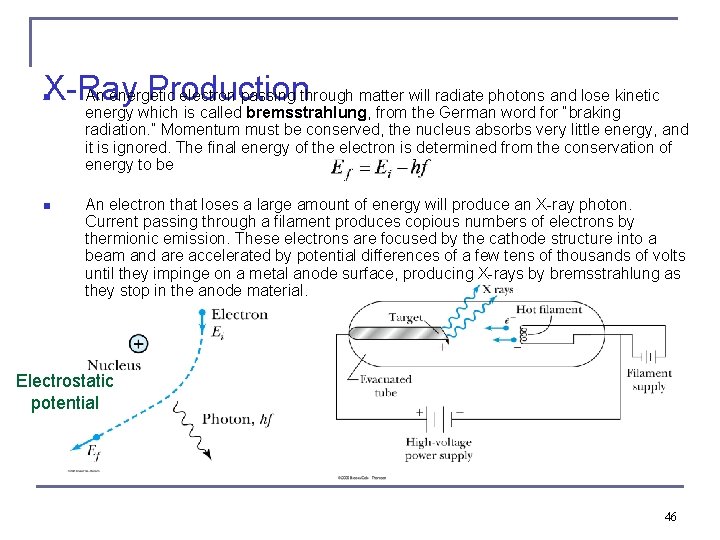
X-Ray Production An energetic electron passing through matter will radiate photons and lose kinetic n energy which is called bremsstrahlung, from the German word for “braking radiation. ” Momentum must be conserved, the nucleus absorbs very little energy, and it is ignored. The final energy of the electron is determined from the conservation of energy to be n An electron that loses a large amount of energy will produce an X-ray photon. Current passing through a filament produces copious numbers of electrons by thermionic emission. These electrons are focused by the cathode structure into a beam and are accelerated by potential differences of a few tens of thousands of volts until they impinge on a metal anode surface, producing X-rays by bremsstrahlung as they stop in the anode material. Electrostatic potential 46

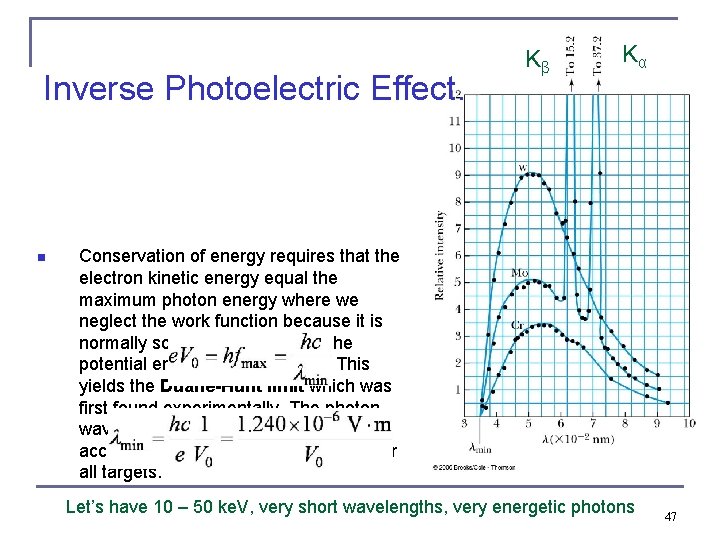
Inverse Photoelectric Effect. n Kβ Kα Conservation of energy requires that the electron kinetic energy equal the maximum photon energy where we neglect the work function because it is normally so small compared to the potential energy of the electron. This yields the Duane-Hunt limit which was first found experimentally. The photon wavelength depends only on the accelerating voltage and is the same for all targets. Let’s have 10 – 50 ke. V, very short wavelengths, very energetic photons 47

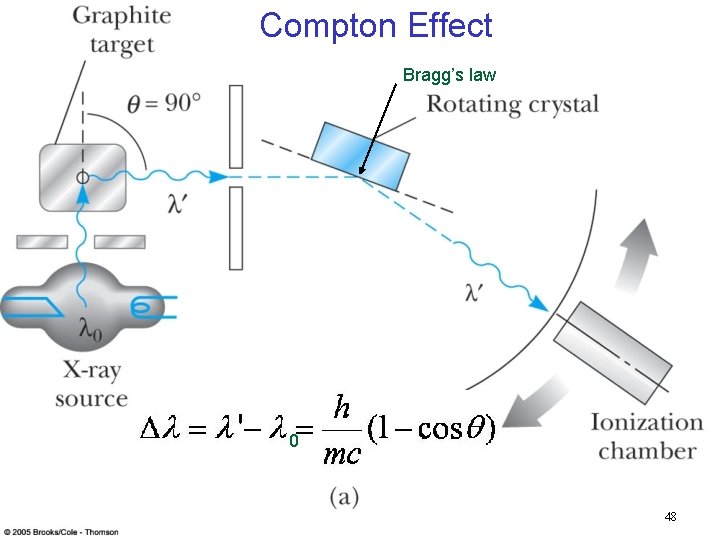
Compton Effect Bragg’s law 0 48

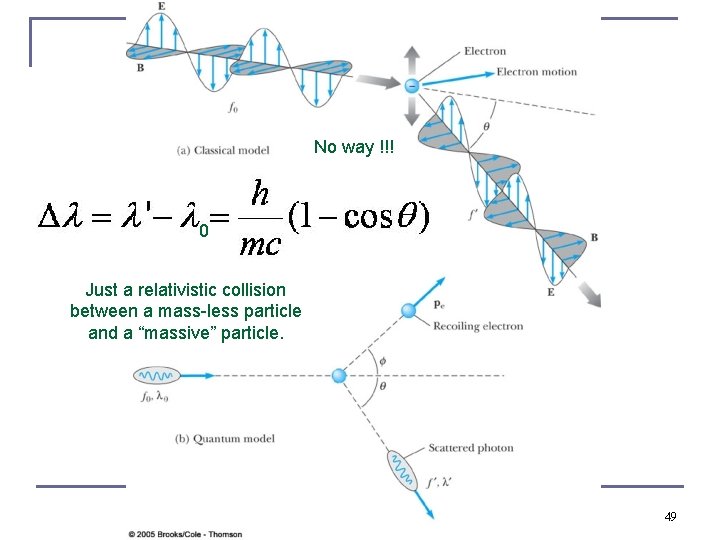
No way !!! 0 Just a relativistic collision between a mass-less particle and a “massive” particle. 49

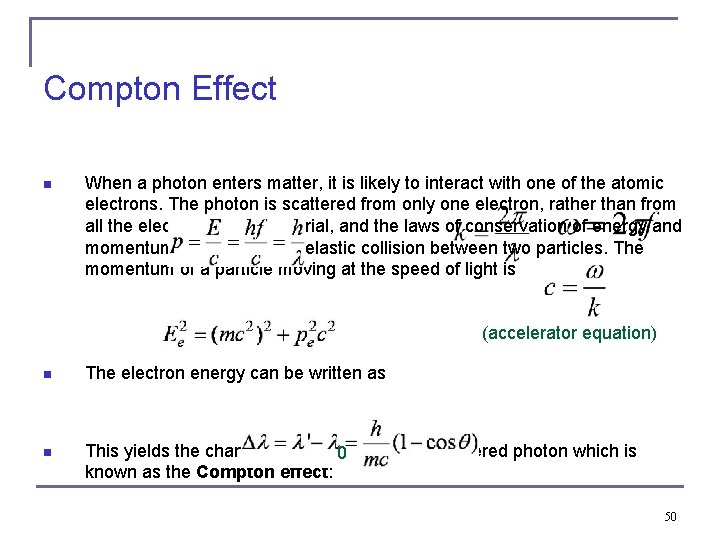
Compton Effect n When a photon enters matter, it is likely to interact with one of the atomic electrons. The photon is scattered from only one electron, rather than from all the electrons in the material, and the laws of conservation of energy and momentum apply as in any elastic collision between two particles. The momentum of a particle moving at the speed of light is (accelerator equation) n The electron energy can be written as n This yields the change in wavelength of the scattered photon which is 0 known as the Compton effect: 50

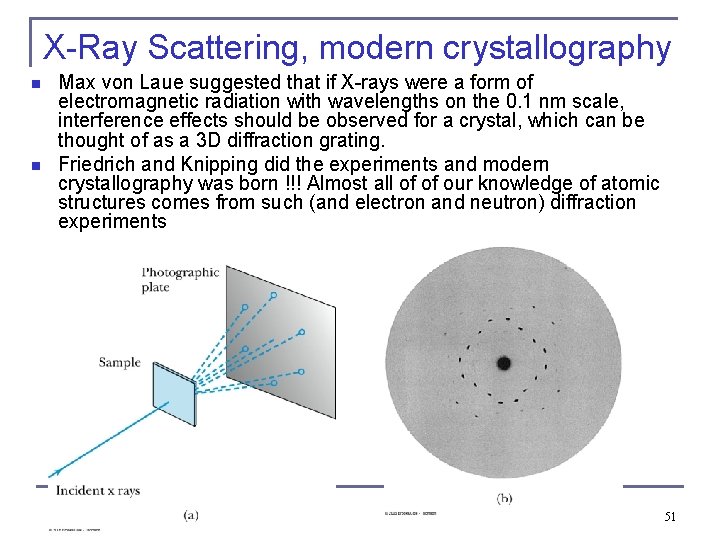
X-Ray Scattering, modern crystallography n n Max von Laue suggested that if X-rays were a form of electromagnetic radiation with wavelengths on the 0. 1 nm scale, interference effects should be observed for a crystal, which can be thought of as a 3 D diffraction grating. Friedrich and Knipping did the experiments and modern crystallography was born !!! Almost all of of our knowledge of atomic structures comes from such (and electron and neutron) diffraction experiments 51

Wave – particle duality, “wavical” Taoism” Taijitu (literally "diagram of the supreme ultimate" No problem, Bohr’s complementarily 52

Dual nature of quantum mechanical objects both/neither particle and/nor wave http: //usatoday 30. usatoday. com/tech/science/genetics/2008 -05 -08 -platypus-geneticmap_N. htm “Australia's unique duck-billed platypus is part bird, part reptile and part mammal according to its gene map. The platypus is classed as a mammal because it has fur and feeds its young with milk. It flaps a beaver-like tail. But it also has bird and reptile features — a duck-like bill and webbed feet, and lives mostly underwater. Males have venom-filled spurs on their heels. ” 53

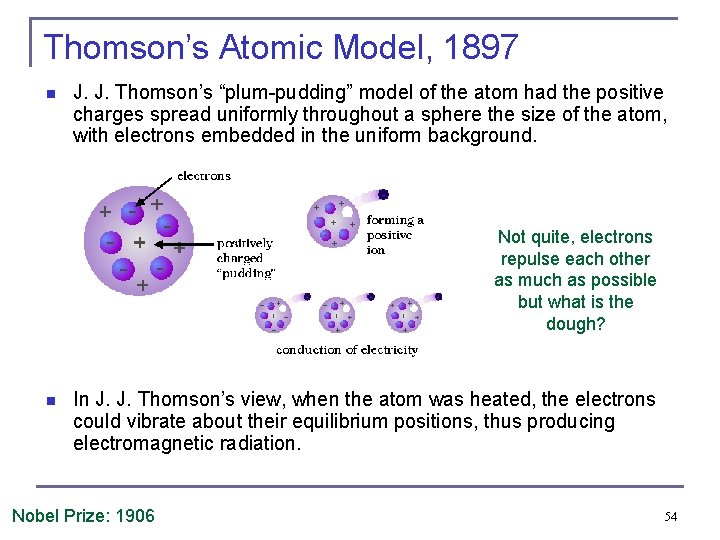
Thomson’s Atomic Model, 1897 n J. J. Thomson’s “plum-pudding” model of the atom had the positive charges spread uniformly throughout a sphere the size of the atom, with electrons embedded in the uniform background. Not quite, electrons repulse each other as much as possible but what is the dough? n In J. J. Thomson’s view, when the atom was heated, the electrons could vibrate about their equilibrium positions, thus producing electromagnetic radiation. Nobel Prize: 1906 54

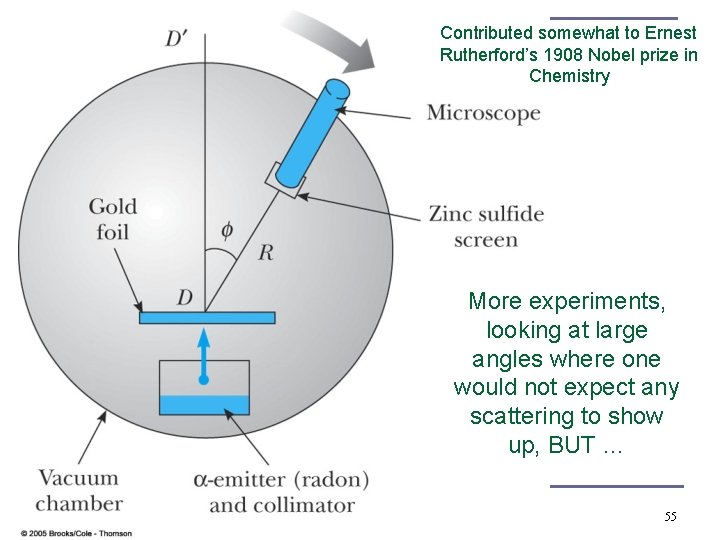
Contributed somewhat to Ernest Rutherford’s 1908 Nobel prize in Chemistry More experiments, looking at large angles where one would not expect any scattering to show up, BUT … 55

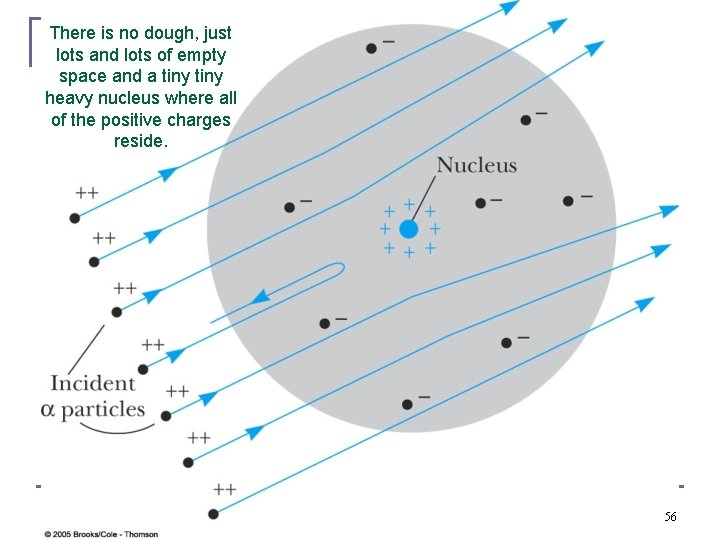
There is no dough, just lots and lots of empty space and a tiny heavy nucleus where all of the positive charges reside. 56

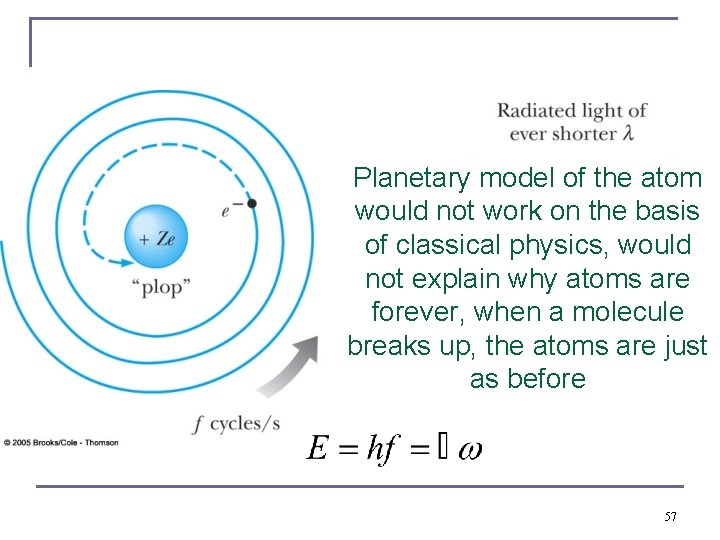
Planetary model of the atom would not work on the basis of classical physics, would not explain why atoms are forever, when a molecule breaks up, the atoms are just as before 57

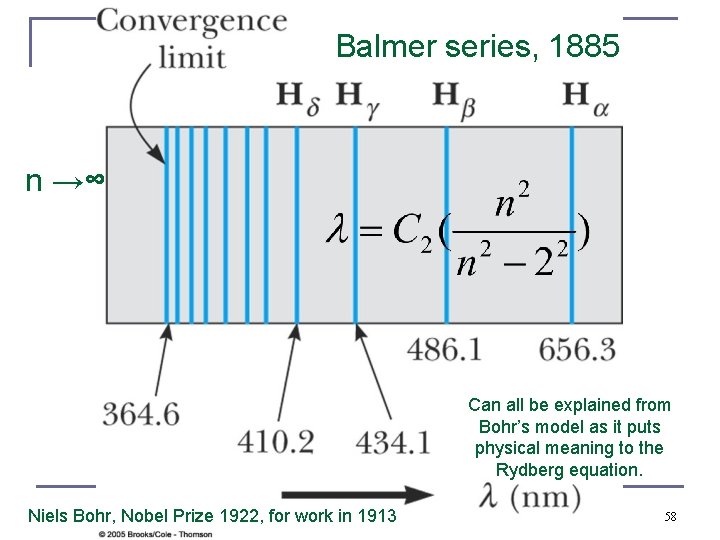
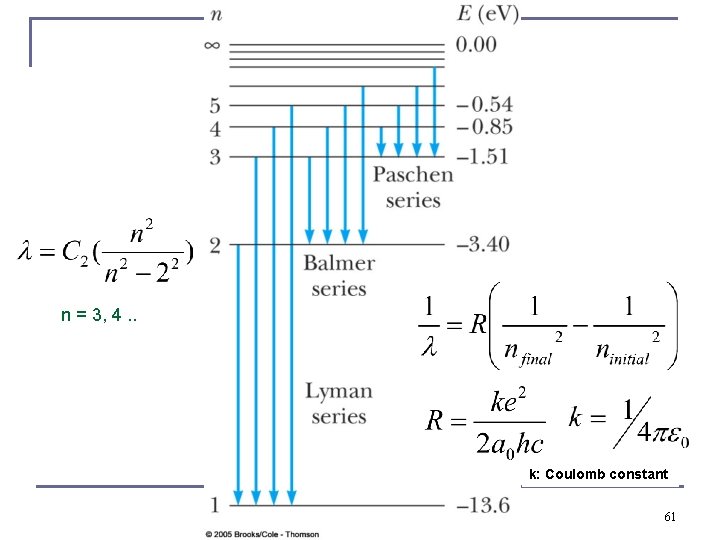
Balmer series, 1885 n →∞ Can all be explained from Bohr’s model as it puts physical meaning to the Rydberg equation. Niels Bohr, Nobel Prize 1922, for work in 1913 58

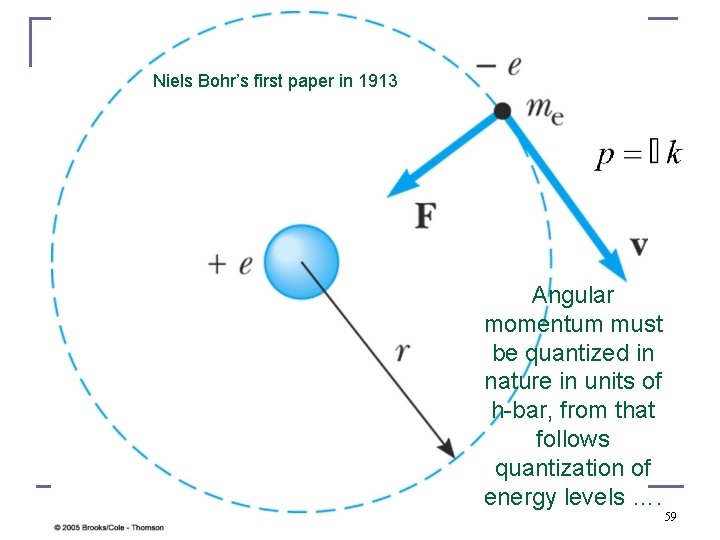
Niels Bohr’s first paper in 1913 Angular momentum must be quantized in nature in units of h-bar, from that follows quantization of energy levels …. 59

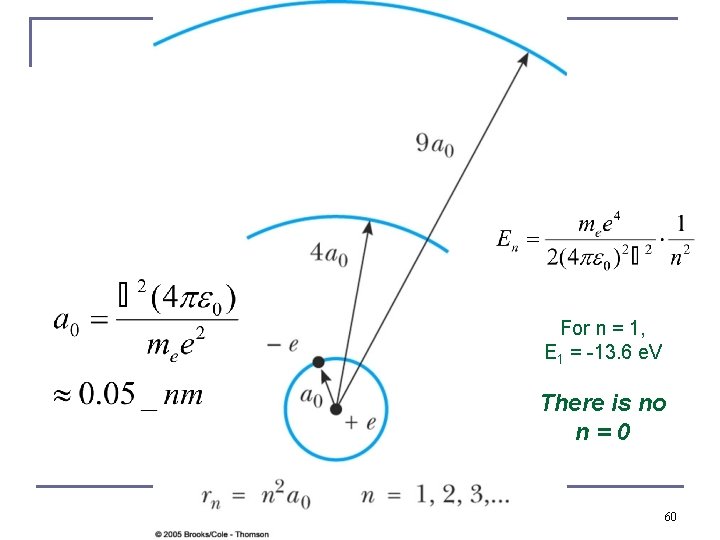
For n = 1, E 1 = -13. 6 e. V There is no n=0 60

n = 3, 4. . k: Coulomb constant 61

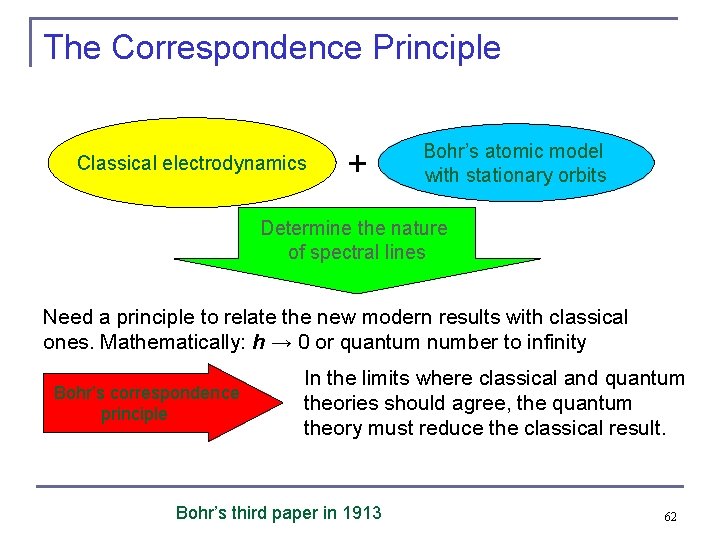
The Correspondence Principle Classical electrodynamics + Bohr’s atomic model with stationary orbits Determine the nature of spectral lines Need a principle to relate the new modern results with classical ones. Mathematically: h → 0 or quantum number to infinity Bohr’s correspondence principle In the limits where classical and quantum theories should agree, the quantum theory must reduce the classical result. Bohr’s third paper in 1913 62

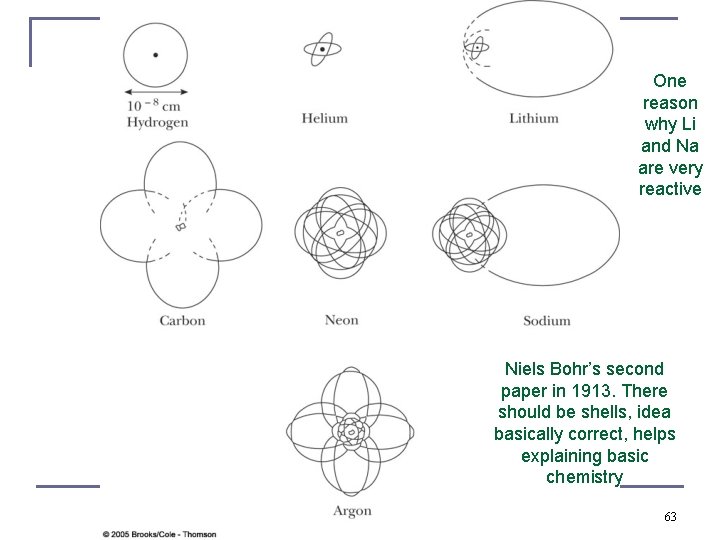
One reason why Li and Na are very reactive Niels Bohr’s second paper in 1913. There should be shells, idea basically correct, helps explaining basic chemistry 63

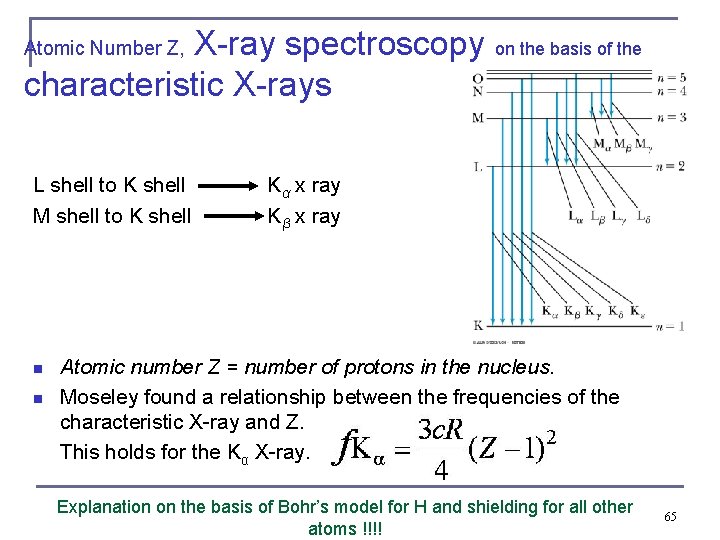
Characteristic X-Ray Spectra and Moseley’s law (kind of rules only when generalized) n Shells have letter names: K shell for n = 1 L shell for n = 2 n The atom is most stable in its ground state. after an inner electron has been kicked out by some process, an electron from higher shells will fill the inner-shell vacancy at lower energy. n n When it occurs in a heavy atom, the radiation emitted is an X ray photon. It has the energy E (X ray) = Eu − Eℓ. 64

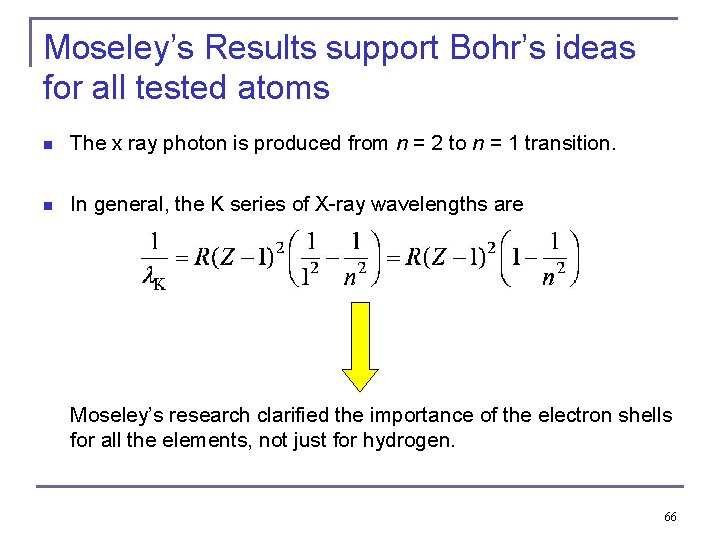
X-ray spectroscopy on the basis of the characteristic X-rays Atomic Number Z, L shell to K shell Kα x ray M shell to K shell Kβ x ray n n Atomic number Z = number of protons in the nucleus. Moseley found a relationship between the frequencies of the characteristic X-ray and Z. This holds for the Kα X-ray. Explanation on the basis of Bohr’s model for H and shielding for all other atoms !!!! 65

Moseley’s Results support Bohr’s ideas for all tested atoms The x ray photon is produced from n = 2 to n = 1 transition. n In general, the K series of X-ray wavelengths are n Moseley’s research clarified the importance of the electron shells for all the elements, not just for hydrogen. 66

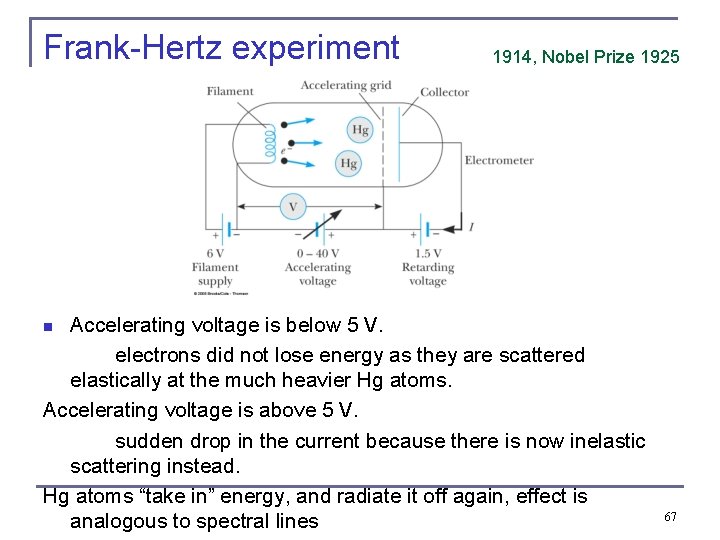
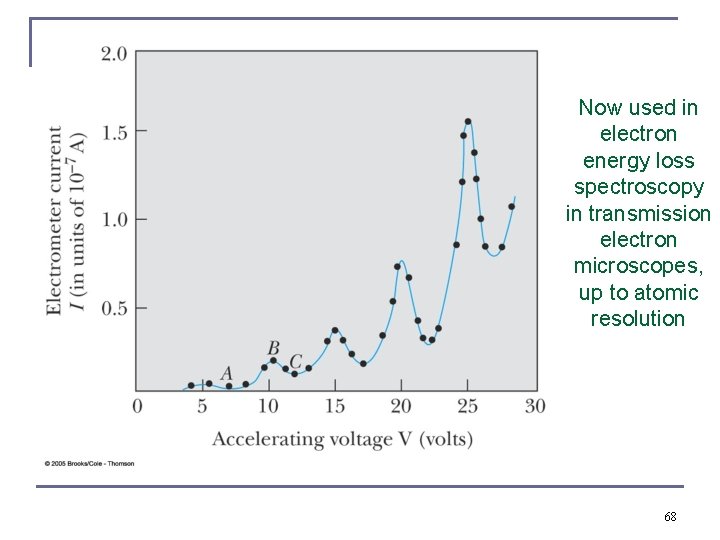
Frank-Hertz experiment 1914, Nobel Prize 1925 Accelerating voltage is below 5 V. electrons did not lose energy as they are scattered elastically at the much heavier Hg atoms. Accelerating voltage is above 5 V. sudden drop in the current because there is now inelastic scattering instead. Hg atoms “take in” energy, and radiate it off again, effect is analogous to spectral lines n 67

Now used in electron energy loss spectroscopy in transmission electron microscopes, up to atomic resolution 68

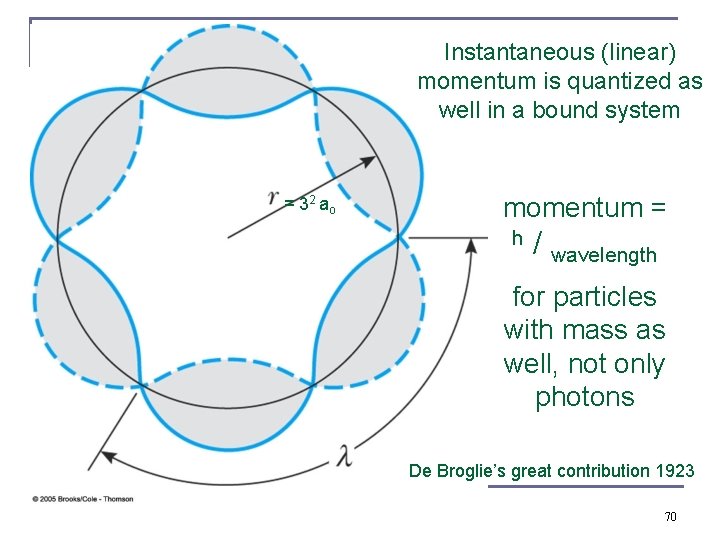
There should also be matter waves, not only classical and electromagnetic waves !!! Louis-Victor de Broglie, Ph. D thesis 1923, Nobel Prize 1929 Wave particle duality for matter leads us into quantum mechanics, condensed matter physics …. 1/ to 2/ of our 3 3 modern economy !!! 69

Instantaneous (linear) momentum is quantized as well in a bound system = 32 ao momentum = h / wavelength for particles with mass as well, not only photons De Broglie’s great contribution 1923 70

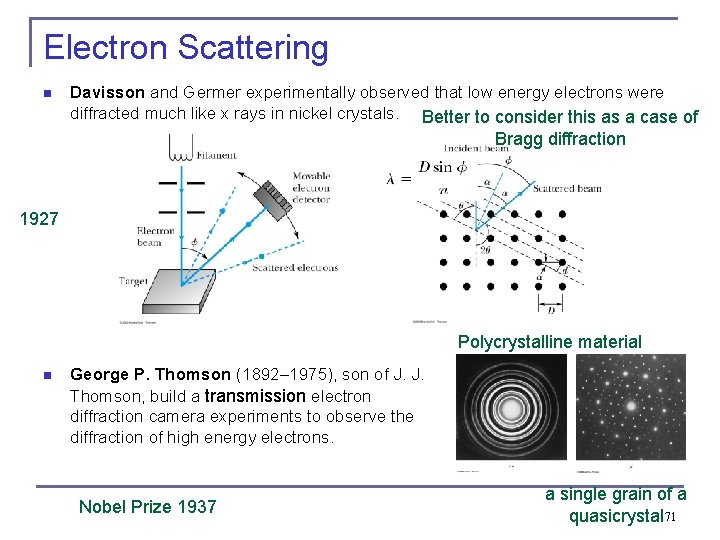
Electron Scattering n Davisson and Germer experimentally observed that low energy electrons were diffracted much like x rays in nickel crystals. Better to consider this as a case of Bragg diffraction 1927 Polycrystalline material n George P. Thomson (1892– 1975), son of J. J. Thomson, build a transmission electron diffraction camera experiments to observe the diffraction of high energy electrons. Nobel Prize 1937 a single grain of a quasicrystal 71

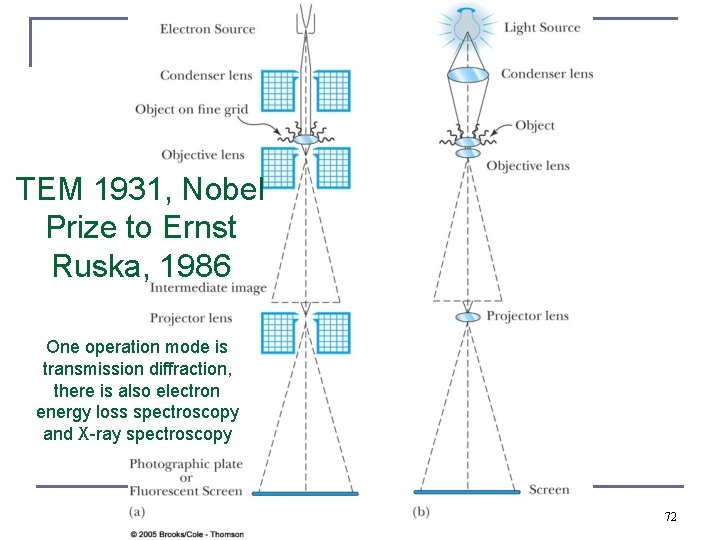
TEM 1931, Nobel Prize to Ernst Ruska, 1986 One operation mode is transmission diffraction, there is also electron energy loss spectroscopy and X-ray spectroscopy 72

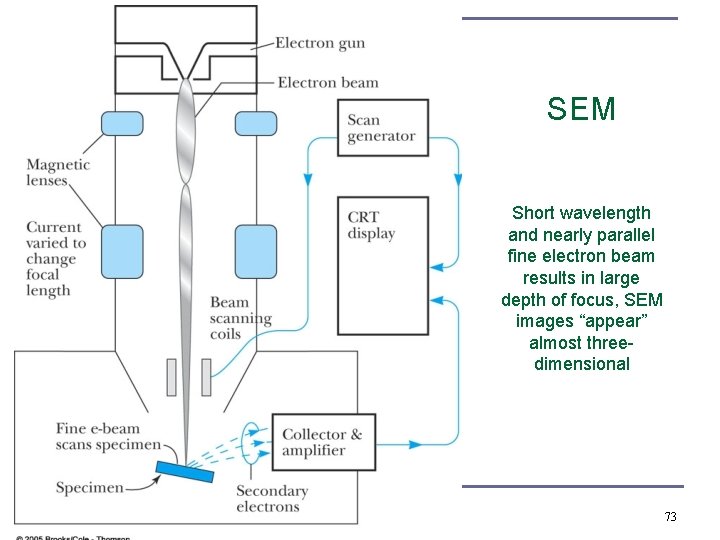
SEM Short wavelength and nearly parallel fine electron beam results in large depth of focus, SEM images “appear” almost threedimensional 73

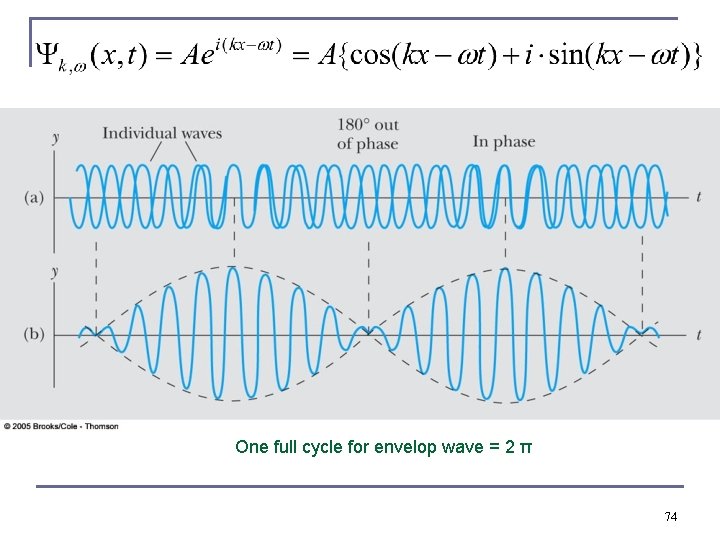
One full cycle for envelop wave = 2 π 74

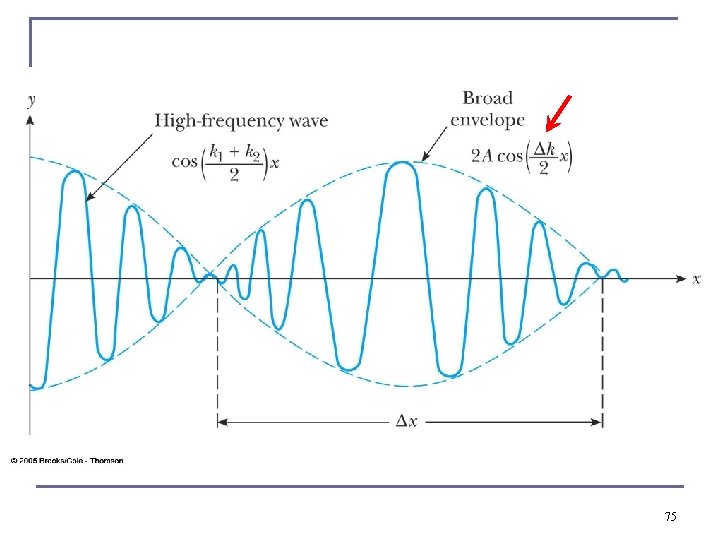
75

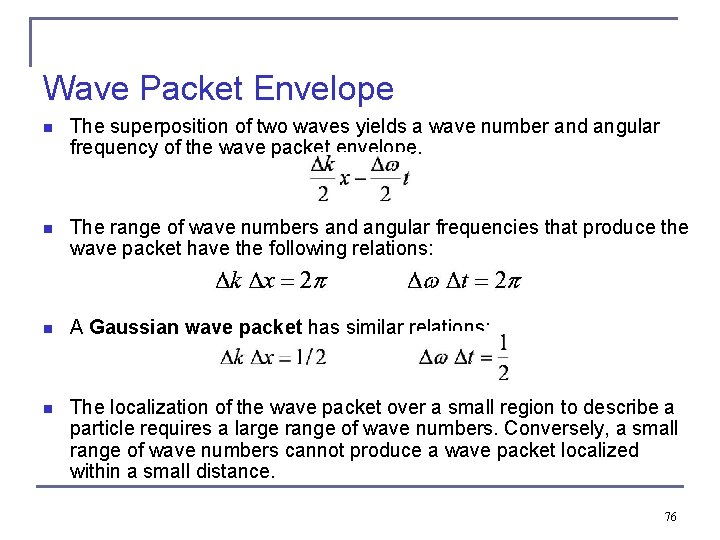
Wave Packet Envelope n The superposition of two waves yields a wave number and angular frequency of the wave packet envelope. n The range of wave numbers and angular frequencies that produce the wave packet have the following relations: n A Gaussian wave packet has similar relations: n The localization of the wave packet over a small region to describe a particle requires a large range of wave numbers. Conversely, a small range of wave numbers cannot produce a wave packet localized within a small distance. 76

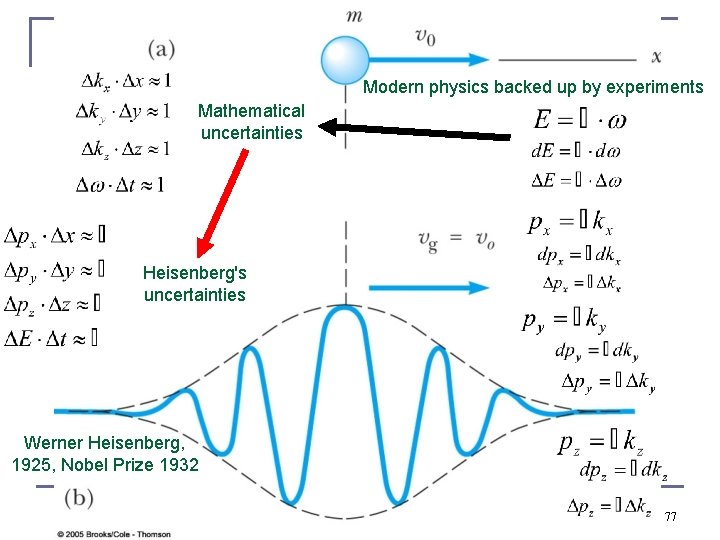
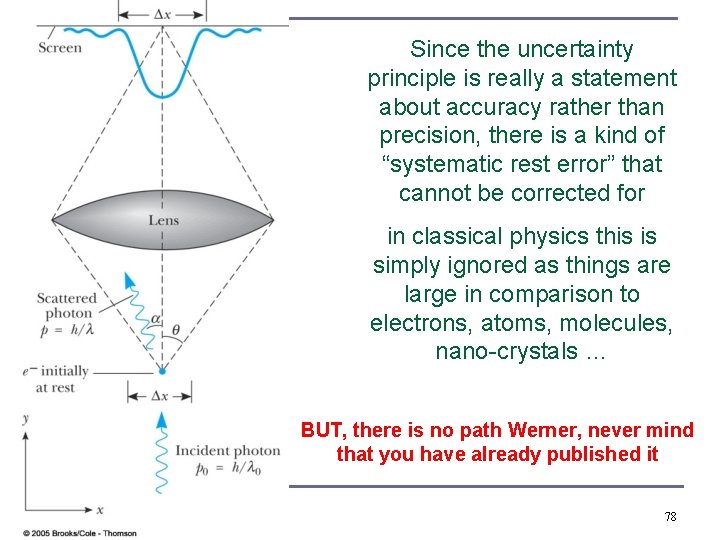
Modern physics backed up by experiments Mathematical uncertainties Heisenberg's uncertainties Werner Heisenberg, 1925, Nobel Prize 1932 77

Since the uncertainty principle is really a statement about accuracy rather than precision, there is a kind of “systematic rest error” that cannot be corrected for in classical physics this is simply ignored as things are large in comparison to electrons, atoms, molecules, nano-crystals … BUT, there is no path Werner, never mind that you have already published it 78

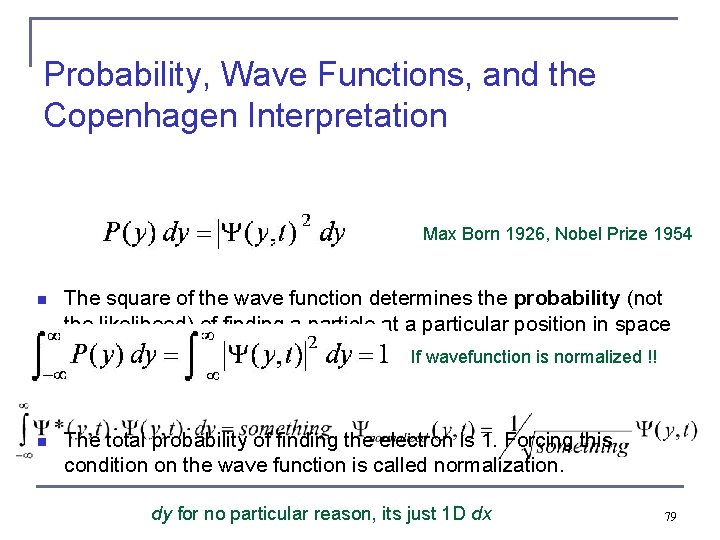
Probability, Wave Functions, and the Copenhagen Interpretation Max Born 1926, Nobel Prize 1954 n The square of the wave function determines the probability (not the likelihood) of finding a particle at a particular position in space at a given time. If wavefunction is normalized !! n The total probability of finding the electron is 1. Forcing this condition on the wave function is called normalization. dy for no particular reason, its just 1 D dx 79

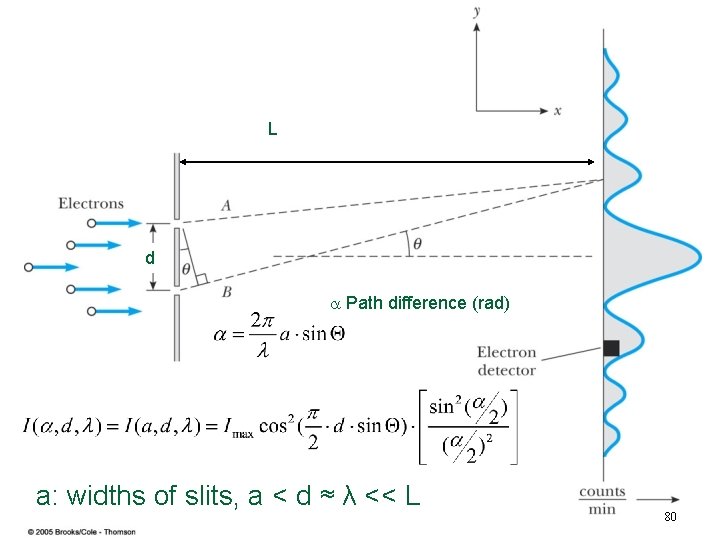
L d Path difference (rad) a: widths of slits, a < d ≈ λ << L 80

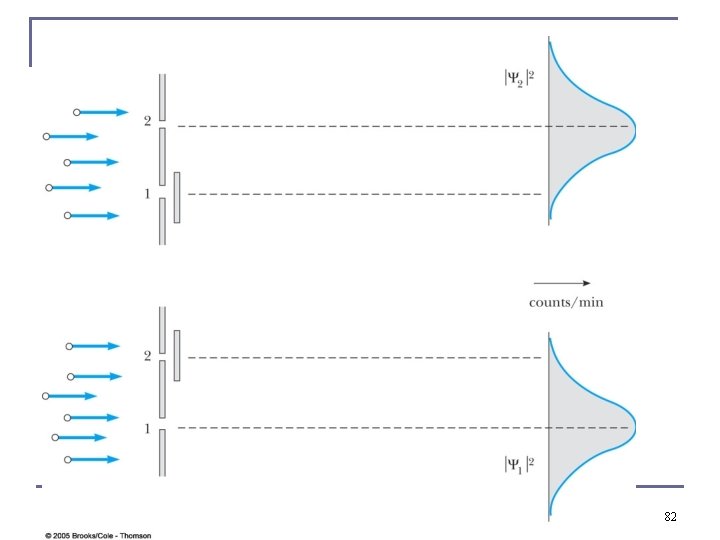
81

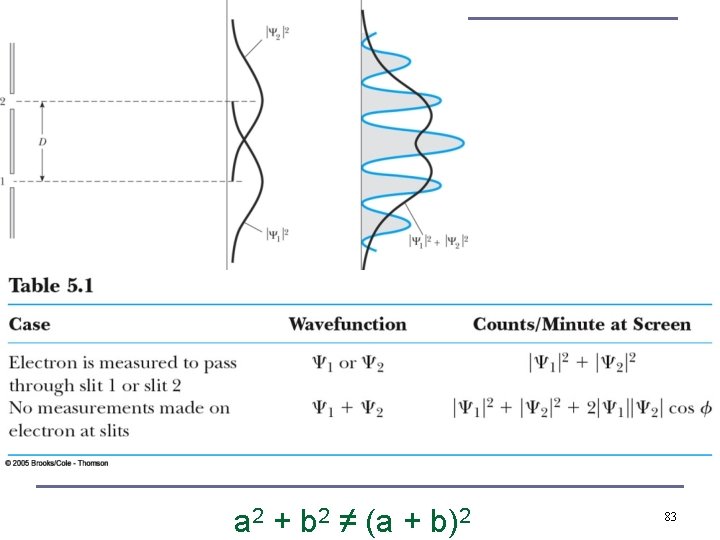
82

a 2 + b 2 ≠ (a + b)2 83

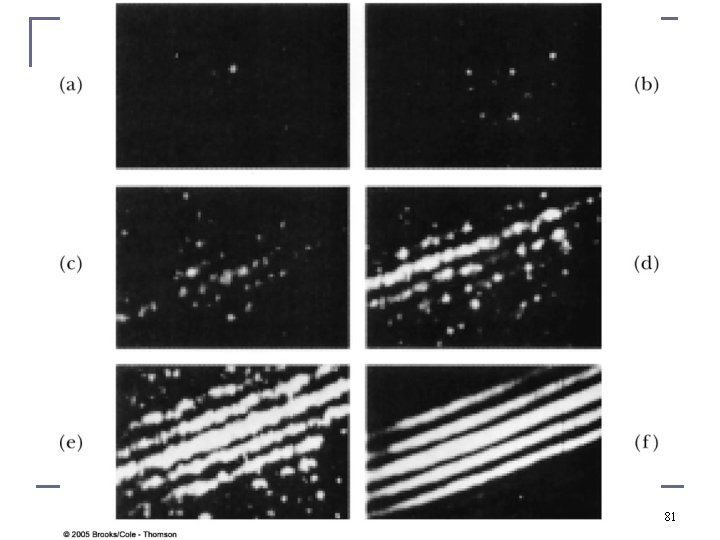
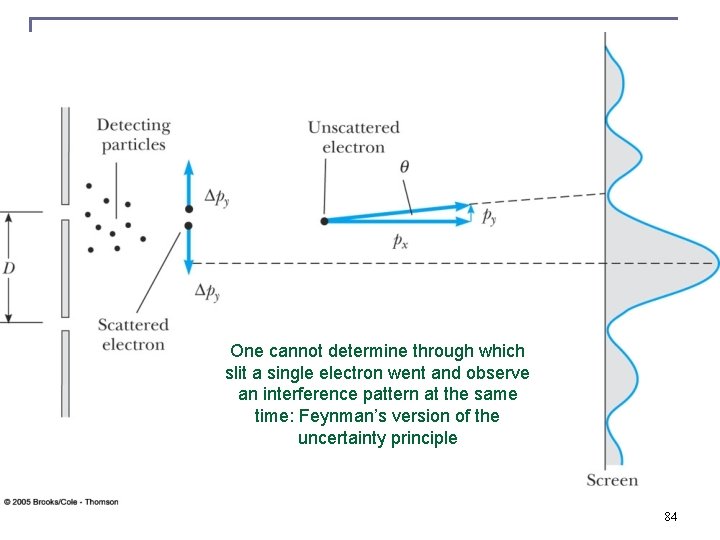
One cannot determine through which slit a single electron went and observe an interference pattern at the same time: Feynman’s version of the uncertainty principle 84

http: //www. feynmanlectures. caltech. edu/I_37. html http: //www. informationphilosopher. com/solutions/scientists/feynman/probability _and_uncertainty. html It is impossible to observe through which slit the quantum mechanical particle went in a double slit experiment and not to destroy its contribution to the double slit interference pattern at the same time. n Feynman’s version of the uncertainty principle: n Direct Quote: “The uncertainty principle “protects” quantum mechanics. Heisenberg recognized that if it were possible to measure the momentum and the position simultaneously with a greater accuracy, the quantum mechanics would collapse. So he proposed that it must be impossible. Then people sat down and tried to figure out ways of doing it, and nobody could figure out a way to measure the position and the momentum of anything—a screen, an electron, a billiard ball, anything—with any greater accuracy. Quantum mechanics maintains its perilous but accurate existence. ” n Last lecture in PH 312, experimentally observed violations of so called Bell inequalities demonstrate that quantum mechanics is complete and Heisenberg’s uncertainty principle is here to stay 85

n n n The Copenhagen Interpretation Copenhagen’s interpretation of the wave function (quantum mechanics in its final and current form) consisted of 3 (to 4) principles: 1) 2) 3) The complementarity principle of Bohr The uncertainty principle of Heisenberg The statistical interpretation of Born, based on probabilities determined by the wave function 4) Bohr’s correspondence principle (for quantum mechanics being reasonable in spite of being counterintuitive) Together these concepts form a logical interpretation of the physical meaning of quantum theory. According to the Copenhagen interpretation, physics needs to make predictions on the outcomes of future experiments (measurement) on the basis of theoretical analysis of previous experiments (measurements) Physics is not about “the truth”, questions that cannot be answered by experiments (measurements) are meaningless to the modern physicist. Philosophers, priests, gurus, … can be asked these questions and often answer them. Problem: they tend to disagree … 86

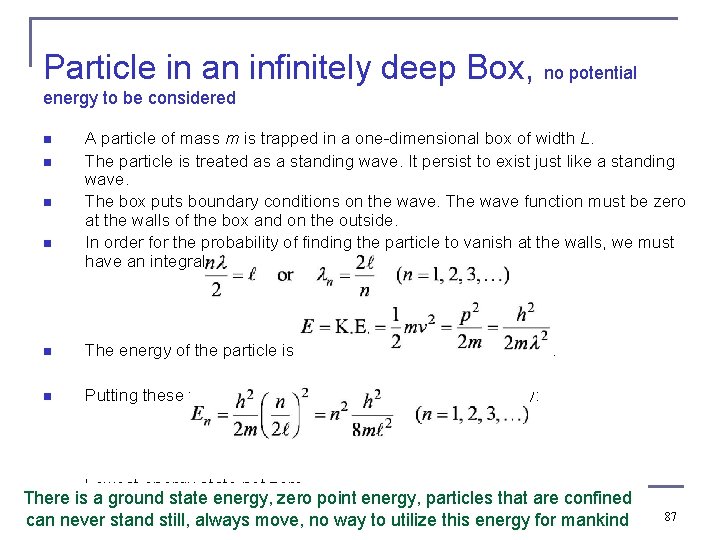
Particle in an infinitely deep Box, no potential energy to be considered n n A particle of mass m is trapped in a one-dimensional box of width L. The particle is treated as a standing wave. It persist to exist just like a standing wave. The box puts boundary conditions on the wave. The wave function must be zero at the walls of the box and on the outside. In order for the probability of finding the particle to vanish at the walls, we must have an integral number of half wavelengths in the box. n The energy of the particle is n Putting these two relations together yields quantized energy: n Lowest energy state not zero. . There is a ground state energy, zero point energy, particles that are confined can never stand still, always move, no way to utilize this energy for mankind 87

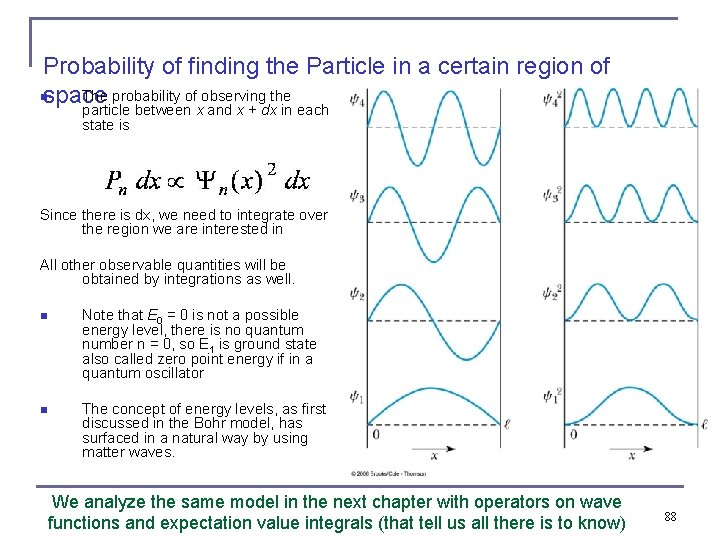
Probability of finding the Particle in a certain region of nspace The probability of observing the particle between x and x + dx in each state is Since there is dx, we need to integrate over the region we are interested in All other observable quantities will be obtained by integrations as well. n Note that E 0 = 0 is not a possible energy level, there is no quantum number n = 0, so E 1 is ground state also called zero point energy if in a quantum oscillator n The concept of energy levels, as first discussed in the Bohr model, has surfaced in a natural way by using matter waves. We analyze the same model in the next chapter with operators on wave functions and expectation value integrals (that tell us all there is to know) 88

time dependent Helmholtz approx. due to the uncertainty principle, we can only make statistical inferences 89

Given the wave particle duality, we need a new way of thinking. The whole physical situation is described by a wave function (which is complex for a traveling matter wave). If the particle is not free, it’s wave function need to account for the physical boundary conditions, which “encodes” the nature of the physical problem. To check if the wave function we came up with makes physical sense, we put it to the “Schrödinger equation test”. (It’s a test if our wave function obeys the conservation of total energy (while ignoring rest energy and with that special relativity – if we need to include that, i. e. v > 0. 01 c, we need to make the Dirac equation test) If our wave function is OK, we can calculate anything we are allowed to know (given the uncertainty principle) about the quantum mechanical system from it. So the first part of PH 312 is all about how to do these things. 90

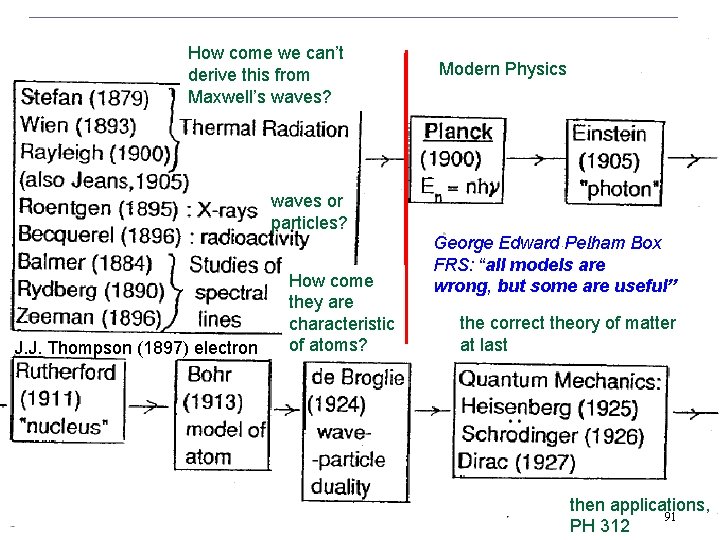
How come we can’t derive this from Maxwell’s waves? Modern Physics waves or particles? J. J. Thompson (1897) electron How come they are characteristic of atoms? George Edward Pelham Box FRS: “all models are wrong, but some are useful” the correct theory of matter at last then applications, 91 PH 312