Chapter 2 Viral Hepatitis and Hepatitis Vaccines Copyright





























- Slides: 60

Chapter 2 Viral Hepatitis and Hepatitis Vaccines Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Viral Hepatitis – Overview • Major HCP occupational concern • At least 7 distinct viruses identified – Short & long incubation intervals – Acute & chronic disease • Variable & subclinical symptomatology Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

RNA Viruses Involved in Human Hepatitis • Picornaviruses (enteroviruses) – HAV, HDV, Coxsackie & Echo viruses • Flaviviruses – HCV • Calciviruses – HEV • Togaviruses – Yellow fever & rubella viruses Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

RNA Viruses Involved in Human Hepatitis (cont’d) • Arenavirus – Junin virus – Machup virus – Lassa virus – Rift Valley Fever virus Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

RNA Viruses Involved in Human Hepatitis (cont’d) • Rhabdoviruses – Marburg virus – Ebola virus • Paramyxovirus – Measles virus Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

DNA Viruses Involved in Human Hepatitis • Hepadnavirus – HBV • Herpesviruses – Cytomegalovirus – Epstein-Barr – Herpes simplex • Varicella-Zoster virus Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Unclassified Viruses Involved in Human Hepatitis • Hepatitis F virus • Hepatitis G virus • Transfusion-Transmitted virus (TTV) Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

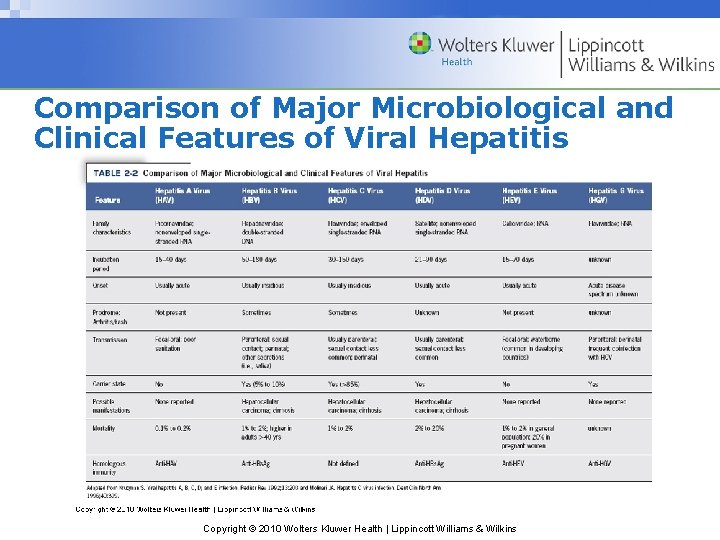
Comparison of Major Microbiological and Clinical Features of Viral Hepatitis Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Classical Division of Hepatitis Conditions • Prodromal phase • Icteric phase – Marked by jaundice • Convalescent phase Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Prodromal Phase • Non-specific respiratory and/or GI symptoms – Malaise, loss of appetite, headache & nausea • Any fever usually low-grade • HBV produces symptoms before seroconversion – Arthritis & maculopapular skin rashes Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Icteric Phase • Jaundice – yellowing – Skin, sclera, mucosa, nail beds & gingiva – By bilirubin & other bile pigments – Hallmark hepatitis manifestation • Dark & foamy urine • Grayish-white stool color Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Icteric Phase (cont’d) • Most infections may not cause jaundice • Elevated liver enzymes before/at clinical onset – Aminotransferase & transaminases • Hepatitic tenderness, hepatomegaly & splenomegaly Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Convalescent (Recovery) Phase • Icteric manifestations disappear • Malaise & fatigue may persist for months • Possible long-term, chronic sequelae Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Question 1 Persons with hepatitis A are most infectious A) During the incubation period. B) Early in the prodromal phase. C) In the last 2 weeks of the prodromal phase. D) Immediately after the onset of jaundice. E) During the convalescent phase. Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Answer Persons with hepatitis A are most infectious C) In the last 2 weeks of the prodromal phase. The greatest infectivity potential occurs during the 2‑week period immediately before the onset of jaundice. Onset of symptoms is rapidly followed by a decrease in both viremia and infectiousness. Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Hepatitis A • Picornavirus family • Small, single-stranded 27 nm RNA agent • Humans are only natural host • More temperature & p. H stable than enteroviruses – Remains infectious at moderate temp & low p. H Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Hepatitis A (cont’d) • Survives in feces & exudates • Stable in environment for months – Inanimate surfaces • Inactivated by high temperature, formalin & chlorine Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Hepatitis A (cont’d) • First described in 1973 • Common in many developing countries • Decreasing incidence in U. S. – 42, 000 new infections in 2005 (CDC estimate) – Still >30% of reported acute hepatitis cases Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Hepatitis A Transmission • Person-to-person contact • Fecal-oral contamination • Indirect via contaminated water or food – Raw or inadequately cooked shellfish • 45% of U. S. cases have no known risk factors Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Risk Factors for Hepatitis A Transmission • Day care center contact • International travel • Intravenous drug use • Men who have sex with men • Persons with chronic liver disease Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Hepatitis A Transmission and Epidemiology • Serial human-human propagation – No carrier state or chronic hepatitis • Incubation period of 15 - 40 days (average 28 – 30) • Highest infectivity in late incubation to early prodromal – Viremia decreases rapidly after symptom onset Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

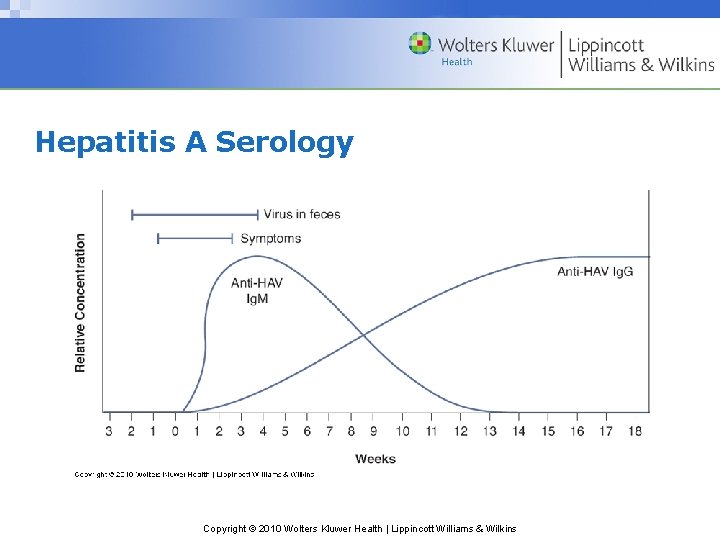
Hepatitis A Serology Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Hepatitis A Serology (cont’d) • Ig. M-class anti-HAV during/soon after acute phase • Ig. G antibodies replace anti-HAV – High concentration in 4 – 6 weeks – Remain detectable thereafter – Confer lifelong protection against HAV Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Hepatitis A Vaccines • Inactivated hepatitis A virus • VAQTA, Havrix (1995) & Twinrix (2001) • For children in high-incidence areas (CDC, 1999) • Recommended for all children (2006) Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Hepatitis B • Seroprevalence varies greatly worldwide • Most transmissions perinatal where endemic • Causes 80% of primary liver cancers • U. S. incidence decreasing – Vaccine & infection control precautions Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Prevalence of Hepatitis B in U. S. • 260, 000 infected in the 1980’s • 78, 000 infected in 2001 • 51, 000 infected in 2005 • 1 million carriers – 20% - 40% will develop life-threatening conditions Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

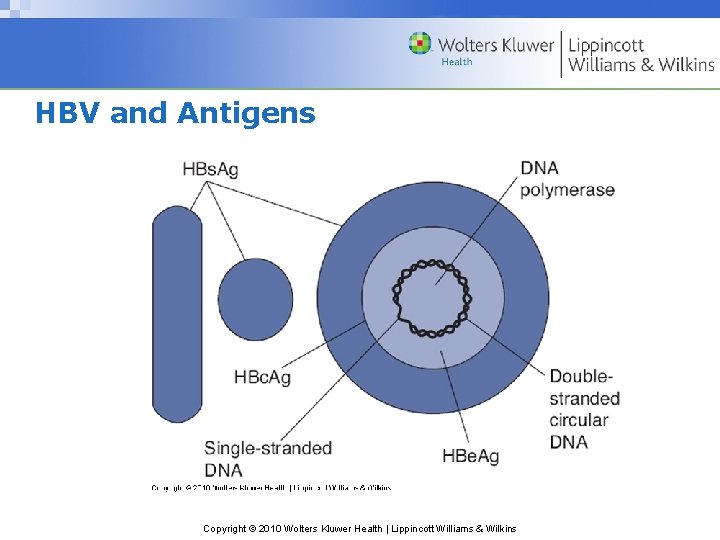
HBV and Antigens Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

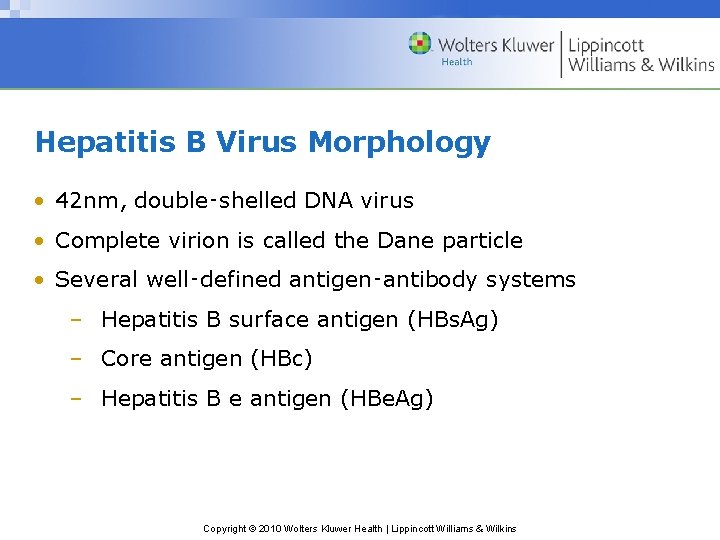
Hepatitis B Virus Morphology • 42 nm, double‑shelled DNA virus • Complete virion is called the Dane particle • Several well‑defined antigen‑antibody systems – Hepatitis B surface antigen (HBs. Ag) – Core antigen (HBc) – Hepatitis B e antigen (HBe. Ag) Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

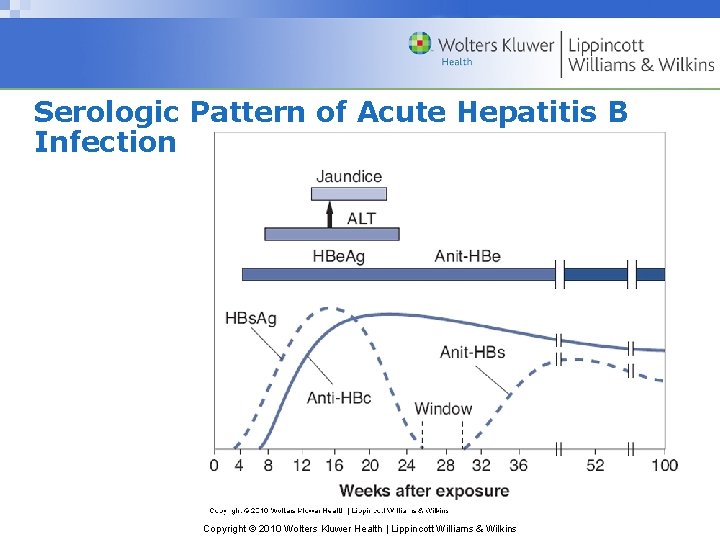
Serologic Pattern of Acute Hepatitis B Infection Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

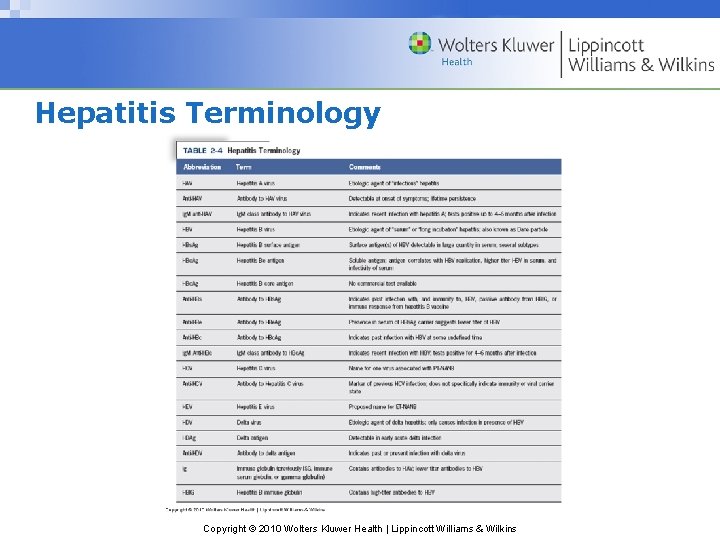
Hepatitis Terminology Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Interpretation of the Hepatitis B Serologic Profiles Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

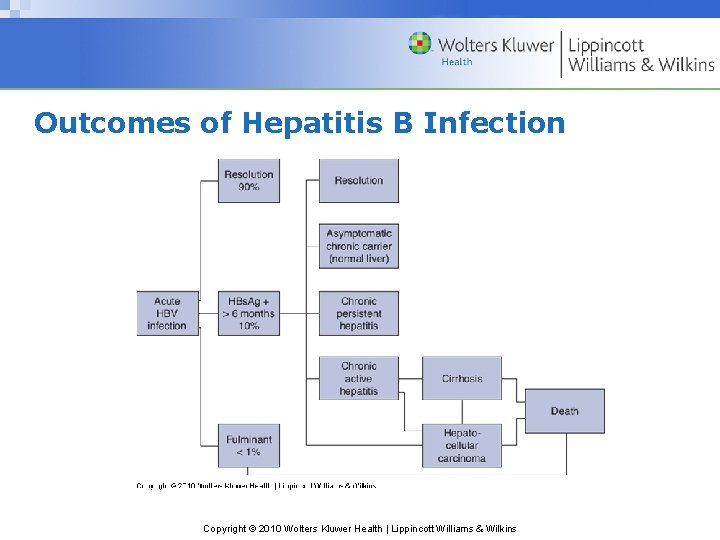
Outcomes of Hepatitis B Infection Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Serologic Profile of HBV Carrier State Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Hepatitis B Clinical Course • Incubation period of 50 – 180 days (average 60 – 120) • Variety of ultimate outcomes • Chronic infection in ~ 5 -10% of adolescents & adults • Most cases anicteric (no jaundice) • As many as 80% of infections undiagnosed Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Clinical Signs And Symptoms of Acute Hepatitis B Infection • Anorexia • Jaundice • Malaise • Skin rashes • Nausea • Arthralgia • Vomiting • Arthritis • Abdominal pain Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Symptoms of Chronic HBV Infection • Disease-specific • Nausea – Cirrhosis • Anorexia – Hepatocellular carcinoma • Upper right quadrant tenderness • Severe fatigue Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

HBV Transmission Modes • Parenteral – Percutaneous – efficient, 7+ day incubation – Non-percutaneous – inefficient, ~54 d incubation • Longer period to transmit disease • Sexual • Vertical (perinatal & parent-child contact) Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Prevalence of Hepatitis B Serologic Markers Among DHCP (1979 -81) Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

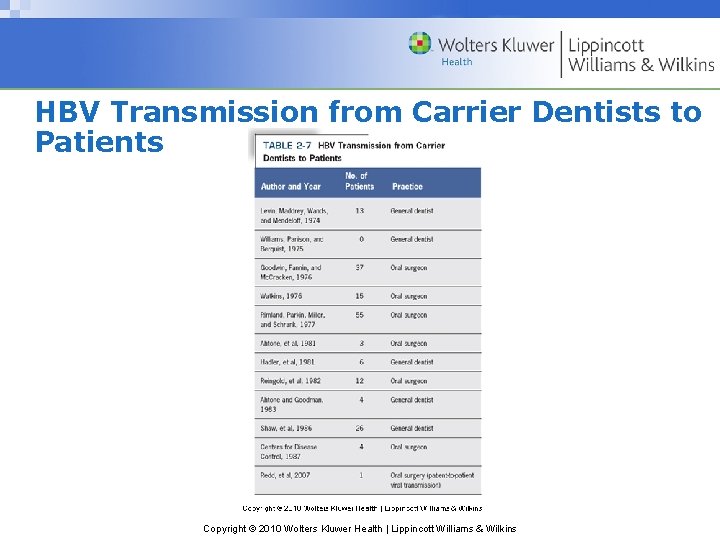
Prevalence of HBV Infection in Dentistry • 13. 6% of general practitioners positive in 1976 • 8. 5% of dentists & 6. 8% of hygienists positive in 2005 • Greater exposure risk than general population – General dentists: 3 x risk – Oral surgeons: 6 x risk Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

HBV Transmission from Carrier Dentists to Patients Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Active Immunity • Antigens stimulate immune response • Long-term protection after a latent period • Conferred by – Host recovery from infection – Vaccination Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Passive Immunity • Antibodies from actively immunized host – Ig (ISG) – against HAV primarily, inexpensive – HBIG – against HBV for 2 months, expensive • Protection immediate & transitory Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Plasma-Derived HBV Vaccine (1982) • Serum from carriers, high HBs. Ag concentration – Boiled 1 minute (Krugman, et al. ) – Lost infectivity & retained antigenicity • Viral coat protein extraction & purification • No HIV transmission risk • Heptavax‑B discontinued in 1989 Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

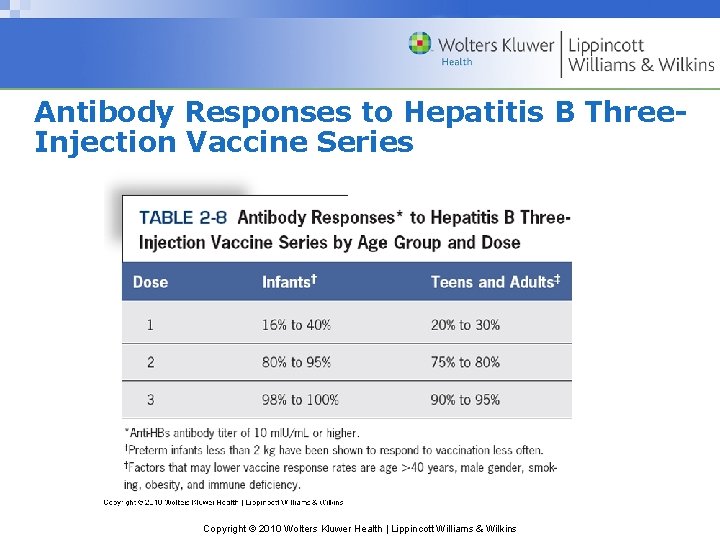
Antibody Responses to Hepatitis B Three. Injection Vaccine Series Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Recombinant DNA HBV Vaccines (1986) • Gene for HBs. Ag inserted into yeast plasmid • HBs. Ag harvested from lysed yeast cells • Final product has 10 μg HBs. Ag protein/m. L • Recombivax‑HB & Engerix‑B Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Pre-Testing (HBV Vaccination) • Cost-effective when prevalence high – Anti-HBs-positive persons don’t need vaccine • Not cost-effective in average dental office – Only 6. 7% of DHCP are positive • May be offered per 1991 OSHA BBP Standard • Significant number of false-positives Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Post-Testing (HBV Vaccination) • Preferably 1 -2 months after 3 rd inoculation • Second 3 -dose course for non-responders • After years, anti-HBs-negative recipient may be – Primary non-responder, susceptible – Antibody level undetectably low, immune Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Question 2 After administration of hepatitis B vaccine, blood should be tested for A) HBs. Ag within 1 -2 months. B) Anti-HBs within 6 months. C) HBs. Ag every 10 years. D) Anti-HBs every year. E) Both A and D Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Answer After administration of hepatitis B vaccine, blood should be tested for B) Anti-HBs within 6 months. HBs. Ag (surface antigen) indicates infection, not immunity. Post‑testing should be scheduled soon after the last inoculation, preferably within 1 -2 months, because antibody levels may become undetectably low over time. Annual testing is not required because the protective anamnestic response is very long-term, even if anti-HBs is no longer detectable. Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

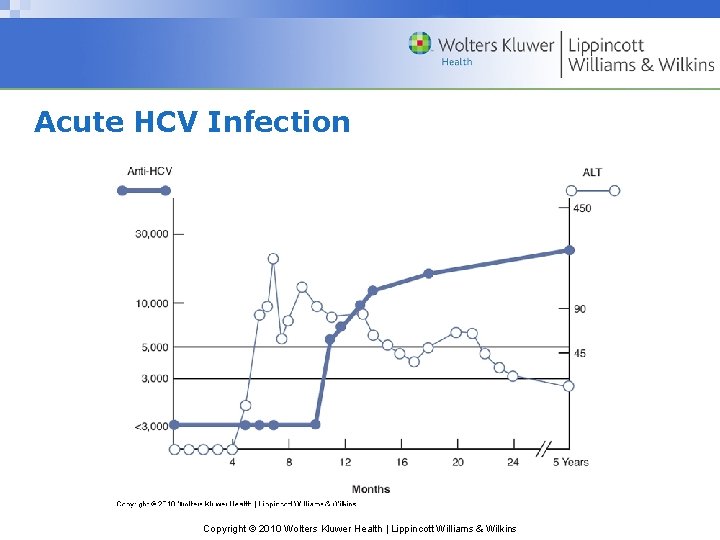
Acute HCV Infection Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Hepatitis C • RNA virus related to genera Flavivirus & Pestivirus • Strains exhibit high genetic diversity – Mutation within host during replication – Very high (85%) rate of chronic infection – No vaccine or PEP available Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Hepatitis C – Transmission • Illegal intravenous drug use – 60% • Other routes have low transmission rates • Transmitted inefficiently by occupational exposures – 1. 8% of percutaneous exposures • Low risk from environmental contamination Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Hepatitis C – Acute Infection • Incubation period 30 – 150 days • 60%-70% have no or nonspecific symptoms • Less hepatic inflammatory response vs. HAV & HBV Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

HCV Infection in Dentistry • Prevalence among HCP has declined • Similar incidence as general population (1%-2%) • Risk of surgeon to patient transmission (0. 17%) • Limited risk of DHCP to patient transmission Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

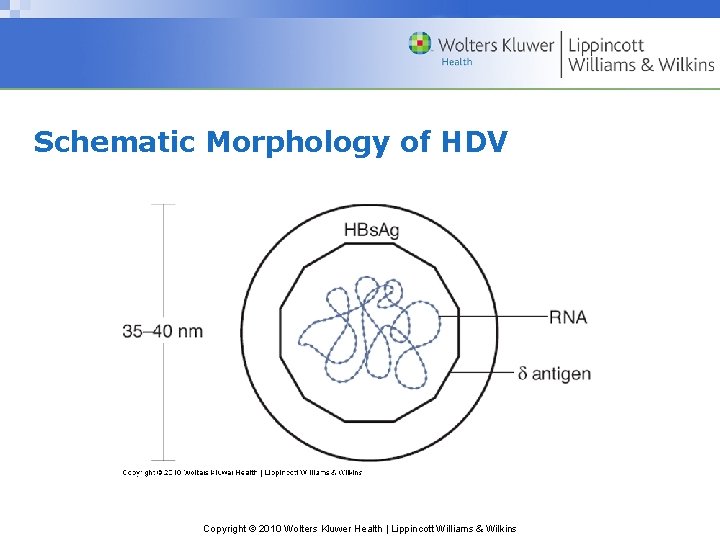
Schematic Morphology of HDV Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Delta Hepatitis • Requires HBV as helper virus – Outer protein coat (HBs. Ag) & replication – Active HBV immunity protects against HDV • Endemic in Mediterranean, parts of Africa & S. America – Transmitted by intimate contact & transmucosally • Transmitted percutaneously where not endemic Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

HDV Infection Modes • Simultaneous infection with HBV and HDV – Often resolves with limited clinical course • Acute delta superinfection in HBV carriers – Already have high titer of circulating HBs. Ag – More serious, fulminating & chronic infections Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Hepatitis E Virus • Single-stranded RNA calcivirus • Transmitted enterically via fecal-oral route • No outbreaks in U. S. , Europe or Australia • Very low transmission risk in occupational exposures • Incubation period 15 – 70 days • Generally self-limiting, except during pregnancy Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Question 3 Which of the following requires an additional etiologic agent for clinical expression? A) Hepatitis A virus B) Hepatitis B virus C) Hepatitis C virus D) Hepatitis D virus E) Hepatitis E virus Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins

Answer Which of the following requires an additional etiologic agent for clinical expression? D) Hepatitis D virus Although HDV is unique and distinct from HBV, it requires HBV as a helper virus for an outer protein coat (HBs. Ag), and thus for replication. Copyright © 2010 Wolters Kluwer Health | Lippincott Williams & Wilkins